Manuscript accepted on :September 08, 2017
Published online on: --
Plagiarism Check: Yes
Pavithira Sekar1, K. Punnagai2 and Darling Chellathai David2
1Department of Pharmacology, Sri Muthukumaran Medical College Hospital and RI, Chikkarayapuram, Chennai-600069.
2Department of Pharmacology, Sri Ramachandra Medical College and Research Institute, Porur, Chennai-600116.
Corresponding Author E-mail: drpavithras06@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1228
Abstract
The study was aimed to compare the safety and efficacy of Gabapentin vs amitriptyline in patients with painful diabetic peripheral neuropathy. Study was conducted in Chennai during the period from October 2014 to May 2015 were a total of 100 patients were randomized to two treatment groups in 1:1 ratio. Gabapentin 600 mg per day and increased upto a maximum of 1800 mg per day, at bed time. Amitriptyline 25 mg per day and increased upto a maximum of 75 mg per day, at bed time. There were four scheduled visits during the study. All study participants were given a diary and necessary scales (Visual analog scale and sleep interference scale). Categorical variables are analysed using Chi square test and Paired Students‘t’ test. With similar baseline for both groups, Gabapentin produced significant improvement versus amitriptyline for mean pain scores (P<0.05); mean sleep interference scores (P<0.05). Additional statistically significant (P<0.05) differences favouring gabapentin treatment were observed in measures of quality of life (Global Impression of Change in both Patient and Clinician perspective). Gabapentin group showed more number of adverse events like increased appetite, somnolence and liver function test increase, while amitriptyline group had increased micturition and dizziness. The study revealed that gabapentin is safer and efficacious compared to amitriptyline in patients with painful diabetic peripheral neuropathy
Keywords
Amitriptyline; Gabapentin; Painful Diabetic peripheral Neuropathy; sleep interference visual analog scale;
Download this article as:| Copy the following to cite this article: Sekar P, Punnagai K, David D. C. Comparative Study of Safety and Efficacy of Gabapentin Versus Amitriptyline in Patients With Painful Diabetic Peripheral Neuropathy, A Randomized open Label Parallel Group Study. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Sekar P, Punnagai K, David D. C. Comparative Study of Safety and Efficacy of Gabapentin Versus Amitriptyline in Patients With Painful Diabetic Peripheral Neuropathy, A Randomized open Label Parallel Group Study. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16647 |
Introduction
In type 2 diabetes, DPN is one of the commonest causes of foot complications leading to pain, loss of sensation, foot ulcers and amputation leading to a reduced functional capacity of an individual.1 In a study to determine the prevalence of foot ulcers and the incidence of amputations in patients with type 2 diabetes, Bruun C et al observed that after 19 years of diagnosis of type 2 diabetes, the incidence of amputations was still high in type 2 diabetes population.2
It is clear that metabolism of nerve cells is altered by increased glucose level in blood. This results from the loss or damage to the sensory nerve fibres. The initial problem is loss of sensation for pain and this increases the likelihood of foot ulcers in the diabetic population. This also accounts for recurrent hospitalization than other complications of type 2 diabetes and is also the most common cause of non‐traumatic amputations. In addition to this, diabetic peripheral neuropathy (DPN) patient’s encounters problems with balance while standing and walking, reduced work capacity and pain in the legs, thus reducing their overall functional output leading to poor quality of life.
Glycemic control is the only currently known therapy to delay the development and progression of Diabetic peripheral neuropathy. Once a patient has been diagnosed with DPN, treatment options focus on symptom control. Previously there had been many studies focusing on drug related prophylaxis for population suffering from DPN such as antidepressants, anticonvulsants, oral hypoglycemic agents but either they have a major side – effect or cannot be continued for long term trials for patients.3 Antidepressants are a common group of drugs used for neuropathic pain. Tricyclic Antidepressants (TCAs), Selective Serotonin Reuptake Inhibitors (SSRIs) and Selective Norepinephrine Reuptake Inhibitors (SNRIs) have been tested for off-label uses in pain management.4 Amitriptyline was the first and most frequently studied TCA to show a significant improvement in pain scores. Later, other TCAs, such as desipramine and nortriptyline proved to have beneficial effects, still the most common and least tolerated adverse effects with tricyclic antidepressant are the anticholinergic effects, which include dry mouth, blurred vision, constipation, urinary retention and cognitive impairment. Other serious side effects associated with these agents relate to cardiovascular toxicity and include orthostatic hypotension, tachycardia and changes in atrioventricular conduction4
Materials and Methods
This study was conducted in accordance with declaration of Helsinki and ICH-GCP (International Conference on Harmonisation – Good Clinical Practice) guidelines with approval of the Institutional Ethics Committee. Statistical software SPSS version 23.
Inclusion Criteria
At screening, Patients pain attributing to diabetic neuropathy based on history, clinical examination and Michigan Neuropathy screening Instrument.
Males and females of age between 18 to 75 yrs.
Glycosylated haemoglobin (HbA1c) in the range of 6.0% to 10.0%
Duration of diabetes ranging from 1 to 25 years.
Diabetic neuropathy from 1 month to 5 years.
Exclusion Criteria
Newly diagnosed cases (diagnosed within past one year) are not included in the study.
Type 1 Diabetes mellitus.
History of hypersensitivity to the study drugs.
Type 2 diabetics with other endocrine disorders like hypo or hyperthyroidism, Cushing’ syndrome, acromegaly.
Any history of acute complications of diabetes mellitus within past 6 months prior to the study.
Type 2 diabetics on drugs like thiazide diuretics, corticosteroids, OCP’s.
Type 2 diabetics with severe renal failure, heart failure, and hepatic failure.
Subject is pregnant or lactating woman.
History of Drug abuse or Alcohol addiction
Study Procedure
After baseline laboratory investigations all the 100 patients selected were randomized and allotted a treatment group. Gabapentin was initiated at 600 mg per day and increased upto a maximum of 1800 mg per day once daily at bed time and Amitriptyline initiated at 25 mg per day and increased upto a maximum of 75 mg per day, once daily at bed time. Baseline laboratory investigations were done. There were four scheduled visits during the study, baseline visit, after 1st month, then after 2nd month and at the 3rd month (end of study visit). Increase in dosage is determined during each visit after examination.
All study participants were given a diary and necessary scales (Primary endpoint: 11 point Visual analog scale and Secondary endpoint: 11 point sleep interference scale). Other secondary endpoints with the following 7 point scale system is used to assess the effectiveness of treatment during the follow-up visits, 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6= much worse, 7= very much worse. The endpoints are Patients – Global Impression of Change (PGIC), where the patient rates their overall status from the beginning of medication and Clinical Global Impression of Change (CGIC), where the clinician rates the improvement of patient based on medical judgement. Adverse event monitoring was done throughout the duration of study.
With ‘Intent to treat principle’, SPSS (Statistical Package for the Social Sciences) software version 23 was used to analyse the data collected Categorical variable analysed using Chi-square test and paired Students ‘t’ test was used to find the significance of difference between treatment group.
Discussion
W.H.Herman et al. studied Michigan Neuropathy Screening Instrument (MNSI) in 1184 subjects with diabetes for assessment of distal symmetrical peripheral neuropathy in 28 different clinical sites and concluded that MNSI is a simple, non-invasive and valid measure of distal symmetrical peripheral neuropathy.5 Moghtaderi A et al validated the MNSI for a period of two years in a cross sectional study in 176 type 2 diabetic patients. Comparison of MNSI score to the evaluation of neurophysiological results were done. It has been found that the accuracy of MNSI scoring makes it a very useful screening test for diabetic neuropathy with a high likelihood ratio and a post-test probability gave a high diagnostic impact. However, it has been suggested that MNSI is considered as a screening test and further evaluation should be done to diagnose the presence of peripheral neuropathy.6
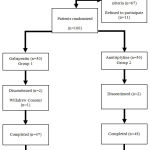 |
Figure 1: Disposition of Study Population
|
Table 1: Mean visual analog score comparison between groups
| VAS | Group 1 Gabapentin | Group 2 Amitriptyline | ‘p’ Value |
| Baseline
(‘0’ Week) |
67.72±16.93 | 65.92±12.89 | 0.559 |
| First Visit
(4th Week) |
55.34±16.94 | 56.02±13.05 | 0.827 |
| Second Visit
(8th Week) |
35.08±16.60 | 46.85±14.14 | 0.00 |
| Third Visit
(12th Week) |
29.51±16.90 | 36.85±14.14 | 0.024 |
This table represents mean change of visual analog score for every visit, p<0.05 is considered significant.
Atli A et al studied the improvement of pain in diabetic neuropathy in a placebo based randomized control study and found that the measurement of Visual analog scale when used correctly is a validated procedure for evaluation of pain in neuropathy.7 KC Yuen et al in a double blind placebo controlled cross over study used visual analog score as mean to measure the pain in diabetic neuropathy.8 Assessment of Visual analog scale showed a significant reduction in pain in both treatment groups (p<0.05). But the extent of the benefit is analysed by comparing the score during the final end point showed gabapentin (67.72±16.93 to 29.51±16.90) has significant reduction in pain compared to amitriptyline (65.92±12.89 to 36.85±14.14). Treatment effect was seen bit earlier on patient group taking Gabapentin. This may be a valid option for patients requiring immediate reduction in pain intensity.
Table 2: Mean visual analog score comparison within groups
| VAS | Baseline
(‘0’ Weeks) |
Third Visit
(12th Week) |
Percentage Change | ‘p’ Value |
| Group 1 Gabapentin | 67.72±16.93 | 29.51±16.90 | -56.42% | 0.000 |
| Group 2 Amitriptyline | 65.92±12.89 | 36.85±14.14 | -44.10% | 0.000 |
This table shows the change of mean visual analog score from baseline to final visit, p<0.05 is considered a significant difference.
One placebo controlled large clinical study reported that Gabapentin is effective in controlling the painful diabetic neuropathy and a calculated need to treat for 50% relief in pain was 3.7. Gabapentin bind to α2δ site of L type, voltage gated Ca channel, which regulates the influx of calcium during depolarization in CNS neurons.9,10
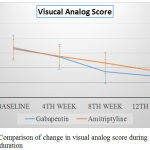 |
Figure 2: Comparison of change in visual analog score during treatment duration
|
Table 3: Mean daily sleep interference score comparison between groups
| Sleep Interference | Group 1 Gabapentin | Group 2 Amitriptyline | ‘p’ Value |
| Baseline
(‘0’ Week) |
6.72±1.91 | 5.96±2.09 | 0.067 |
| First Visit
(4th Week) |
4.74±2.03 | 5.61±2.13 | 0.043 |
| Second Visit
(8th Week) |
4.20±1.95 | 4.69±2.21 | 0.252 |
| Third Visit
(12th Week) |
3.73±1.78 | 4.46±2.23 | 0.079 |
This table represents mean change of daily sleep interference score for every visit, p<0.05 is considered significant.
One of the main characteristics of neuropathic pain is, it interferes with daily sleep. Zelman DC et al conducted a sleep data analysis in patients with diabetic peripheral neuropathy and has found that it is considerably associated with sleep impairment.11 Also a meta-analytic study conducted by Roth T, provided evidence from data of 2399 patients, that there is a significant improvement in sleep quality from medications for peripheral neuropathy in diabetes.12 Daily sleep interference score analysis in our study has been performed. Both treatment group showed a great deal of potential in improving the sleep (p<0.05). But Gabapentin showed a very high significance in sleep improvement at a very early stage (6.72±1.91 to 4.74±2.03 within four weeks compared to amitriptyline, 5.96±2.09 to 5.61±2.13). Both these drugs are candidates in causing sleep related adverse effects that had proven to be beneficial in this condition but reports suggested that residual sleep was present in some subjects, two in amitriptyline group and four in gabapentin group. This was more in amitriptyline.
Table 4: Mean PGIC and CGIC score comparison between groups
| PGIC | CGIC | |||||
| Group 1 Gabapentin | Group 2 Amitriptyline | ‘p’ Value | Group 1 Gabapentin | Group 2 Amitriptyline | ‘p’ Value | |
| First Visit
(4th Week) |
4.10±1.36 | 3.90±1.20 | 0.459 | 4.02±0.93 | 4.12±0.95 | 0.607 |
| Second Visit
(8th Week) |
2.59±1.56 | 3.59±1.21 | 0.001 | 3.60±0.93 | 3.67±0.99 | 0.712 |
| Third Visit
(12th Week) |
2.16±1.45 | 3.11±1.2 | 0.001 | 3.10±0.94 | 3.17±1.00 | 0.740 |
This table represents mean change of PGIC score for every visit, p<0.05 is considered significant.
Any chronic illness always have a tendency to take toll on the quality of life and diabetic neuropathy is no exception. Benbow SJ et al observed the effect of this disease affecting the quality of life and urged to have intensive research for effective management.13 Amanda Boyd et al validated a questionnaire for measuring the quality of life in diabetic neuropathy.14 AE Bunner et al used global impression of change as a scale to analyse the quality of life in diabetic neuropathy patients.15 Global impression of change was obtained in both perspectives (patient and clinician). While the results of clinician’s global impression of change improved significantly (p<0.05) from the initial value, they didn’t find any significant difference in both treatment groups. But patient’s global impression of change suggested that patients in gabapentin group (4.10±1.36 to 2.16±1.45) experienced an increased sense of wellbeing compared with that of amitriptyline group (3.90±1.20 to 3.11±1.2) and both group showed significant improvement in wellbeing.
The main drawback of treating a complication of chronic disease is the dose limiting adverse drug reaction. This happened in our study also. Tolerability data available from our study is consistent with previous similar studies. Tricyclic antidepressants is usually limited with highly intolerable adverse effects like increased sedation, retention of urine, postural hypotension, or cardiac arrhythmias as evident from Moore R.A et al.16 But drug tolerance was never a factor for discontinuation or treatment non-compliance in our study. Even though adverse events has been reported in our study, there were no need of any study discontinuation. Some patients in gabapentin group experienced side effect like weight gain. But amitriptyline group patients experienced day time drowsiness and increased frequency of micturition. Both study drugs increased the mean liver function test compared to baseline but only gabapentin group (two patients) showed a clinically significant increase in liver function tests (p<0.05).
In both study groups there has been no change in the glycated haemoglobin value from baseline, this suggest that the beneficial effects in neuropathic pain can be completely attributed to the study drugs and not to pharmacotherapy of diabetes mellitus.
Although this study has given us an understanding the benefits and shortcomings of both the drugs we had our own limitations, primarily based on the design, this is an open label study and no cross-over was done. Educated patients may be prone for bias due to the amount of content available on the World Wide Web. Also we couldn’t use a placebo arm in this study due to the obvious logistics issue. Future study may extend the present therapy and include an add-on therapy such as topical applications and verify the benefit and any dose reduction of the main therapy.
Conclusion
This study revealed that gabapentin is safer and efficacious compared to amitriptyline in patients with painful diabetic peripheral neuropathy. Both treatment group provided clinically meaningful relief of pain with tolerable side effects. But the induction of pain relief was very much superior in gabapentin when compared with amitriptyline. The same holds for the improvement in quality of sleep and for day to day quality of life. The incidence and severity of adverse effect is less in gabapentin compared with amitriptyline
To conclude newer therapy based on the actions of gabapentin provide a promising role in reducing pain and improving the quality of life in such chronic painful condition.
References
- Merskey H., Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms, 2nd ed. SeattleWA : IASP Press. 1994.
- Bruun C., Siersma V., Guassora A. D., Holstein P., deOlivarius F. Amputations and foot ulcers in patients newly diagnosed with Type 2 diabetes mellitus and observed for 19 years. The role of age, gender and co-morbidity. Diabet Med. 2013;00:1–9.
- Wong M. C., Chung J. W., Wong T. K. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ. 2007;335:87.
- Donnan J., Ledger S. An update on the treatment and management of diabetic peripheral neuropathy: continuing education series. The CANNT Journal. 2006;16:32-35.
- Herman W. H., Pop-Busui R., Braffett B. H., Martin C. L., Cleary P. A., Albers J. W., Feldman E. L. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29(7): 937–944.
- Moghtaderi A., Bakhshipour A., Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006;108(5):477-81.
- Atli A., Dogra S. Zonisamide in the treatment of painful diabetic neuropathy: a randomized, double-blind, placebo-controlled pilot study. Pain Med. 2005;6(3):225-34.
- Yuen C. J. K., Baker R. N., Rayman G. Treatment of Chronic Painful Diabetic Neuropathy With Isosorbide Dinitrate Spray. Diabetes Care. 2002;25(10):1699-1703.
- Chong M. S., Hester J. Diabetic painful neuropathy: current and future treatment options. Drugs. 2007;67:569-85.
- Backonja M., Beydoun A., Edwards K. R., Schwartz S. L., Fonseca V., Hes M., et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus a randomized controlled trial. JAMA. 1998;280:1831-6.
- Zelman D. C., Brandenburg N. A., Gore M. Sleep impairment in patients with painful diabetic peripheral neuropathy. Clin J Pain. 2006;22(8):681-5.
- Roth T., van Seventer R., Murphy T. K. The effect of pregabalin on pain-related sleep interference in diabetic peripheral neuropathy or postherpetic neuralgia: a review of nine clinical trials. Curr Med Res Opin. 2010;26(10):2411-9.
- Benbow S. J., Wallymahmed M. E., MacFarlane I. A. Diabetic peripheral neuropathy and quality of life. QJM. 1998;91(11):733-7.
- Boyd A., Casselini C., Vinik E., Vinik A. Quality of Life and Objective Measures of Diabetic Neuropathy in a Prospective Placebo-Controlled Trial of Ruboxistaurin and Topiramate. J Diabetes Sci Technol. 2011;5(3):714–722.
- Bunner A. E., Wells C. L., Gonzales J., Agarwal U., Bayat E., Barnard N. D. A dietary intervention for chronic diabetic neuropathy pain: a randomized controlled pilot study. Nutrition & Diabetes. 2015;5:e158.
- . Moore R. A., Derry S., Aldington D., Cole P., Wiffen P. J. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;6;7:CD008242.







