Kaveripakam Saisruthi and Adikay Sreedevi
Pharmaceutical Chemistry, Institute of Pharmaceutical Technology, Sri Padmavathi Mahila Visvavidyalayam (Women’s University), Tirupati, Andhra Pradesh, India.
Corresponding Author E-mail: sruthisai7@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1250
Abstract
The present study was aimed to evaluate the nephroprotective potential of ethanolic extract of roots of Anthocephalus cadamba. Ethanol extract of roots of Anthocephalus cadamba was prepared by hot extraction and subjected to preliminary phytochemical and acute toxicity studies. The nephroprotective potential of extract was evaluated in Wistar rats at two dose levels of 200 and 400 mg/kg b. w. Nephrotoxicity was induced by injecting cisplatin (5 mg/kg b. w. i.p). Nephroprotective potential was assessed by determining serum creatinine, Blood urea nitrogen, Urinary total protein, Creatinine clearance, lipid peroxidation and antioxidant levels. Extract significantly attenuated nephrotoxicity induced by cisplatin which was confirmed by reducing levels of serum markers, urinary total protein, lipid peroxidation and increased creatinine clearance. Extract also compensated deficits in the renal antioxidant system. Histological studies substantiated the biochemical parameters. Thus it can be concluded that Anthocephalus cadamba has a potential role in the abatement of cisplatin-induced nephrotoxicity.
Keywords
Anthocephalus cadamba Cisplatin;Nephroprotective;
Download this article as:| Copy the following to cite this article: Saisruthi K, Sreedevi A. Amelioration of Cisplatin - Induced Nephrotoxicity by Roots of Anthocephalus Cadamba. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Saisruthi K, Sreedevi A. Amelioration of Cisplatin - Induced Nephrotoxicity by Roots of Anthocephalus Cadamba. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16555 |
Introduction
Over the centuries, the use of medicinal herbs has become an important part of daily life despite the progress in modern medical and pharmaceuticals research. Through observation and experimentation, human beings have learnt that plants promote health and well-being. Much of the world’s population, especially within developing countries depends on traditional medicine to meet daily health requirements.1 For more than 80% of Asia’s population, medicinal plants are accessible, affordable and culturally appropriate sources of primary health care.2 There are several attempts were made to explore indigenous knowledge on sue of common medicinal plants for the treatment of diseases related to various systems of human beings. Many medicinal plants claimed and scientifically evidenced for their therapeutic potential in treatment of various ailments including nephrotoxicity.3
Nephrotoxicity is a poisonous effect of some substances, both toxic chemicals and medication on the kidneys. The kidney while playing its primary role as eliminator of numerous endogenous and exogenous substances including drugs, accumulate large amount of these substances particularly in the proximal tubules. A number of drugs including the cisplatin, doxorubicin, Gentamicin are nephrotoxic.4 Cisplatin [cis-dichlorodiammineplatinum (II)] is a potent chemotherapeutic drug that is used in the treatment of various solid tumors including testis, breast, lung, and uterine cervix carcinomas. Though the potency of the drug is highly effective, its serious nephrotoxic nature as adverse side effect restricts its long-term use and effective dose selection in cancer chemotherapy.5 Thus, there is a continuous search for agents that provide nephroprotection against cisplatin and other platinum drugs.
In traditional medicine various medicinal plants had been used for the treatment of renal diseases. Anthocephaluscadamba (F: Rubiaceae), which grows mainly in South Asia and South East Asia is used widely in traditional formulations, and it has been a remedy in the treatment of eye infection, skin diseases, dyspepsia as well as to cure the gum re- lated troubles, stomatitis, cough, fever, anaemia, blood disorders, stomach pain and urinary disorders. In traditional system of medicine roots of this plant claimed to possess diuretic property and protective against urinary complaints.6 But till today the roots of the plant was not scientifically explored for its nephroprotective activity. Hence the present study is aimed to evaluate the nephroprotective potential of ethanolic extract of roots of Anthocephalus cadamba.
Materials and Methods
Chemicals
Cisplatin was purchased from Varsha medicine house, Tirupati, India. Blood Urea Nitrogen (BUN) and serum creatinine kits were obtained from Erba Diagnostics, Himachal Pradesh, India. All other chemicals were of analytical grade procured from reputed Indian manufacturers.
Collection and Authentication of Plant Material
The roots of Anthocephalus cadamba were collected from Tirumala hills, Chittor district of Andhra Pradesh. The plant was authenticated by Botanist Dr. Madhavachetty, Herbarium keeper, Department of botany, Sri Venkateswara University, Tirupati, India and a specimen (No.1856) has been deposited in Department of Botany, Sri Venkateswara University, Tirupati, India. Roots were washed, shade dried and powdered in Wiley mill.
Preparation of Extract
The root powder was defatted with petroleum ether (60-800C). The defatted marc was air-dried and macerated with ethanol for 24 h. Macerated material was refluxed for 3h and then filtered. The procedure was repeated twice and obtained filtrate was combined and subjected to distillation under reduced pressure.
Preliminary Phytochemical Studies
Ethanolic extract of Anthocephalus cadamba was subjected to Preliminary phytochemical studies using standard procedures to identify the presence of various phytoconstituents.7
Animals
Male Wistar Albino rats (body weight 150–200 g) were purchased from Sri Venketeswara Enterprises, Bangalore, India and were kept under standard conditions of temperature and humidity in the center’s animal house facility. The animals were provided with standard rat chow and water ad libitum. All animal experiments in this study were carried out with the prior approval of the Institutional Animal Ethics Committee (IAEC) and were conducted strictly adhering to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) constituted by the Animal Welfare Division of the Government of India (CPCSEA approval No. 1677/PO/Re/S/2012/CPCSEA/09).
Acute oral Toxicity Studies
Oral acute toxicity studies were conducted for EEAC at 2000 mg/kg body weight as per OECD guidelines no.423. Any changes in body weights of the rat, changes in skin and fur, eyes and mucous membranes, salivation, nasal discharge, urination and behavioral (sedation, depression), neuromuscular (tremors, convulsions), cardiovascular changes, lethargy, sleep and coma were observed. The animals were kept under observation for 14 days.8
Experimental Design
Screening of Nephroprotective Activity
Animals were divided into five groups of six each. Nephrotoxicity was induced by single intra peritoneal administration of cisplatin at a dose of 5mg/kg b. w. The following treatment schedule was followed:
Group-I: Vehicle from day1 to day 10
Group-II: Vehicle from day1 to day 10 and Cisplatin 5mg/kg b. w. on day 5.
Group-III: Extract 200mg/kg b. w. from day 1 to 10 and Cisplatin 5mg/kg b. w. on day-5
Group-IV: Extract 400mg/kg b. w. from day 1 to 10 and Cisplatin 5mg/kg b. w. on day-5
Group-V: Cystone 5ml/kg b. w. from day 1 to 10 and Cisplatin 5mg/kg b. w. on day-5
Prior to the termination of experiment urine was collected with the help of metabolic cages and the urine samples were subjected for estimation of urinary functional parameters. On day 11 blood samples were collected by retro orbital puncture and serum was separated by centrifugation at 3000rpm at 40C used for estimation of serum markers. Then the animals were sacrificed by cervical decapitation and both kidneys were harvested; one of the kidneys was used for antioxidant studies and other was subjected to histological examination.
Assessment of Renal Function
Serum and Urinary Parameters
Renal function was assessed by measuring biochemical parameters such as Blood Urea nitrogen (DAM method), Serum creatinine (Jaffe’s Alkaline picrate method), Urinary total protein (Turbidity method) and Urinary creatinine (Alkaline picrate method) using commercial kits in semi automatic analyzer9.
Renal Oxidative Stress Markers
A 10% homogenate of rat kidney was prepared in ice cold 0.1 M phosphate buffer (pH 7.8). The homogenates were centrifuged at 6000 rpm for 15 mins at 4 °C. The clear supernatant was used immediately for estimation of levels of lipid peroxidation, reduced glutathione, catalase and superoxide dismutase.10-13
Histological Evaluation
The formalin fixed kidney tissues were dehydrated in a graded series of alcohol, cleared in xylene and then embedded in paraffin. Tissue embedded paraffin blocks were sectioned at 5μm thickness, stained with hematoxylin and eosin and examined using a light microscope.
Statistical Analysis
Values were represented as mean ± SEM. Data were analyzed using one-way analysis of variance (ANOVA) and group means were compared using the Tukey-Kramer Multiple Comparison Test using GraphPad Prism software. P values < 0.05 were considered significant.
Results
Preliminary Phytochemical Studies
Preliminary phytochemical studies of EESS revealed the presence of flavanoids, saponins, phenolic compounds and tannins.
Acute Toxicity Studies
Animals received extract at a dose of 2000mg/kg b. w. had not shown any signs of toxicity and mortality up to day 14.
Effect of EEAC on Serum and Urinary Parameters
Cisplatin alone administered group showed significant increase in levels of blood urea nitrogen (BUN), Serum creatinine (SC), Urinary total protein (UTP) and decreased the levels of creatinine clearance (Crcl). Treatment with EEAC in dose dependent manner attenuated the levels of BUN, SC and UTP and restored the levels of creatinine clearance when compared to the disease control (Table 1).
Table 1: Effect of EEAC on serum and urinary parameters:
| Group | BUN (mg/dl) | SC (mg/dl) | UTP (mg/24hrs) | Creatinine clearance ((ml/hr/100g b.w) |
| I | 14.80±0.85 | 0.82±0.05 | 2.10±0.37 | 22.20±1.96 |
| II | 34.23±1.43* | 2.32±0.08* | 6.62±0.32* | 4.34±0.53* |
| III | 27.01±1.21# | 1.70±0.05# | 4.87±0.21# | 11.02±0.60# |
| IV | 22.01±1.39# | 1.43±0.04# | 3.25±0.31# | 14.00±1.44 # |
| V | 20.27±0.85# | 1.30±0.04# | 2.86±0.27# | 15.71±1.23# |
Each value represents mean ± SEM of six animals in each group. *: P<0.05 when compared with Group-I; #: P<0.05 when compared with Group-II.
Effect of EEAC on Renal Oxidative Stress Markers
Rats from cisplatin alone administered groups presented with significant elevation of tissue lipid peroxidation (LPO) and decrease in reduced glutathione (GSH), Super oxide dismutase (SOD) and catalase (CAT) enzyme activities. Treatment with EEAC significantly suppressed lipid peroxidation and attenuated the depletion of antioxidant defense system GSH, SOD and CAT levels in dose dependent manner when compared to cisplatin alone treated group (Table 2).
Table 2: Effect of EEAC on renal oxidative stress markers:
| Group | LPO (nmol/g of tissue) | GSH (nmol/g
of tissue) |
SOD (units/mg of tissue) | CAT (units/mg of tissue) |
| I | 2.75±0.07 | 1.25±0.02 | 32.00±0.61 | 31.26±0.92 |
| II | 7.48±0.55* | 0.58±0.01* | 9.32±0.46* | 13.63±1.29* |
| III | 4.11±0.36# | 0.75±0.01# | 14.04±0.50 # | 20.96±1.11# |
| IV | 3.75±0.37 # | 0.87±0.03 # | 18.36±0.76 # | 25.86±0.97 # |
| V | 2.88±0.09 # | 0.98±0.03# | 21.90±1.47 # | 29.48±1.13 # |
Each value represents mean ± SEM of six animals in each group. *: P<0.05 when compared with Group-I; #: P<0.05 when compared with Group-II.
Effect of EEAC on Histopathological Changes
The histopathological examination of kidney sections from normal rats showed regular architecture of cortex without any evidence of injury. The kidneys of cisplatin-treated rats showed diffuse acute tubular necrosis, tubular dilatation, and denudation of epithelium, interstitial edema, and infiltration of inflammatory cells. EEAC at 200 mg/kg b.w showed mild regenerative changes and group treated with EEAC at 400mg/kg b.w showed marked regenerative changes and thereby significant protection against cisplatin- induced renal injury (Figure 1).
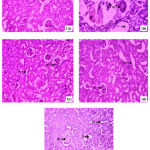 |
Figure 1: Photomicrographs of rat kidney: |
1(a): Group-I- Section of normal rat kidney showing normal organization; 1(b): Group-II- Section of rat kidney of disease control showing necrotic changes in kidney tissues and congestion; 1(c): Group-III- Section of rat kidney showing moderate regenerative changes; 1(d): Group-IV- Section of rat kidney showing moderate regenerative changes; 1(e):Group-V- Section of rat kidney showing regenerative changes and almost normal organization. RT-Renal tubule; BC- Bowman’s capsule; NC-Nerotic changes; V-Vacuolization; DC-Degeneartive changes; D-Distal tubule; PT-Proximal tubule.
Discussion
Drug induced nephrotoxicity is very common and among all cisplatin nephrotoxicity leads to considerable health and economical burden worldwide.14 The anticancer mechanism of cisplatin includes the formation of highly reactive platinum complexes which binds with nucleophilic DNA via intra-strand and inter- strand crosslinking with guanine nucleotide. These events lead to denaturation of DNA and consequently cell cycle arrest. However, the mechanism of cisplatin nephrotoxicity differs from its anticancer activity.15 Cisplatin via cytochrome P450 (CYP) in microsome and mitochondrial dysfunctioning leads to the formation of reactive oxygen species (ROS) and damage the renal tissue. This leads to acute renal failure by induction of oxidative damage, inflammatory cytokines, tubule-interstitial inflammation and apoptosis/necrosis of renal tubular cells.16
Many different adjuvants including antioxidants, modulators of nitric oxide and anti-apoptotic agents have been investigated for their beneficial effect on cisplatin- induced renal injury.17 However, none of them were found safe for clinical application. Recently a plethora of pharmacological and molecular studies in preclinical studies revealed the promising potential of herbal medicine against cisplatin injury.18
In the present study, we demonstrated ameliorating potential of ethanol extract of roots of Anthocephalus cadamba against cisplatin-induced nephrotoxicity in rats. A single dose of cisplatin (5 mg/kg, ip) to the rats resulted in noticeable nephrotoxicity in cisplatin control rats which was in good agreement with previous reports.19 Cisplatin caused significant raise in SC, BUN, urinary total protein and decline in creatinine clearance compared to normal control rats which may be due to Cisplatin induced impairment in renal reabsorption and enhanced reno-vascular resistance and the findings were in agreement with the reported references.20-22 Administration of EEAC ameliorated cisplatin induced alterations in various serum/urine parameters and led to significant recovery of both serum and urinary parameters.
One of the most important mechanisms involved in cisplatin toxicity is oxidative stress. Under normal physiological conditions generation and elimination of reactive oxygen species (ROS) in cells are controlled by an endogenous scavenging system including catalase, superoxide dismutase, and reduced glutathione. Under oxidative stress conditions, ROS levels are elevated and many cellular structures (proteins, lipids, DNA) can be damaged. ROS play a crucial role in the pathogenesis of cisplatin- induced nephrotoxicity. Increased oxidative stress can produce cellular injury and necrosis in the kidney and other tissues.23 Previous studies stated the key role of lipid peroxidation and antioxidant enzymes such as SOD and CAT in the free radical metabolisms.24 The antioxidants enzymes such as catalase and SOD scavenges free radicals or converts them in to non-toxic end products and considered as first line cellular defense mechanism against oxidative damage. The second line of antioxidant defense consists of the non-enzymatic scavenger’s such as GSH, which scavenge remaining free radicals escaping from the first line of antioxidant enzymes defense.25 The results of current study shown that cisplatin administration significantly raised the levels of lipid peroxidation and caused depletion of GSH, SOD and CAT. These findings suggested increased level of oxidative stress, which is in agreement with earlier findings.26 While EEAC administration significantly reduced the renal LPO level and restored the antioxidant enzyme levels to the normal.
Cisplatin induced nephrotoxicity was further evidenced from histopathological studies. The inhibition of the deleterious effects produced by free radicals, enhanced supply of antioxidants and regeneration of glomerular region in the kidney might result from the potential therapeutic phytocompounds like flavonoids, saponins and phenolic compounds present in EEAC.
Conclusion
Based on the experimental findings it can be concluded that Anthocephalus cadamba has a potential role in the abatement of cisplatin-induced nephrotoxicity. Furthermore the present study provides corroborative scientific evidence for ethno-medicinal use of these roots in renal disorders.
Conflicts of Interest
No conflicts of interest
References
- Prakasha H. M., Krishnappa M., Krishnamurthy Y. L and Poornima S. V. Folk medicine of NR PuraTaluk in Chikamaglur district of Karnatka. Indian Journal of Traditional Knowledge. 2010;9(1):55-60.
- National policy on traditional medicine and regulation of herbal medicines: Report of a WHO global survey. World Health Organization, Geneva, Switzerland. 2005.
- Janakiraman M and Jayaprakash K. Nephroprotective potential of medicinal plants: A Review. Int j sci res. 2015;4(9):543-547.
- Hartmann J. T and Lipp H. P. Toxicity of platinum compounds.Expert Opin. Pharmacother. 2003;4:889–901.
CrossRef - George S. P and Anushree C. S. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014;7:457–68.
- Atul D., satish N and Goupale D. C. Anthocephalus Cadamba: A Review. Pharmacognosy Journal. 2013;2(18):71-76.
- Harbone J. P. Phytochemical Methods, A Guide to Modern Technique of Plant Analysis. Chapmann and Hall, London. 1973;1-271.
- Organization for Economic Cooperation and Development (OECD). Guideline 423 for testing chemicals: Paris. 2001;1-14.
- Godkar P. B. Kidney function tests. In: Text book of Medicinal laboratory. Bombay: Bhalani publishing house. 1994;1022-28.
- Wright J. R., Colby H. D and Miles P. R. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Archives of Biochemistry and Biophysics. 1981;206:296–304.
CrossRef - Ellman and Georg L. Tissue sulfahydryl group. Arch. Biochem. Biophys. 1959;82:70–77.
CrossRef - Claiborne A. Catalase activity. In: CRC Handbook of Methods for Oxygen Radical Research. 1985:283–284.
- Misra H. P and Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175.
- Frezza M., Hindo S., Chen D., Davenport A., Schmitt S., Tomco D and Ping Q. Novel metals and metal complexes as platforms for cancer therapy. Curr. Pharm. Des. 2010;16(16):1813–1825.
CrossRef - H and Devarajan P. Cisplatin nephrotoxicity molecular mechanisms. Cancer Therapy. 2003;1-47.
- Saisyo A., Nakamura H., Fang J., Tsukigawa K., Greish K., Furukawa H and Maeda H. pH-sensitive polymeric cisplatin-ion complex with styrene-maleic acid copolymer exhibits tumor-selective drug delivery and antitumor activity as a result of the enhanced permeability and retention effect. Colloids Surf. B: Biointerfaces. 2016;138:128–137.
CrossRef - Ali B. H and AlMoundhri M. S. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds a review of some recent research. Food Chem Toxicol. 2006;44:1173–1183.
CrossRef - Abdel Moneim A. E., Othman M. S and Aref A. M. Azadirachta indica attenuates cisplatin-induced nephrotoxicity and oxidative stress. Biomed. Res Int. 2014;647131.
- Naziroglu M., Karaoglu A and Aksoy A. O. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicol. 2004;195: 221-30.
CrossRef - Mondi S., Kvsrg P., Jhansi D., Vijay R and Rao V. U. Prophylactic and curative effect of ethanolic extract of Bassiamala baricabark against cisplatin induced nephrotoxicity. Asian J Pharm Clin Res. 2014;7(4):143-6.
- Ingale K. G., Thakurdesai P. A and Vyawahare N. S. Protective effect of Hygrophila spinosaagainst cisplatin induced nephrotoxicity in rats. Indian J Pharmacol. 2013;45(3):232-6.
CrossRef - Prakasha H. M., Krishnappa M., Krishnamurthy Y. L and Poornima S. V. Folk medicine of NR PuraTaluk in Chikamaglur district of Karnatka. Indian Journal of Traditional Knowledge. 2010;9(1):55-60.
- Naqshbandi A., Rizwan S and Khan F. Dietary supplementation of flaxseed oil ameliorates the effect of cisplatin on rat kidney. J. Funct. Foods. 2013;5:316– 326.
CrossRef - Kumar M., Kasala E. R., Bodduluru L. N., DahiyaV and Lahkar M. Baicalein protects isoproterenol induced myocardial ischemic injury in male Wistar rats by mitigating oxidative stress and inflammation. Inflamm. Res. 2016;1–10.
CrossRef - Zheng W., Huang L. Z., Zhao L., Wang B., Xu H. B., Wang G. Y., Wang Z. L and Zhou H. Superoxide dismutase activity and malondialdehyde level in plasma and morphological evaluation of acute severe hemorrhagic shock in rats. Am. J. Emerg. Med. 2008;26(1):54–58.
CrossRef - Ognjanovi B. I., Djordjevi N. Z., Mati M. M., Obradovi J. M., Mladenovi J. M., Stajn A. S and Sai Z. S. Lipid peroxidative damage on cisplatin exposure and alterations in antioxidant defense system in rat kidneys a possible protective effect of selenium. Int. J. Mol. Sci. 2012;13(2):1790–1803.
CrossRef







