Marzieh Norouzi1, Zahra Mousavi2 and Hamed Shafaroodi2
1Herbal Medicines Research Center, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran-Iran, HMRC.
2Department of Pharmacology and Toxicology, Faculty of Pharmacy, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran – Iran, IAUPS.
Corresponding Author E-mail: mosavi50@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1215
Abstract
Aripiprazole is an atypical antipsychotic drug mainly characterized by partial agonist activity at dopamine D2 and serotonin-1A receptors with minimal side effects. Based on typical antipsychotic pharmacological activity, including antinociception effect and disruption opioid anti-nociceptive tolerance, Aripiprazole activity and its interaction with morphine on nociception was evaluated by tail flick and hot plate assay in the mouse. In experiment 1, mice received aripiprazole (5, 10 and 20 mg/kg IP), saline (1 ml/kg, IP) and morphine (5 mg/kg, IP) 30 minutes prior to the test. The tail flick and hot-plate methods were used for pain evaluation. In order to assess the effect of aripiprazole on morphine antinociception in experiment 2, it was administered 30 min after morphine injection and then the test was assessed. Also, in experiment 3, the effect of aripiprazole (10 and 20 mg/kg IP), on acute morphine tolerance was studied. Comparisons between the groups were carried out using the Analysis of Variance (ANOVA), and post hoc Tukey's test. P<0.05 was considered statistically significant. The results revealed that aripiprazole, at doses that had no affected themselves (10–20 mg/kg), were significantly (P<0.001) effective on prolonging the morphine antinociceptive effect by tail flick test in mice. Also Aripiprazole (20 mg/kg) significantly increased the duration of morphine antinociception effect by hot plate test, but it did not significantly influence the morphine antinociception time course at 5 and 10 mg/kg of drug. Pretreatment with aripiprazole (20 mg/kg IP) prevented the acute morphine tolerance in hotplate test. These results also suggest that aripiprazole might have therapeutic value in combination to morphine as an adjuvant analgesic. It was also shown that partial agonist properties of D2 and 5-HT1A as well as antagonist properties of 5-HT2A in aripiprazole likely account for the potentiation of morphine antinociception.
Keywords
Aripiprazole; antipsychotics; antinociceptive effect; morphine; mice
Download this article as:| Copy the following to cite this article: Norouzi M, Mousavi Z, Shafaroodi H. Aripiprazole Prolongs Morphine Antinociception Effect and Disrupts Acute Morphine Tolerance. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Norouzi M, Mousavi Z, Shafaroodi H. Aripiprazole Prolongs Morphine Antinociception Effect and Disrupts Acute Morphine Tolerance. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16184 |
Introduction
The role of antipsychotic drugs as adjuvant analgesics has been studied in human and animals, but the existing data are contradictory.1,2 Aripiprazole is a unique atypical antipsychotic that seems to act as a partial agonist at 5-HT1A and D2 receptors of dopamine and also as an antagonist at 5-HT2A receptors.3-6 However, like most central nervous system drugs, the actual mechanism of its action is not entirely understood. Aripiprazole has a low tendency for extrapyramidal side effects. It causes minimal sedation or weight gain and produces no elevation in serum prolactin levels or cardiovascular side-effects.7 Dopamine receptor’s antagonists such as haloperidol are used to stand such adverse effects of opioids as hallucination and delirium8; though, most of these drugs have other side effects such as extrapyramidal.9 A previous study reported that morphine-induced hyper-locomotion, reward and dopamine release in the nucleus accumbens were suppressed by aripiprazole pretreatment. In this regard, co-administration morphine with aripiprazole might be valuable for decreasing the severity of morphine-induced dopamine-related side effects.10 On the other hand, different antipsychotic drugs did not seem to have similar effects on morphine antinociception. Haloperidol has been reported to potentiate the morphine antinociception effect in rats,11 but aripiprazole did not appear to have any effect on morphine antinociception activity in mice.12 Dopamine and opioid systems interactions have been studied extensively.13,14 The critical role of dopamine in descending inhibition pathway has also been demonstrated. In clinical studies, abnormalities in dopaminergic neurotransmission have been objectively shown in painful clinical conditions such as burning mouth syndrome, restless legs syndrome and fibromyalgia.15 In addition, convincing evidence from animal studies showed that spinal cord and different brain nuclei dopaminergic system are involved in nociception. For example, it was reported that amphetamine- and morphine-induced analgesia are involved in increasing dopamine levels in nucleus accumbens.16 Increasing the dopamine release in nucleus accumbens has an antinociceptive effect which is mediated through D1 and D2 receptors.17 In addition to its stimulus-induced antinociception, dopamine may also inhibit nociception in the mesolimbic/mesocortical circuits tonically, because the lesion of dopaminergic neurons of Ventral Tegmental Area (VTA) results in hyperalgesic responses.18 In VTA, Dopaminergic neurons are particularly involved in both endogenous and morphine-induced antinociception. It has been indicated that D1 and D2 dopamine receptors in NAc and D1 receptors in VTA are involved in developing the sensitization to morphine in rats.19 Recently, Reisi et al. revealed that D1 and D2 antagonist receptors microinjection into NAc and D1 antagonist receptor into VTA can prevent the morphine antinociceptive effects in the tail flick test.
Materials and Methods
Animals
In the present study, male albino mice (20–25 g) were housed in groups of six to eight and were allowed free access to food and water. All experiments were conducted between 10 am and 3 pm with 12 hours of regular light/dark cycle and constant temperature (22±1°C) according with the guidelines of Pharmaceutical Sciences Branch of Islamic Azad University for animal care and use.
Drugs
Aripiprazole (Sigma, USA) was suspended in saline. Powdered morphine (Temad Co.) was dissolved in saline.
Experiments
Tail-flick and hot plate tests were carried on separate animals groups. The first experiment was conducted to observe whether aripiprazole (2, 5, 10 and 20 mg/kg IP) could change the basal nociception. The animals were injected with aripiprazole or saline and placed in the Tail flick apparatus and/or hot plate device.
In order to determine the effective doses of morphine٫ the second experiment was conducted in which the animals received 5 mg/kg morphine (with strong antinociceptive and minor sedation effect) and were tested in the Hotplate and Tail flick. To assess the effect of D2 receptor partial agonist on the anti-nociceptive response of µ receptor agonist (morphine), aripiprazole (10 and 20 mg/Kg IP) was then administered 30 min after receiving 5 mg/kg IP morphine. They were immediately placed in the analgesia meter.
Nociceptive Tests
Tail-Flick
The tail-flick latency (TFL) was recorded by the tail-flick apparatus (Borj Sanat, Iran). Reaction time between the onset of heat stimulus and movement of the tail away from the noxious stimulus was determined by an automatic sensor as TFL. To avoid tissue injury٫ cut-off point was set in 9.0 and 10.0 (S). To estimate the animals’ sensitivity to nociceptive stimulus٫ the individual TFL was considered as a pain threshold before and after (15, 30, 60 and 180 min) drug treatment.
Hot-Plate
Animals were placed on a thermostatically controlled hot-plate (Borj Sanat, Iran), set at 55 ± 0.5 °C. The time between placement animals on platform of hot-plate apparatus and shaking or licking of the paws or jumping was recorded as latency of pain response. In order to avoid the animals paw injury, cut-off time set to 15 seconds. Latency response was recorded before treatment and at 15, 30, 60 and 180 min after drugs/vehicles administration.
Acute Morphine Tolerance
To induce acute morphine tolerance, mice were given morphine (100 mg/kg SC, time 0). Tolerance to morphine developed within hours and peaked at approximately 4 to 6 h 20. Morphine tolerance was considered by monitoring reduced antinociception of morphine test dose (10 mg/kg SC, given at 4 h). In all mice, hot plate responses had returned to normal value at that time. To prevent morphine tolerance, aripiprazole (10 and 20 mg/kg IP) was given 30 min before the induction dose of morphine (100 mg/kg SC).
Animals’ response is presented as the percentage of maximal possible effect (MPE).
MPE% 100 (postdrug latency predrug latency)(cutoff predrug latency).
Statistical Analysis
Data were expressed as mean ± SEM. The effect of antinociception was measured and the mean latencies in all animal groups were subjected to one-way ANOVA followed by protected Tukey’s test for multiple comparisons. P<0.05 were considered to be statistically significant.
Results
Tail Flick Test
In the first experiment, the aripiprazole effect on pain threshold was assessed in the tail flick test. The results for TFLs revealed that there are no significant differences in TFLs at any time intervals among the vehicle and aripiprazole (5, 10 and 20 mg/kg) dose groups. Though, aripiprazole (2 mg/kg) would provide a significant reduction TFLs in compared to vehicle group at 30 and 60min after treatment (p<0.05, p<0.01 respectively).
Furthermore, no significant drug effect was observed, indicating that aripiprazole did not alter pain threshold in the higher doses administered in the current study.
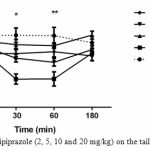 |
Figure 1: Effect of aripiprazole (2, 5, 10 and 20 mg/kg) on the tail flick response in mice
|
No difference was found between the pre-drug response latency (0 min) and post-injection (30, 60 and 180 min) for different doses (5, 10 and 20 mg/kg) of aripiprazole. Aripiprazole (2 mg/kg) would provide a significant reduction TFLs in compared to vehicle group at 30 and 60min after treatment (p<0.05, p<0.01 respectively). (n=6-12).
The second experiment was designed to analyze the aripiprazole effects on the morphine antinociception time course. The results (Fig. 2) revealed that antipsychotic doses (10, 20) were significantly effective in prolonging the action of morphine (P<0.001).
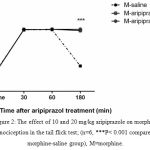 |
Figure 2: The effect of 10 and 20 mg/kg aripiprazole on morphine antinociception in the tail flick test; (n=6, ***P< 0.001 compared to morphine-saline group), M=morphine.
|
Hot-Plate Test
Aripiprazole alone at 5, 10 and 20 mg/kg had no effect on the hot plate response latency (Fig.3).
The second experiment was designed to analyze the aripiprazole effects on morphine antinociception time course, morphine produced significant antinociception, which peaked at 30 min and lasted for 60 min (P<0.05). Morphine plus aripiprazole at 20 mg/kg significantly (P<0.05) increased the duration of morphine antinociception effect for 180 min, but it did not significantly influence the morphine antinociception time course at 5 and 10 mg/kg doses (Fig. 4).
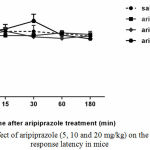 |
Figure 3: Effect of aripiprazole (5, 10 and 20 mg/kg) on the Hot plate response latency in mice
|
No difference was found between the pre-drug response latency (0 min) and post-injection (15, 30, 60 and 180 min) for different doses of aripiprazole (n=6-8).
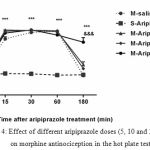 |
Figure 4: Effect of different aripiprazole doses (5, 10 and 20 mg/kg) on morphine antinociception in the hot plate test
|
Aripiprazole (20mg/kg) significantly increased the duration of morphine antinociception effect, but it did not significantly influence the morphine antinociception time course at 5 and 10 mg/kg. (n=6, ***P< 0.001; &&& P<0.001 compared to saline-aripiprazole and morphine-saline group respectively).
Prevention of Acute Morphine Tolerance by Aripiprazole
We investigated aripiprazole effect on the development of morphine tolerance in acute model of opioid tolerance. Mice received an induction dose of morphine (100 mg/kg SC) and were exhibit significantly reduced antinociception for 4 h later (2.6% MPE versus 96.4% MPE in saline-pretreated mice, p<0.0001) by a test dose of morphine (10 mg/kg SC), indicative of the development of acute morphine tolerance (Fig. 5). In mice pretreated with aripiprazole (20 mg/kg IP) 30 min before the induction dose of morphine, morphine-antinociception keep on largely intact (91.95%, respectively; not significantly different from control). While at the lower dose used, aripiprazole (10 mg/kg IP) was unable to prevent morphine tolerance. These results confirmed that aripiprazole blocked the development of morphine tolerance in higher dose.
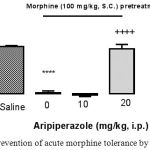 |
Figure 5: Prevention of acute morphine tolerance by aripiprazole
|
Animals received aripiprazole (10 and 20 mg/kg IP) or saline 30 min before morphine administration (100 mg/kg SC) and 4 hours later, all groups received a test dose of morphine (10 mg/kg SC). The antinociception was assessed by the hot plate test 30 min later. Development of morphine tolerance was prevented by aripiprazole (20 mg/kg). (n=6; ****, p<0.0001; ++++, p<0.0001 in compared to saline; in compared to morphine group respectively).
Discussion
The purpose of this study was to evaluate the effect of aripiprazole, an antipsychotic drug, on the morphine antinociception effect in two pain assessment models in mice. It was demonstrated that aripiprazole at doses that had no affected themselves (10–20 mg/kg) prolonged the morphine antinociception effect. Also in this study, we revealed that aripiprazole disrupted morphine antinociceptive tolerance.
Interestingly, it was observed that aripiprazole (10 and 20 mg/kg IP) increased the action time of morphine antinociception in the tail flick test. Also there was an immediate decrease in pain threshold after the administration of aripiprazole (2 mg/kg IP) in this test.
In hotplate test, aripiprazole (20 mg/kg IP) significantly prolonged the morphine antinociception effect. Though, the lower dose of aripiprazole (5 and 10 mg/kg) did not significantly change the morphine antinociception time course. Also in this study, we demonstrated that aripiprazole (20 mg/kg IP) prevented morphine antinociceptive tolerance in hot plate model.
There is little evidence regarding the possible interaction in antinociceptive effect of morphine and aripiprazole. An experimental study showed that aripiprazole did not affect the antinociceptive action of morphine. Aripiprazole prevented the stimulant action of morphine, without interfering with basal motor activity.12 Also, it was reported that aripiprazole did not induce place preference or aversion by itself; however, it inhibited both the development and expression of morphine-induced CPP.12 Previous studies have revealed that aripiprazole pretreatment reduced the reinstatement of CPP induced by morphine, but had no effect on the expression of morphine-induced CPP or locomotor activity.21 Moreover, former studies showed that aripiprazole did not reduce the spontaneous locomotion and had no marked sedation in the dose study in mice and rats.22,23
One reason for unpredictable reports on analgesic interaction of aripiprazole and morphine might be the use of different methods for testing the analgesia and dose of aripiprazole. This discrepancy could be attributed to the used dose of aripiprazole. In this study, the antinociceptive potentiation effect of aripiprazole was observed in higher doses.
Previous studies on other antipsychotics such as haloperidol indicate that their interaction with morphine on the antinociception action is dose-dependent and may also differ among animal species. It was reported that haloperidol (0.1–1 mg/kg IP) by itself neither produce any antinociception effect nor alter morphine antinociception in mice 24. However, another study showed that haloperidol could potentiate the antinociception of morphine in rats, possibly by acting as a σ-receptor antagonist.11
The interaction between dopamine and opioid systems is well documented.14 Significant evidence suggests that dopamine activity affects the opioid system by modulating opiate peptide transcripts,25 synthesis,26 release and biotransformation.27 In contrast, opioids modify the dopamine system by several mechanisms, such as dopamine synthesis,28 release,29,30 biotransformation31 and activity of dopaminergic neurons.32,33 Moreover, behavioral evidence suggests that changes in dopaminergic and/or opioidergic systems are involved in the behavioral sensitization to morphine. Since morphine increases both dopamine synthesis and release in the dopaminergic system via activation of μ-opioid receptors, it is likely that morphine sensitization might be caused by a similar mechanism.34 It has been stated that there is a functional relationship among morphine and dopaminergic system.35-37 Morphine locomotion 38 may be mediated by dopaminergic system. In addition, dopaminergic system has also been implicated in antinociceptive effect and expression of morphine withdrawal signs.39 It has been proposed that sulpiride, a D2 receptor antagonist, decreased the response to morphine (6 and 9 mg/kg) in the formalin test, whereas SCH 23390 did not influence the morphine antinociception.40 In contrast, Ozdemir et al., demonstrated that eticlopride, D2 antagonist significantly increased the morphine analgesic effect.41 Considering the animal studies focusing on pain behavior, clinical data and genetic associations, a common suggestion is that dopamine is antinociceptive by D2 receptors.13 Animal studies have directed that the administration of D2⁄D3 receptor agonists in the striatum suppresses pain-related responses, whereas D2⁄D3 receptor antagonists in the striatum increase the pain.42-44 Subsequently aripiprazole has a unique pharmacological profile that includes partial agonism at D2 receptors with actions on both postsynaptic D2/D3 receptors and presynaptic dopamine auto-receptors with varying degrees of efficacy. Additionally, aripiprazole acts as a partial agonist at 5HT1A receptors 45 and an antagonist at 5HT2A receptors.4,46 Considering the above-mentioned studies, the partial activation of D2 receptors can be considered as the main mechanism through which this compound stimulates the effects of morphine and alters dopaminergic receptor functions in pain pathway.
The main finding in the present study is that aripiprazole (at doses used here) significantly prolonged morphine antinociception effect and disrupted morphine antinociceptive tolerance in mice. Although the detailed mechanism by which aripiprazole affect the morphine antinociception is yet unclear, the partial activation of D2 receptors can be considered as the main mechanism aripiprazole on prolongation of morphine antinociception effect.
However, further pharmacological researches are needed to elucidate the actual mechanism of aripiprazole on modulating morphine-induced antinociception in animal models of pain.
Acknowledgments
Supports from the Pharmaceutical Sciences Branch of the Islamic Azad University, Tehran, Iran are gratefully acknowledged. The authors also thank the personnel of the Pharmacology and Toxicology laboratories for their help.
Footnotes
Author’s Contribution
Study concept, design and critical revision of the manuscript for important intellectual content: Zahra Mousavi, Marzieh Norouzi, Hamed Shafaroodi.
Funding/Support:
This study was supported in part by the Pharmaceutical Sciences Branch of the Islamic Azad University, Tehran, Iran.
References
- Patt R. B., Proper G., Reddy S. The neuroleptics as adjuvant analgesics. J Pain Symptom Manage. 1994;9(7):446-453
CrossRef - Seidel S., Aigner M., Ossege M., Pernicka E., Wildner B., Sycha T. Antipsychotics for acute and chronic pain in adults. J Pain Symptom Manage. 2010;39(4):768-778.
CrossRef - Hirose T., Kikuchi T. Aripiprazole a novel antipsychotic agent: dopamine D2 receptor partial agonist. J Med Invest. 2005;52:284-290.
CrossRef - Davies M. A., Sheffler D. J., Roth B. L. Aripiprazole a novel atypical antipsychotic drug with a uniquely robust pharmacology. CNS Drug Rev. 2004;10(4):317-336.
CrossRef - Kasper S., Lerman M. N., McQuade R. D., Saha A., Carson W. H., Ali M., et al. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol. 2003;6(4):325-337.
CrossRef - Mamo D., Graff A., Mizrahi R., Shammi C., Romeyer F., Kapur S. Differential effects of aripiprazole on D 2, 5-HT 2, and 5-HT 1A receptor occupancy in patients with schizophrenia a triple tracer PET study. Am J Psychiatry. 2007;164(9):1411-1417.
CrossRef - Naber D., Lambert M. Aripiprazole a new atypical antipsychotic with a different pharmacological mechanism. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(8):1213-1219.
CrossRef - Ross D. D., Alexander C. S. Management of common symptoms in terminally ill patients: Part II. Constipation, delirium and dyspnea. Am Fam Phys. 2001;64(6):1019-1026.
- Tonini M., Cipollina L., Poluzzi E., Crema F., Corazza G., De Ponti F. Clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther. 2004;19(4):379-390.
CrossRef - Narita M., Takei D., Shiokawa M., Tsurukawa Y., Matsushima Y., Nakamura A., et al. Suppression of dopamine-related side effects of morphine by aripiprazole a dopamine system stabilizer. Eur J Pharmacol. 2008;600(1):105-109.
CrossRef - Chien C. C., Pasternak G. W. Sigma antagonists potentiate opioid analgesia in rats. Neurosci lett. 1995;190(2):137-139.
CrossRef - Almeida-Santos A. F., Gobira P. H., Souza D. P., Ferreira R. C., Romero T. R., Duarte I. D., et al. The antipsychotic aripiprazole selectively prevents the stimulant and rewarding effects of morphine in mice. Eur J Pharmacol. 2014;742:139-144.
CrossRef - Taylor A. M., Becker S., Schweinhardt P., Cahill C. Mesolimbic dopamine signaling in acute and chronic pain implications for motivation analgesia and addiction. Pain. 2016;157(6):1194.
CrossRef - Unterwald E. M., Cuntapay M. Dopamine–opioid interactions in the rat striatum a modulatory role for dopamine D 1 receptors in delta opioid receptor-mediated signal transduction. Neuropharmacol. 2000;39(3):372-381.
CrossRef - Wood P. B. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8(5):781-797.
CrossRef - Franklin K. Analgesia and the neural substrate of reward. Neurosci Biobehav Rev. 1989;13(2-3):149-154.
CrossRef - Altier N., Stewart J. Dopamine receptor antagonists in the nucleus accumbens attenuate analgesia induced by ventral tegmental area substance P or morphine and by nucleus accumbens amphetamine. J Pharm Exp Ther. 1998;285(1):208-215.
- Saadé N. E., Atweh S. F., Bahuth N. B., Jabbur S. J. Augmentation of nociceptive reflexes and chronic deafferentation pain by chemical lesions of either dopaminergic terminals or midbrain dopaminergic neurons. Brain res. 1997;751(1):1-12.
CrossRef - Reisi Z., Bani-Ardalan M., Zarepour L., Haghparast A. Involvement of D1/D2 dopamine receptors within the nucleus accumbens and ventral tegmental area in the development of sensitization to antinociceptive effect of morphine. Pharmacol Biochem Behav. 2014;118:16-21.
CrossRef - Tang L., Shukla P. K., Wang L. X., Wang Z. J. Reversal of morphine antinociceptive tolerance and dependence by the acute supraspinal inhibition of Ca2+/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther. 2006;317(2):901-909.
CrossRef - Li S. x., Zou Y., Liu L. j., Wu P., Lu L. Aripiprazole blocks reinstatement but not expression of morphine conditioned place preference in rats. Pharmacol Biochem Behav. 2009;92(2):370-375.
CrossRef - Leite J. V., Guimarães F. S., Moreira F. A. Aripiprazole, an atypical antipsychotic prevents the motor hyperactivity induced by psychotomimetics and psychostimulants in mice. Eur J Pharmacol. 2008;578(2):222-227.
CrossRef - Hirose T., Uwahodo Y., Yamada S., Miwa T., Kikuchi T., Kitagawa H., et al. Mechanism of action of aripiprazole predicts clinical efficacy and a favourable side-effect profile. J Psychopharmacol. 2004;18(3):375-383.
CrossRef - Yang C., Chen Y., Tang L., Wang Z. J. Haloperidol disrupts opioid-antinociceptive tolerance and physical dependence. J Pharm Exp Ther. 2011;338(1):164-172.
CrossRef - Morris B., Hunt S. Proenkephalin mRNA levels in rat striatum are increased and decreased respectively, by selective D 2 and D 1 dopamine receptor antagonists. Neurosci lett. 1991;125(2):201-204.
CrossRef - Voorn P., Docter G. J., Jongen‐Rêlo A. L., Jonker A. J. Rostrocaudal subregional differences in the response of enkephalin, dynorphin and substance P synthesis in rat nucleus accumbens to dopamine depletion. Eur J Neurosci. 1994;6(3):486-496.
CrossRef - Hong J., Yoshikawa K., Kanamatsu T., Sabol S. Modulation of striatal enkephalinergic neurons by antipsychotic drugs. Fed Proc. 1985;44(9):2535-2539.
- Alper R., Demarest K., Moore K. Morphine differentially alters synthesis and turnover of dopamine in central neuronal systems. J Neural Transm. 1980;48(3):157-165.
CrossRef - Di Chiara G., Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharm Exp Ther. 1988;244(3):1067-1080.
- Devine D. P., Leone P., Pocock D., Wise R. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharm Exp Ther. 1993;266(3):1236-1246.
- Yonehara N., Clouet D. H. Effects of delta and mu opiopeptides on the turnover and release of dopamine in rat striatum. J Pharm Exp Ther. 1984;231(1):38-42.
- Walker J. M., Thompson L. A., Frascella J., Friederich M. W. Opposite effects of μ and κ opiates on the firing-rate of dopamine cells in the substantia nigra of the rat. Eur J Pharmacol. 1987;134(1):53-9.
CrossRef - Johnson S., North R. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12(2):483-488.
CrossRef - Azizi P., Haghparast A., Hassanpour-Ezatti M. Effects of CB1 receptor antagonist within the nucleus accumbens on the acquisition and expression of morphine-induced conditioned place preference in morphine-sensitized rats. Behav Brain Res. 2009;197(1):119-124.
CrossRef - Rezayof A., Amini R., Rassouli Y., Zarrindast M. R. Influence of nitric oxide on morphine-induced amnesia and interactions with dopaminergic receptor agents. Physiol behav. 2006;88(1):124-131.
CrossRef - Chartoff E. H., Mague S. D., Barhight M. F., Smith A. M., Carlezon W. A. Behavioral and molecular effects of dopamine D1 receptor stimulation during naloxone-precipitated morphine withdrawal. J Neurosci. 2006;26(24):6450-6457.
CrossRef - Chartoff E. H., Barhight M. F., Mague S. D., Sawyer A. M., Carlezon Jr W. A. Anatomically dissociable effects of dopamine D1 receptor agonists on reward and relief of withdrawal in morphine-dependent rats. Psychopharmacol. 2009;204(2):227-239.
CrossRef - Zarrindast M. R., Zarghi A. Morphine stimulates locomotor activity by an indirect dopaminergic mechanism: possible D-1 and D-2 receptor involvement. Gen Pharmacol. 1992;23(6):1221-1225.
CrossRef - Dizgah I. M., Karimian S. M., Zarrindast M. R., Sohanaki H. Attenuation of morphine withdrawal signs by a D1 receptor agonist in the locus coeruleus of rats. Neuroreport. 2005;16(15):1683-1686.
CrossRef - Zarrindast M. R., Asgari-Afshar A., Sahebgharani M. Morphine-induced antinociception in the formalin test: sensitization and interactions with D1 and D2 dopamine receptors and nitric oxide agents. Behav pharmacol. 2007;18(3):177-84.
CrossRef - Ozdemir E., Bagcivan I., Gursoy S. Role of D1/D2 dopamin receptors antagonist perphenazine in morphine analgesia and tolerance in rats. Bosn J Basic Med Sci. 2013;13(2):119-125.
CrossRef - Pertovaara A., Martikainen I. K., Hagelberg N., Mansikka H., Någren K., Hietala J., et al. Striatal dopamine D2/D3 receptor availability correlates with individual response characteristics to pain. Eur J Neurosci. 2004;20(6):1587-1592.
CrossRef - Lin M., Wu J., Chandra A., Tsay B. Activation of striatal dopamine receptors induces pain inhibition in rats. J Neural Transm. 1981;51(3-4):213-222.
CrossRef - Magnusson J. E., Fisher K. The involvement of dopamine in nociception: the role of D 1 and D 2 receptors in the dorsolateral striatum. Brain res. 2000;855(2):260-266.
CrossRef - Jordan S., Koprivica V., Chen R., Tottori K., Kikuchi T., Altar C. A. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT 1A receptor. Eur J Pharmacol. 2002;441(3):137-140.
CrossRef - Stark A. D., Jordan S., Allers K. A., Bertekap R. L., Chen R., Kannan T. M. Interaction of the novel antipsychotic aripiprazole with 5-HT1A and 5-HT2A receptors: functional receptor-binding and in vivo electrophysiological studies. Psychopharmacol. 2007;190(3):373-382.
CrossRef








