I. Made Suma Wirawan1, Zainul Muttaqin2, Novitasari3, I. Gde Raka Widiana4, I. Gst Alit Artha5 and I. Nyoman Mantik Astawa6
1Doctoral of Program, Udayana University, Denpasar, Bali, Indonesia.
2Biomedical Research Unit, West Nusa Tenggara General Hospital, Lombok Indonesia.
3Pathology Anatomy Laboratorium, Wangaya Hospital, Denpasar, Bali, Indonesia.
4Department of Nephrology and Hypertension Division, Faculty of Medicine Udayana University, Denpasar Bali, Indonesia.
5Pathology Anatomy Department of Faculty Medicine,Udayana University, Bali, Indonesia.
6Veterinary Faculty of Udayana University, Denpasar, Bali, Indonesia.
Corresponding Author E-mail: suma_wirawan@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1245
Abstract
Helicobacter pylori infection (H. pylori) is associated with gastric carcinoma and various of gastrointestinal disease. The prevalence of H. pylori infection in Indonesia is still controversial. There is no data about H. pylori Bali isolates and the Cag A virulence factor from patients with dyspepsia in Bali.H. pylori Bali isolates provided from Laboratorium of Microbiology, Biomedical Research Unit, West Nusatenggara General Hospital, which have been isolated from gastric antral biopsies collected from 3 gastric ulcer Balinese patients. This research revealed that all of threeH. pylori Bali isolates examined positive for CagA (Cytotoxin associated antigen A)gene. Based on the result of the gel electrophoresis, a 640 bp single band observed similar in each sample. All H. pylori Bali isolates have EPIYA-ABD motif with unique motifs EPIYT on second EPIYA sequence.Three of H. pylori Bali isolates named as BLI 01, BLI 02 and BLI 03 that have a CagA genes. All the H. pylori Bali isolates have an unique EPIYT motifs (substitution A to T) in CagA genes. The degree of gastric inflammation is more severe in the 4th week of the study. There was a significant difference of gastric inflammation degree in all of three groups of mice. H. pylori BLI 03 isolate with positive CagA gene, have a greatest degree of inflammation.
Keywords
Cag A; EPIYA H. pylori;
Download this article as:| Copy the following to cite this article: Wirawan I. M. S, Muttaqin Z, Novitasari N, Widiana I. G. R, Artha I. G. A, Astawa I. N. M. Comparison of Gastric Inflammation in Balb/C Inoculated by Three Unique EPIYT Motif Caga Gene Helicobacter Pylori Bali Isolates Based on Update Sidney System Criteria. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Wirawan I. M. S, Muttaqin Z, Novitasari N, Widiana I. G. R, Artha I. G. A, Astawa I. N. M. Comparison of Gastric Inflammation in Balb/C Inoculated by Three Unique EPIYT Motif Caga Gene Helicobacter Pylori Bali Isolates Based on Update Sidney System Criteria. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16155 |
Introduction
Helicobacter pylori infection (H. pylori) is associated with gastrointestinal diseases including gastritis, peptic ulcer, gastric carcinoma and gastric mucosa-associated lymphoid-tissue lymphoma (MALT Lymphoma). H. pylori bacteria have been classified into class 1 carcinogen in humans by the International Agency for Research on Cancer Consensus Group since 1994.1Based on the involvement of inflammatory cells in the gastermucosa, histologically, and onset of occurrence, the gastritis can be divided into acute gastritis and chronic gastritis. The main cause of chronic gastritis and peptic ulcer is infection of H. pylori.2 H. pylori infects half of the world’s population.3,4 The prevalence of H. pylori infection in Indonesia stillcontroversial. Studies by Syam AF et al in dyspepsia patients in Java, Papua, Sulawesi, Borneo and Sumatera found that the prevalence of H. pylori infection was 22.1%. People from Papua, Batak and Bugisethnicshave a higher risk H. pylori infection than Dayak, Chinese and Javanese.5 There is no data about H. pylori Bali isolates and the Cag A virulence factor from patients with dyspepsia in Bali. The research purposes were to obtain H. pylori Bali isolates, and to study the development of gastric inflammation caused by H. pylori Bali isolates in Balb / c.
Material and Methods
Preparation of H. Pylori Bali Isolates
We used Fujinon 2500 series to do esophagogastroduodenoscopy. Biopsies performed in 7 patients with gastric ulcer diagnoses, biopsies were performed 2 in anthrums and 2 in gastric corpus. We collected 3 Bali isolates of H. pylori of that patients. Biopsy results are inserted into CarryBlaire transport media, then sent to Biomedical Research Unit of West Nusa Tenggara Provincial Hospital for cultures. H. pyloricultures were performed using a blood agar plate with 7% of sheep blood, adding Skirrow supplements and Dent supplements buried using an microaerophilic anaerobic jar.H. pylori Bali isolates provided from Laboratorium of Microbiology, Biomedical Research Unit, West Nusatenggara General Hospital, which have been isolated from gastric antral biopsies collected from 3Balinese patients (Gastric ulcer) stored at -80o Celsius.6 Medical records of patients provided from Endoscopy Unit, Wangaya Hospital, Denpasar, Bali.
Amplification of the 3’ Region of the cagA Gene
DNAZol Kit (Invitrogen) used to extraction DNA Genomic according to the manufacturer′sinstructions. Primers P1 (5′-GA TAACAGGCAAGCTTTTTGAGG-3′) and P2 (5′CTGCAAAAGATTGTTTGGCAG-3′) used to amplification of the cagA 3′ variable region.The amplification steps were done under the conditions: 94°C for 1min; 34 cycles of 94°C for1min and 55°C for 1min, and 72°C for 1 min. Final extension was done at 72°C for 5 min. The mixture was stored at 4°C. PCR products were separated by 2% agarose gel electrophoresis and examined under UV illumination. The PCR products were then sequenced.
Prevalence of CagA Gene
All of the threeH. pylori Bali isolates examined were positive for CagAgene. Based on the result of the gel electrophoresis, a 640 bp single band were similar in each sample. Once confirmed by PCR that the growing isolate wasH. pylori, it was then sequenced in Singapore to determine the sequence of the DNA (Deoxyribo Nucleic Acid) and the EPIYA motifs of CagA of the three H. pylori Bali isolates.Three of H. pylori Bali isolates named as BLI01, BLI 02 and BLI 03
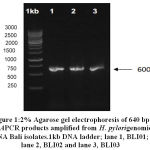 |
Figure 1: 2% Agarose gel electrophoresis of 640 bp cagAPCR products amplified from H. Pylorigenomic DNA Bali isolates.1kb DNA ladder; lane 1, BLI01; lane 2, BLI02 and lane 3, BLI03
|
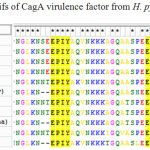 |
Table 1: EPIYA motifs of CagA virulence factor from H. pylori Bali isolates |
Alignments of the deduced amino acid sequence of the C-terminus of CagA between the BLI01, BLI02 and BLI03 strain and the representative East Asian CagA genotype shown at table 1. Subtitution A àT in EPYA motif indicated in red eclipse.Alignments of the deduced amino acid sequence of the C-terminus of CagA between the BLI01, BLI02 and BLI03 and the representative East Asian CagA genotype shown at Figure 1. It was found that low variation of the amino acid sequence C-terminus contained EPIYA motif of CagAH. pylori Bali isolates. All H. pylori Bali isolates have EPIYA-ABD motif with unique motifs EPIYT on second EPIYA sequence.
Inoculation H. pylori Bali Isolates in Balb/c
Three of H. pylori Bali isolates were used to study the gastric inflammation in Balb/c mice. H. pylori Bali isolates were inoculated into three groups of Balb/c to determine the degree of gastric inflammation. We used twelve malesBalb/c aged 2-4 months. Mice were fasted for 24 hours before inoculation. Inoculation was done after the mice were given orally 0.25 ml of 0.2 M NaHCO3 solution to neutralized gastric acid. Each group of mice were inoculated with different H. pylori Bali isolates. The first group was inoculated withH. pyloriBLI 01, the second group was inoculated withH. pylori BLI 02 and the third group was inoculated with H. pyloriBLI 03.Mice were previously given H. pylori 1×1010 CFU/ml given as much as 3 x intermittent one day. Each group consisting of 4 mice. All mices feces cultured were positive infected by H. pylori after 2 weeks. Three mice were killed, one in each a group at first week, 2nd week, 4th week to see the development of gastric inflammation.
Results
Patient Characteristics
The H. Pylori Bali isolates cultured from gastric biopsies of patients undergoing endoscopic examination at Wangaya Hospital, Denpasar, Bali Province. All patients were Balinese people. One was male and two were females. The upper gastrointestinal diagnosis was gastric ulcer in all patients. The average age was 45 years.
 |
Figure 2: Bali Island map
|
Pyloribaliisolates Comparison With Neighbouring Countries
Phylogenetic analysis of 3’ variable regions of cagA gene of H. pylori Bali isolates were compared with isolates from Japan, China, Korea, Philippines, Malaysia and Thailand. It was showed that there was a different clustered between Bali isolates than other countries.(Figure 3)
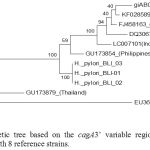 |
Figure 3: Phylogenetic tree based on the cagA3’ variable regions of H. pyloriBali isolates compared with 8 reference strains.
|
Maximum Likelihood trees of 5% partial conservation sequence of H. pylori isolate Bali BLI_01, BLI_02 and BLI_03 compared with isolates from several countries, namely strains with access numbers GU173854 (Philippine), LC097101 (Indonesia / North Sulawesi), DQ306710 (Central China), FJ458163 (Korea), AB017923 (Japan), KF028589 (China), GU173879 (Thailand) and EU369652 (India-Malaysia).
The sequenced amino acid from DNA sequences show EPIYA motifs of cagA H. pylori Bali isolates have an unique EPIYT motifs (substitution A → T), compare with the others countries. Three of H. pylori Bali isolates have a cagA virulence factor.
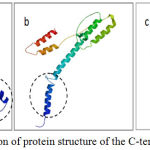 |
Figure 4: Prediction of protein structure of the C-terminal part Cag AH.
|
pylori (a) Bali isolate BLI-01 strain has the motive EPIYA-ABD (East Asian) of patients with gastric ulcer. (b)Western strainEU369652 have EPIYA-ABC type of gastritis patient and (c) Chinese isolate KF028589. Part of C-terminaltail(marked by acirclebroke up) is thought to bethe sitefor interactionwithSHP-2.
Phylogenetic Analysis and Determination of the EPIYA Pattern
The analysis included cagA 3′ region nucleotide sequences of 3 isolates of which 3 Bali isolates and 8 from other countries retrieved from GeneBank. To determine the phylogenetic relationship between Lombok isolates and those from other countries the nucleotide sequences of cagA3′ region was multiple aligned and Maximum Likelihood phylogenetic tree constructed using the CLUSTALW program. The translated 3′ region sequences of our Balinese isolates were then aligned using multiple alignments with MUSCLE program. Unrooted phylogenetic trees depicting relationships among CagA amino acid sequences were built using ML algorithm in MEGA v6 software.7 To predict structure of the C-terminal CagA protein we use SWISS-MODEL program.8
Histopathological Assessment With Update Sidney System
Each gasterbiopsiassessed for histopathology. The measurement focus on: H. pylori, the number of polymorphonuclearinflammatory cells (activity) and lymphocyte inflammatory cells (chronicity), present of gland atrophy and intestinal metaplasia as the update Sidney system classification.Scores of all variables are summed to obtain gastritis degrees base on visual analoque scale.9
The assessment is more detailed in Table 2.
Table 2: Inflammatory score base on update Sidney system
| Variable | First week | Second week | Fourth week | |||||||||||||
| BLI 01 | BLI 02 | BLI 03 | BLI 01 | BLI 02 | BLI 03 | BLI 01 | BLI 03 | |||||||||
| Cor | Ant | Cor | Ant | Cor | Ant | Cor | Ant | Cor | Ant | Cor | Ant | Cor | Ant | Cor | Ant | |
| H. Pylori | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Neutrofil Activity | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 3 |
| Chronic inflammation | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | >1 | >1 | 2 | 2 | 3 | 3 | 3 | 3 |
| Atrofi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Intestinal metaplasia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Macroscopically
In thefirst week, 3 gaster size in all groups still in normal size. The size of the gaster becomes maximal at the second week. At fourth week, the size of the gaster back shrinks even smaller than the first week. In the first week, the gasterrugae infected by BLI01 and BLI02 isolates still normal appeared, while the gastric mice rugaeinoculated by BLI03 appeared thinning since the first week. All stomach rugae thinned as well as by the fourth week all the gasterrugae thins out at fourth week.
Microscopically
At the first week, chronic cells inflammation appeared in all of 3 gastric biopsies infected by H. pylori Bali BLI01, BLI 02 and BLI 03isolates. At the second week, Acutaand chronic inflammatory cells appeared in all gaster mice inoculated byH. pylori BLI01, BLI 02 and BLI 03isolates. The most inflammatory cells were found in gastric mice inoculated by H. pylori BLI 03isolate.At the fourth week, one mice in group 2 dead, so the analysis only done in the group 1 and group 3. We found colony of H. pylori by Giemsa staining with equal density in the group 1 and group 3. Robust inflammatory cells found at the fourth week, acuta and chronic cell inflammations. Neither metaplasia intestinal nor atrophy in gaster mice until fouth week of the study.
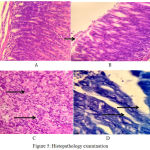 |
Figure 5: Histopathology examination
|
Lymphocyte cell and plasma cell, no activity yet, at the first week(HE, 400x)
Small activity of neutrofil, at the second week (HE, 400x)
Robust activity of neutrofil extra and intraepithelial, at the fourth week (HE, 400x)
H pylori colony in anthrum at fourth week.
Discussion
Pylori infection manifestation are associated with a complex combination of host susceptibility, environmental factors and bacterial virulence. Infections involving H. pylori strains that possess the virulence factor CagA have a worse clinical outcome than CagAnegative strains. CagA-positive H. pylori increase the risk for gastric cancer than CagA negative.3,10,11 CagA behaves as a bacterial oncoprotein playing a key role in H. pylori-induced gastric cancer. There are 2 types of H. pylori isolates: CagAgene- positive strains and CagAgene-negative strains. Almost all H. pylori isolates from East Asia are CagApositive, and approximately 20% to 40% of isolates from Europe and Africa are CagAnegative.12 CagA is an oncoprotein that play a key role in H. pylori-induced gastric cancer. Activation of oncogenic signaling pathways and inactivation of tumor suppressor pathways are two crucial events in the development of gastric cancer. CagA shows the ability to affect the expression or function of vital protein in oncogenic or tumor suppressor signaling pathways via several molecular mechanisms.13
All the H.pylori Bali isolates in this study have a CagA gene positive, and this results need to reevaluate in the further study to know the development to gastric cancer. CagA genecould mainly classified into 2 types (East Asian type and Western type) according to the sequence located in the of CagA and classified the repeat regions into 2 types, the first repeat and the second repeat, and found that the sequence of the second-repeat region was considerably different between East Asian strains and Western strains. Each region contains the Glu-Pro-Ile-Tyr-Ala (EPIYA) motifs, which includes a tyrosine phosphorylation site. Recently, it has been more common to name the first- repeat regions as EPIYA-A and EPIYA-B segments and the second-repeat region in Western and East Asian strains as EPIYA-C and EPIYA-D segments, respectively.3 We found different level of gastric inflammation due to 3 Bali isolates H. pylori in this study. It may due to another virulence factor or bacterial determinants between three of Bali isolates H. pylori that we do not explore yet, such as Vac A, 𝛾-glutamyltranspeptidase (gamma GT), the duodenal ulcer-promoting gene (dupA), or peptidoglycan have been also shown to be important inducers of gastric inflammation.
Zhang et al reveal a strong non-random distribution of the EPIYA B motif polymorphisms (including EPIYT and EPIYA) in Western H. pylori isolates, and provide evidence that the EPIYT are significantly less associated with gastric cancer than the EPIYA. By constructing a series of H. pylori cagA isogenic mutants and isogenic complementation plasmids, generating specific antibodies, co-culturing with human AGS cells, performing biochemical and modeling analysis, they demonstrate that CagA B-motif phosphorylation status is essential for its interaction with host PI3-kinase during colonization and that CagA with an EPIYT B-motif had significantly attenuated induction of interleukin-8 and the hummingbird phenotype, had higher affinity with PI3-kinase, and enhanced induction of AKT compared to the EPIYA.
Gastric biopsy after a few days of infection show signs of inflammation, appearance of acute inflammatory cells such as neutrophils. Two weeks after infection occurs infiltration of lymphocyte and monocyte cells. Within 4 weeks there was an increase in the number of CD4 + and CD8 + lymphocytes that indicated the onset of an adaptive immune response.15,16,17 In our research, the lymphocyte cells appearance in the first week, although still lack of theinflammation activity due to polymorphonuclear.The adaptive immune response due to H. pylori infection in mice is similar to that occurring in human. The hallmark of the immune response to H. pylori infection is the presence of gastric mucosal infiltration by T cells, plasma cells, and neutrophil cells in the gastric mucosa simultaneously.17,18 Our study found the same appearance of inflammatory cells, that the acuta and the chronic inflammatory cells found simultaneously in the gastricmucosa.
Conclusions
We obtained three H. pylori Bali isolated named as BLI01, BLI 02 and BLI 03 that have a CagAgenes. All the H. pylori Bali isolates havean unique EPIYT motifs (substitution A to T) in CagA genes. The degree of gastric inflammation is more severe in the 4th week of the study. There was a significant difference ofgastric inflammation degree in all three groups of mice. H. pylori BLI03 isolate with positive CagA gene, have a greatest degree of inflammation.
Acknowledgment
Authors of the current article take this opportunity to thanks to patients who warmly co-operated in this research program. This research was financially by I Made Suma Wirawan, MD.
Conflict of Interests
The authors declare that there is no conflict of interest regarding the publication of this paper
References
- Yang J. C., Lu W., Jung L. C. Treatment of Helicobacter pylori infection: Current status and future concepts World J Gastroenterol. 2014;20(18):5283-5293.
- Redéen S., Petersson F., Kechagias S., Mårdh E and Borch K. Natural history of chronic gastritis in a population-based cohort. Scandinavian journal of gastroenterology. 2010;45(5):540-549.
- Yamaoka Y. Pathogenesis of Helicobacter pylori-related gastroduodenal diseases from molecular epidemiological studies. Gastroenterology research and practice. 2012.
- Hagymasi. Helicobacter pylori infection new pathogenic and clinical aspect. World J Gastroenterol. 2014;20:6386-6399.
- Syam A. F., Miftahussurur M., Makmun D., Nusi I. A., Zain L. H., Akil F., Uswan W. B., Simanjuntak D., Uchida T., Adi P and Utari A. P. Risk Factors and Prevalence of Helicobacter pylori in Five Largest Islands of Indonesia: A Preliminary Study. PloS one. 2015;10(11):e0140186.
- Suharjono Z., Aulanni’amAulanni’am. Phylogenetic analysis of 3’ region of Helicobacter pylori cagA gene of Lombok isolates and the association with gastric pathology. IOSR Journal of Pharmacy and Biological Sciences. 2016;11(1 ):01-06. Ver. II (Jan. – Feb.).
- Tamura K., Stecher G., Peterson D., Filipski A., and Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725-2729.
- Biasini S., Waterhouse B. A., Arnold K. et al., SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Research. 2014;42(1):252-258. doi: 10.1093/nar/gku340.
- Ariefiany D., Hassan A. H., Dewayani B. M., Yantisetiasti A. Analisis Gambaran Histopatologi Gastritis Kronikdengandan TanpaBakteri Helicobacter pylori MenurutSistem Sydney: In Majalah Patologi. Mei. 2014;23(2).
- Yong X.,Tang B., Bo-Sheng L., Xie R., Chang-Jiang H.,Luo G., QinY., Dong H and Shi-Ming Y. Helicobacter pylori virulence factor CagA promotes tumorigenesis of gastric cancer via multiple signaling pathways. Cell Communication and Signaling. 2015;13:30.
- Backert S., Clyne M. Pathogenesis of Helicobacter pylori in- fection. Helicobacter. 2011;16(1:):19-25. [PMID: 21896081 29 DOI: 10.1111/j.1523-5378.2011.00876.x]
- Yamaoka., Kodama T., Gutierrez O., Kim J. G., Kashima K and Graham D. Y. Relationship between Helicobacter priori iceA, cagA, and vacAstatus and clinical outcome studies in four different countries. Journal of Clinical Microbiology. 1999;37(7):2274–2279.
- Hardbower D. M., Peek., Mal R. M & Wilson K. T. At the Bench: Helicobacter pylori, dysregulated host responses, DNA damage, and gastric cancer. Journal of leukocyte biology. 2014;96(2):201-212.
- Zhang X. S., Tegtmeyer N., Traube L., Jindal S., Perez-Perez G., Sticht H., Backert S and Blaser M. J. A specific A/T polymorphism in Western tyrosine phosphorylation B-motifs regulates Helicobacter pylori CagA epithelial cell interactions. PLoS. Pathog. 2015;11(2):e1004621.
- Kusters J. G., van Vliet A. H and Kuipers E. J. Pathogenesis of Helicobacter pylori infection. Clinical microbiology reviews. 2006;19(3):449-490.
- Hagymasi. Helicobacter pylori infection: new pathogenic and clinical aspect. World J Gastroenterol. 2014;20:6386-6399.
- Moreno E., McGregor E. G., Perini L., et al. Helicobacter pylori: bacte- rial factors and the role of cytokines in the immune response. Curr Microbiol. 2010;60(2):143-55.
- Raghavan S and Quiding-Jarbrink M. Immune modulation by regulatory T cells in Helicobacter pylori-associated diseases. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders). 2012;12(1):71-85.







