Yurnadi Hanafi Midoen1, Dita Rany Anggraeni2, Dwi Anita Suryandari1 and Hans-Joachim Freisleben3
1Department of Medical Biology, Faculty of Medicine, Universitas Indonesia.
2Master Program Biomedical Sciences, Faculty of Medicine, Universitas Indonesia.
3Medical Research Unit, Faculty of Medicine, Universitas Indonesia, Jalan Salemba Raya 6, Jakarta Pusat, 10430 Indonesia.
Corresponding author E-mail: yurnadi.kes@ui.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1162
Abstract
To reduce population growth, the Indonesian government promotes a Family Planning Program for fertile couples. Depot-medroxyprogesterone acetate (DMPA) combined with testosterone or herbal androgens as testosterone substitute is intended for hormonal male contraception because it is expected that these combinations can suppress gonadotropin and inhibit spermatogenesis. Various medicinal herbs contain androgens, e.g., Javanese long pepper (JLP) (Piper retrofractum Vahl). Traditionally, JLP chili extract (JCE) is used to cure impotency and was proven to increase blood testosterone levels in hypogonad men. The aim of this study is to combine increasing amounts of JCE (0.94 – 3.76 mg) with DMPA (1.25 mg) and to study the effects on testicular parameters using male Sprague-Dawley rats: testis weight, seminiferous tubule diameter, the subpopulations of spermatogenic cells, and Leydig cells. Our randomized study design used six equally sized groups with 6 animals, each. Testis weight only decreased vs. controls in two groups, DMPA + placebo and DMPA + 3.76 mg JCE. Significant decrease occurred in the diameter of seminiferous tubules and at 3.76 mg JCE, in Leydig cell population. Within spermatogenic cell population spermatogonia A, spermatocyte-I preleptotene, spermatocyte-I pachiten, and spermatid population decreased. The combination of 1.25 mg DMPA and 3.76 mg JCE appears to be the minimal dose and a potent candidate for male contraception in our rat model.
Keywords
DMPA; Leydig cells; Piper retrofractum Vahl; seminiferous tubules; spermatogenic cells
Download this article as:| Copy the following to cite this article: Midoen Y. H, Anggraeni D. R, Suryandari D. A, Freisleben H. Finding The Minimal Effective Dose of A Combination of Depot Medroxyprogesterone Acetate and Javanese Long Pepper for Male Contraception on Testicular Fertility Parameters in Male Sprague Dawley Rats. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Midoen Y. H, Anggraeni D. R, Suryandari D. A, Freisleben H. Finding The Minimal Effective Dose of A Combination of Depot Medroxyprogesterone Acetate and Javanese Long Pepper for Male Contraception on Testicular Fertility Parameters in Male Sprague Dawley Rats. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=15028 |
Introduction
In 2025, the Indonesian population is expected to reach 273 million inhabitants even with population growth below 1.5%. To effectively reduce these numbers, the Indonesian government launched a Family Planning Program for couples in fertile age.1 By far less male contraceptives have been available until today as compared to female contraception methods. Until mid-20th century, male contraceptive methods were used by 30% of couples in the world, such as condoms, vasectomy, coitus interruptus, and sexual abstinence.2
In our efforts searching for male contraceptives which are safe, effective, easy to use, reversible, and acceptable by the society.3 We used depot medroxyprogesterone acetate (DMPA), a synthetic progesterone derivative with a long acting and safe effect, when administered via IM injection. DMPA is a potent inhibitor of pituitary gonadotropin secretion and widely used for female contraception.4-6 Combined with testosterone it is good prospect for development of male contraceptives providing sufficient effective time which is required for inhibiting the secretion of gonadotropin hormones FSH and LH, thus suppressing spermatogenesis and plasma testosterone levels. This decrease in testosterone levels can be overcome by the administration of exogenous testosterone.7-8
Applying a combination of progestin and testosterone was shown to cause a decrease in testicular volume or weight and shrinkage of the diameter of the tubulus seminiferous.9-10 Moreover, this combination decreased the population of spermatogenic cells11 by disrupting their development due to constraints on gonadotropin hormones FSH and LH7. Progesterone acetate derivatives injected into rams did not only lead to the cessation of spermatogenesis but also to atrophy of Leydig cells.12
Java chili fruits are traditionally used to cure male impotence, stomach weakness, strong transpiration etc. They contain piperine, piperatin, beta-sitosterol, free amino acids, essential oils, resins, saponins, polyphenols, and other compounds.13-14 Infusion of Javanese chili fruit extract (JCE) at a dose of 2.1 mg / 10 g body weight of rats showed androgenic and anabolic effects.14 In the Ames test JCE did not show any mutagenic effects and was considered safe for consumption.15 On male chicks, the androgenic effect of 3.75 mg / 100 g body weight of ethanolic JCE extract was not significantly different from the standard dose of 500 mg TU / 100 g BW.16 In 2006, a clinical trial was carried out to determine the androgenic effects of JCE on 9 hypogonadal men.17 The results showed that JCE could increase blood testosterone levels in 7 out of 9 hypogonadal men (78%), did not reduce the levels of FSH and LH, could increase the frequency of coitus and did not cause serious side effects.17
In previous studies, the minimal effective dose of 1.25mg DMPA had been determined by the concentration and viability of spermatozoa in Vas deferens of rats.18-19 However, testosterone levels decreased by more than 50% and JCE was administered to maintain the testosterone level. The combination of 1.25 mg DMPA with various amounts of JCE had shown to keep the number and viability of spermatozoa low, but only the combination with JCE 3.76 mg kept the testosterone level at 100% of controls.20-21 According to these studies DMPA can suppress spermatogenesis, while JCE can maintain testosterone levels decreased by DMPA. It is necessary to further investigate the combined effects of DMPA and JCE on spermatogenesis using rats as experimental animals. The purpose of this study is to confirm the results of preliminary studies by investigating testicular fertility parameters.
Materials and Methods
Healthy and fertile male rats (Rattus norvegicus L) Sprague-Dawley, aged 40-60 days with 250 grams of body weight and Java chili extract (JCE) in the form of gelatin capsules were obtained from BPOM RI.
Most chemicals (benzene, benzyl benzoate, entellan, eosin Y, ethanol, ether, glycerol, paraffin, and xylol) were purchased from Merck AG, Darmstadt, Germany (subsidiary Jakarta), at highest grade available, all other chemicals and instruments from local distributors in Jakarta or Singapore of the companies given in brackets: solution of 1% Na-CMC (Sino-CMC), DMPA (Depogeston-Biowet), picric acid (JT-Baker), aquabidest (IKA), physiological saline (Baxter), hematoxiline (MCB), measure cups (Iwaki), syringes (Terumo), surgical tools (Yamaco-Inox), digital scales (Citizen), rotary microtome (Spencer), beaker glasses and bottles (Schott-Duran), electronic binocular microscope (Nikon), micrometer objective and micrometer ocular (B&L), laboratory counter (Clay-Adams), glass slides and cover glasses (Assistent), razor blades and staining jar (Termo-Sandon). The stomach tubes were handmade in our animal house.
Experimental Design
This study used a completely randomized design (CRD) with equal sample size (n=6), 6 groups consisting of the control group (C), a sham control group (CP = rats injected with DMPA and given placebo), treatment group I (TI = rats injected with DMPA and JCE 0.94 mg), treatment group II (TII = rats injected with DMPA and JCE 1.88 mg), treatment group III (TIII = rats injected with DMPA and JCE 2.82 mg), treatment group IV (TIV = rats injected with DMPA and JCE 3.76 mg).
Treatment of the Experimental Animals
The rats were acclimatized in their cages for 15 days, fed standard diet with free access to drinking water ad libitum. Rats were injected with DMPA in accordance with the predetermined dose of 1.25 mg, alternately into the right or left thigh. Injections were done twice, the first one at week 0 and the second one at week 12, because the effective time of DMPA in suppressing the secretion of gonadotropin hormones FSH and LH is 12 weeks.
In addition, JCE was administered to the treatment groups from week 7 to week 18. The reason for the start of JCE administration at week 7 was the effective time of DMPA to suppress spermatogenesis. KP group was administered with placebo, group TI with 0.94 mg of JCE, group TII with 1.88 mg of JCE, group TIII with 2.82 mg of JCE, and group TIV 3.76 mg of JCE. Administration of placebo and JCE was accomplished by means of a stomach tube equipped with a modified syringe tip, every morning at 8 o’clock Western Indonesian Time (WIB).
By the end of experiments at week 18, the rats were anesthetized with ether and dissected: right and left testicles were taken, weighed and then prepared for further data retrieval.
Data Collection
Weight of right and left testicles was determined by Digital Citizen Scales.
The diameter of the seminiferous tubules was measured with an electronic microscope equipped with micrometer objective and ocular at magnification 10×10.
Leydig cells were observed and calculated per one optical field under the electronic microscope at magnification 40×10.
Spermatogenic cells comprising spermatogonia-A, spermatocytes-I preleptoten, spermatocytes-I pachytene and spermatids were observed using the electric microscope at magnification 40×10.
Calculations of spermatogenic cells were performed in the seminiferous tubules in phase VII and VIII of the seminiferous epithelium cycle, cut one round slice per optical field. Stage VII / VIII was selected because it is long lasting, relatively common in testicular cross section, and the type of spermatogenic cells are found more complete than in other stages. To calculate the actual number of spermatogenic cells, the obtained data were then corrected by the Abercrombie formula22:
![]()
Legend: P = average number of nuclei per slice (true count), A = number of rough calculation of nuclei per slice (crude count), M = thickness of incision (microns), L = average diameter of nuclei (microns).
Data Analysis
Quantitative data were statistically evaluated using computer software Statistical Product and Service (SPSS) release 15 with the following test sequence23-24:
Shapiro and Wilk Normality Test and Bartlett Homogeneity of Variance Test.
If the data were normally distributed and variance homogeneous, one-way Analysis of Variances (ANOVA) was applied. If the obtained value of p was <0.05, post hoc Bonferroni test followed for the average differences between the two groups. For normally distributed data and variance not homogeneous (or the other way around), data had to be transformed. After data transformation, it turned out that the fixed data were still not normally distributed or inhomogeneous, non-parametrical Kruskal-Wallis test was applied. If the obtained value of p was <0.05, post hoc Mann-Whitney analysis followed.
Results
Testicle Weight
The average weight of the testicles decreased from 1.59±0.08g in controls (group C) to 1.51±0.24g with 1.25 mg DMPA (group CP). In group TIII, testicle weight slightly decreased to 1.53±0.13g and only in group TIV the weight was a low as in group CP (1.51±0.23g). In groups TI and TII, testicle weight did not decrease but increased in both groups to 1.67g. In other words, only the combination of 1.25 mg DMPA and 3.76 mg JCE is as effectve as 1.25 mg DMPA alone.
The Diameter of the Seminiferous Tubules
Figure 1 shows the results of the average diameter determination of the seminiferous tubules: group C, 235.5±17.41µm; CP, 205.9±6.62µm; TI, 201.9±11.45µm; TII, 195.2±7.51µm; TIII, 193.0±11.47µm; TIV, 179.1±9.95µm. Data management and calculation with one way ANOVA and Bonferroni post-hoc test demonstrated significant differences in diameter of seminiferous tubules between control and all other groups. Sham group (CP) differed significantly from treatment group TIV. The latter differed also significantly from TI group. Treament groups TII, TIII, and TIV did not differ significantly. The most effective dose combination with significant decrease in the diameter of seminiferous tubules was DMPA 1.25 mg and JCE 3.76 mg (group TIV).
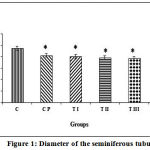 |
Figure 1: Diameter of the seminiferous tubules
|
Spermatogonia-A Cells
Figure 2 shows the results of the calculation of average spermatogonia-A cell population for group C, 37.25±3.63; CP, 30.75±1.81; TI, 27.00±2.26; TII, 25.30±2.76; TIII, 22.00±2.98; TIV, 21.25±1.97. Data management and calculation with Kruskal-Wallis (p = 0.000) and Mann-Whitney post-hoc tests demonstrated that the control group differed significantly from all treatment groups (TI, TII, TIII, and TIV). Sham group CP did not differ significantly from control, but significantly differed from groups TII, TIII, and TIV. TI group differed significantly from group TIV. The most effective dose combination with significant reduction of the spermatogonia-A cell population was DMPA 1.25 mg and JCE 3.76 mg (group TIV).
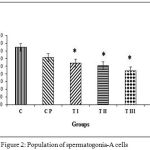 |
Figure 2: Population of spermatogonia-A cells
|
Spermatocytes-I Preleptoten
Figure 3 shows the calculation results of the average population of spermatocytes-I preleptotene for group C, 27.53±4.86; CP, 21.10±2.33; TI, 20.48±1.51; TII, 20.38±2.22; TIII, 17.35±1.94; TIV, 16.65±2.27. Data management and calculation with one way ANOVA (p = 0.000) and Bonferroni post-hoc test demonstrated significant differences in the population of spermatocytes-I preleptoten between control and all treatment groups (CP, TI, TII, TIII, and TIV). CP and TI also differed significantly from TIV. The most effective dose combination with significant reduction of spermatocytes-I preleptoten population was DMPA 1.25 mg and JCE 3.76 mg (group TIV).
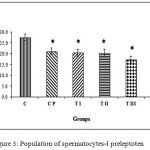 |
Figure 3: Population of spermatocytes-I preleptoten
|
Spermatocytes-I Pachytene
Figure 4 shows the calculation results of the average population spermatocytes-I pachytene for group C, 24.30±1.83; CP, 20.70±2.57; TI, 20.73±0.47; TII, 19.17±1.59; TIII, 16.80±1.13; TIV, 14.08±1.44. Data management and calculation with Kruskal-Wallis (p = 0.000) and Mann-Whitney post-hoc tests demonstrated that the control group differed significantly from treatment groups TII, TIII, and TIV. Sham group CP and treatment group TI did not differ significantly from control. Treatment group TIV differedsignificantly from sham group CP and treatment groups TI and TII; TI also differed significantly from group TIII. The most effective dose combination with significant reduction of spermatocytes-I pachytene population was DMPA 1.25 mg and JCE 3.76 mg (group TIV).
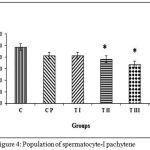 |
Figure 4: Population of spermatocyte-I pachytene
|
Spermatid Cells
Figure 5 shows the calculation results of the average spermatid cell population for group C, 44.58±9.40; CP, 33.25±5.20; TI, 27.83±2.70; TII, 24.75±1.13; TIII, 19.50±0.63; TIV, 18.67±3.33. Data management and calculation with Kruskal-Wallis (p = 0.000) and Mann-Whitney post-hoc tests demonstrated that the control group differed significantly from treatment groups TII, TIII, and TIV. Sham group CP and treatment group TI did not differ significantly from control. Treatment group TIV differed significantly from sham group CP and treatment groups TI and TII; TI also differed significantly from group TIII. Sham group CP differed significantly from treatment groups TII, TIII, and TIV. Treatment group TI differed significantly from groups TIII and TIV and group TII differed significantly from group TIII. The most effective dose combination with significant reduction of spermatid cell population was DMPA 1.25 mg and JCE 3.76 mg (group TIV).
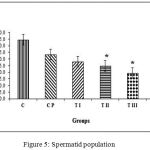 |
Figure 5: Spermatid population
|
Leydig Cells
Figure 6 shows the results of the calculation of the average population of Leydig cells for group C, 508.67±23.32; CP, 482.67±16.94; TI, 473.50±42.67; TII, 473.50±42.67; TIII, 475.50±45.85; TIV, 364.67±48.80. Data management and calculation with one way ANOVA and Bonferroni post-hoc test demonstrated significant differences in diameter of seminiferous tubules between treament group PIV and all other groups. The sham group CP and treatment groups TI, TII and TIII did not significantly differ from the control group. The most effective dose combination with the only significant decrease in the number of Leydig cells was DMPA 1.25 mg and JCE 3.76 mg (group TIV).
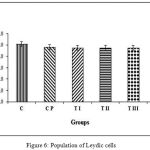 |
Figure 6: Population of Leydic cells
|
Discussion
DMPA is a progestin derivative already known for long in hormonal contraception.8 It lowers the concentration of androgens in the testes by reducing androgen biosynthesis, altering the metabolism of androgens and competing directly at the androgen receptor (AR) with androgen binding.4
By binding to plasma albumin DMPA concentration in serum is generally maintained for about 3 months, only decreasing gradually25. DMPA enters the cell and binds to receptors located between the nucleus and cytoplasm. This ligand-receptor complex forms a dimer before bound to DNA.26 The mechanisms of DMPA to inhibit spermatogenesis are multifactorial8; it can decrease serum concentrations of sex hormone-binding globuline (SHBG), gonadotropins and testosterone. Combined with testosterone DMPA can decrease spermatogenesis, thereby lowering sperma production.27-28
As reported in previous studies13-14 JCE has androgenic effects. Javanese chili contains β-sitosterol which is converted into testosterone to replenish the loss due to the provision of DMPA.13
In previous studies, Yurnadi et al.18-21 investigated the concentration and the viability of spermatozoa in Vas deferens and the testosterone levels with DMPA 1.25 mg alone and in combination with JCE. The essentials are summarized in Table 1. Furthermore, the authors investigated safety parameters of the combination of DMPA and its combination with JCE.19,21
Table 1: Summary of preliminary studies
| Control | 1.25 mg DMPA | 1.25 mg DMPA + JCE 3.76 mg | % of control | |
| Spermatozoa | DMPA/ + JCE | |||
| Concentration in Vas deferens | 94.7×106 / mL | 22.3×106 / mL | 22.1×106 / mL | 23 / 23 |
| Viability | 53.8% | 32.8% | 29.4% | 61 / 55 |
| Testosterone | 2.26 ng / mL | 1.08 ng / mL | 2.28 ng / mL | 48 / 100 |
Note: The data in Table 1 are taken from Yurnadi et al.18-21
From the preliminary results, only the combination of 1.25 mg DMPA + JCE 3.76 mg fulfilled the desired effects (Table 1). Thus, we now investigated whether these results can be confirmed with testicular parameters.
The body weight (BW) of the rats did not differ significantly between all groups.21 The estimation of the dose administered in group TIV (1.25 mg DMPA + 3.76 mg JCE per 250 g BW) is equivalent to 5 mg DMPA + 15 mg JCE per Kg BW, in general.
Testicular Weight
Berndston and Thompson29 reported that there are many factors that affect the weight of the testes. Increasing age is directly proportional to the weight of Sprague Dawley rat testes. Total body weight gain was also positively correlated with an increase in testicular weight; the older rats and the greater their weight, the greater also the weight of their testes.
According to Amatayakul et al.30 a decrease in testis weight is closely connected with decreasing diameter of the seminiferous tubules and production of spermatogenic cells. Marson et al.31 claimed that gonadal function is positively correlated with the volume of the gonads. Bercovitch and Rodriguez32 reported that testis weight of male rhesus monkeys was positively correlated with sexual activity. This means that the increase in gonadal function would lead to an increase in testicular weight. Research by Kusmana9 showed that a combination of DMPA and TE injections caused a decrease in testicular volume of short-tailed male macaques (Macaca nemestrina L) and confirmed the same pattern of decline in testicular volume that had been reported by Sutyarso10 with long-tailed macaques (Macaca fascicularis L).
In our study the combination of 1.25 mg DMPA + JCE 3.76 mg reduced the testicular weight to the same value as 1.25 mg DMPA + placebo in the sham control.
Seminiferous Tubules
Soeharsono33 reported that medroxyprogesterone acetate (MPA) injections at a dose of 8 mg per rat reduced the number of sperm cells and caused shrinkage of the seminiferous tubules. Kusmana9 showed that administration of TE and DMPA caused a decrease in the diameter of the seminiferous tubules and the score of spermatogenesis. This occurred because of the damage of the histological structure of the testes. Such damage could be classified from mild to severe hypoplasia of the germ cells.
The decrease in seminiferous tubule diameter treated by DMPA and JCE may be caused by hyalinisation as discussed by Nistal and Paniagua.34 According to the authors it is usually caused by hormonal mechanisms of interference on the axis hypothalamus-pituitary-testes.
Spermatogenic Cells and Sub-Populations
Spermatogenic cell population and all subgroups investigated (Figures 4,5,6,7) gradually decreased in a dose-dependent manner with the most significant effect in group TIV. In general, it appears that the combination of DMPA and 3.76 mg of JCE is most effective in our entire study.
McLahlan et al.11 reported decline in the number of spermatogenic cells subjected to DMPA + testosterone. After 12 weeks of treatment, there was a significant reduction in all sub-types of spermatogenic cells from spermatogonia A to spermatids vs controls. Kusmana9 observed with the scoring method of Johnson that DMPA + TE treatment of male macaque monkeys caused bottleneck spermatogenesis if compared with controls. Population decline of spermatogenic cells in experimental animals given DMPA combination and JCE may occur due to constraints on the process of spermatogenesis.
DMPA and JCE cooperate in reducing the concentration of intra-testicular testosterone through a negative feedback mechanism in the hypothalamic-pituitary-testicular axis. It is known that testosterone is necessary in controlling spermatogenesis and a decrease in testosterone level leads to constraints of spermatogenesis by disrupting the process of the cells to develop spermatogenic.35-36 A decrease in FSH may disturb the Sertoli cells to produce androgen-binding protein (ABP) which is very important for the binding of testosterone. According to Lui et al.37 FSH receptor containing Sertoli cells generate support and nutrition for spermatogenic cells during spermatogenesis.
During spermatogenesis, spermatogenic activity of cells is very high, which occurs through both, morphological and biochemical changes. To support these activities, spermatogenic cells are highly dependent on energy sources derived from Sertoli cells. Lue et al.38 proved that a decline in FSH leads to significantly reduced numbers and activity of Sertoli cells. Glucose is an important substrate for the survival of spermatogenic cells. The decline in the population of Sertoli cells can block the transport of glucose into the testicles, causing a decrease in the population of spermatogenic cells and vacuolization of seminiferous tubules. Limitation to glucose transport into spermatogenic cells can cause limitation of protein biosynthesis by spermatocytes and spermatids. Instead of glucose pachytene spermatocytes and spermatids also utilize energy sources in the form of lactate and pyruvate, which are supplied by Sertoli cells. In other words, apart from controlling survival, proliferation and differentiation by hormones spermatogenic cells are also controlled by Sertoli cells. Lactate and pyruvate production by Sertoli cells is affected by FSH, namely through increased intracellular level and activity of cAMP.10
Leydig Cells
Low content of LH can also suppress the production of testosterone by Leydig cells and thus, spermatogenic cell proliferation. Leydig cells express LH receptors and in response to LH produced by pituitary gland they produce testosterone.39 Meistrich and Shetty40 reported that suppression of gonadotropins and testosterone cause disturbances in differentiation and development of spermatogenic cells. Under normal conditions spermatogenesis is directly related to the differentiation and survival of spermatocytes and spermatids. The decline of FSH level can inhibit the differentiation of spermatogonia. Testosterone also affects normal mitotic spermatogonia and the successful completion of meiosis.41 FSH plays a role in the stimulation of mitosis in type B spermatogonia and in spermatocytes preleptoten activity in preventing apoptosis of pachytene spermatocytes and spermatids in the vicinity. Low levels of testosterone and FSH together affect the adhesion of spermatogenic cells to Sertoli cells and eventually cause increased spermatogenic cell apoptosis.42
DMPA also inhibits Leydig cells in their process of steroidogenesis.4 Leydig cells play a role in the process of steroidogenesis by producing testosterone which is required for spermatogenesis.43 Biosynthesis of testosterone by the Leydig cells involve the action of a carrier protein cascade and enzymes of steroidogenesis. Mutations of genes regulating steroidogenesis in Leydig cells can result in testosterone deficiency. The decline in Leydig cell population can cause limited spermatogenesis.44 Ericson and Dutt12 injected rams with progesterone acetate derivatives and reported not only the cessation of spermatogenesis but also atrophy of Leydig cells.
The decline of Leydig cell population is likely due to apoptotic mechanisms. Morris et al.45 reported that apoptosis occurs because of interference of Leydig cells with their extracellular environment. A decrease in the concentration of gonadotropin (LH) seems to be positively correlated with a decrease in Leydig cell population, but the exact relationship between hormonal effects and the decline of Leydig cell population has not yet been explained.
Our results showed differences in Leydig cell population among treatment groups and controls. As seen in most other results, also population decline of Leydig cells in seminiferous tubules was most effective and significant vs control in group TIV at a dose of 3.76 mg JCE. That means, the combination of DMPA and JCE impedes the development of Leydig cells gradually in a dose-dependent manner, because there is no significant difference of groups CP, TI, TII, and TIII vs control.
Safety and Final Remarks
During recent years, novel interventions for male contraception have been investigated with an extract from Justicia gendarussa leaves in Indonesia46 and with Vasalgel™ in India.47 A clinical trial with intramuscular (IM) injection of a combination of 200 mg norethisterone enanthate (NE) and 1000 mg testosterone undecanoate (TU) turned out effective to almost complete and reversible suppression of spermatogenesis, however, the study was terminated early because of adverse events, such as acne, injection site pain, increased libido, and mood disorders.48
The safety and tolerability of DMPA and its combination with JCE in the range of the dosage used in our studies was reported previously from the examination of hematological and biochemical parameters in rats.19-21
Table 2: Compilation of results of the combination of DMPA 1.25 mg + JCE 3.76 mg
| Testicular parameter | Control | 1.25 mg DMPA | 1.25 mg DMPA + JCE 3.76 mg | % of control DMPA/+JCE |
| Testis weight | 1.59±0.08g | 1.51±0.24g | 1.51±0.23g | 95 / 95 |
| Diameter of seminiferous tubules [µm] | 235.5±17.41 | 205.9±6.62 | 179.1±9.95 | 87 / 76 |
| spermatogonia-A cells | 37.25±3.63 | 30.75±1.81 | 21.25±1.97 | 83 / 57 |
| Spermatocytes-I preleptoten | 27.53±4.86 | 21.10±2.33 | 16.65±2.27 | 77 / 60 |
| Spermatocytes-I pachytene | 24.30±1.83 | 20.70±2.57 | 14.08±1.44 | 85 / 58 |
| Spermatid cells | 44.58±9.40 | 33.25±5.20 | 18.67±3.33 | 75 / 42 |
| Leydig cells | 508.67±23.32 | 482.67±16.94 | 364.67±48.80 | 95 / 72 |
Summary and Conclusion
The minimal effective dose for the tested combination is DMPA 1.25 mg and JCE 3.76 mg (Table 2). Further histological and long-term observational fertility studies on effectivity and safety with experimental animals should follow before this combination will be tested in humans.
Acknowledgement
This work is dedicated to late Prof. Dr. Nukman Moeloek, who was one of the initiators of male contraception research at Universitas Indonesia. The authors would like to express their gratitude to the Directorate of Research and Community Service (Direktorat Penelitian dan Pengabdian Masyarakat), Directorate General of Higher Education (Dirjen Dikti), and the Ministry of National Education (Depdiknas) for providing funds to this research.
References
- Kementerian Koordinator Bidang Kesejahteraan Rakyat. Penduduk Indonesia 273 juta pada 2005. http://menkokesra.go.id /content/view/1975/39/. Accessed on 12 Juni 2008. (Indonesian) [Ministry Coordinator Sector Population Prosperity. Indonesian inhabitants 273 Million for 2005].
- Hair W. M and Wu F. C. Male contraception: prospect for the new millenium. Asian J. Androl. 2000;2:3-12.
- Sutyarso and Moeloek N. Prospek kontrasepsi hormonal pada pria dengan menggunakan testosteron. Majalah Kesehatan Masyarakat Indonesia,(Indonesian) [Prospect of hormonal contraception for men with the use of testosterone. Journal of Indonesian Public Health]. 1995;4: 241-245.
- Handelsman D. J., Conway A. J., Howe C. J., Turner L., Mackey M. A. Establishing the minimum effective dose and additive effects of depot progestin in suppresion of human spermatogenesis by a testosteron depot. Clin. Endocrinol. Metab. 1996;81:4113-4121.
- Boroditsky R and Guilbert E. Injectable medroxyprogesterone acetate for contraception. SOGC. 2000;94:305-307.
- Black A. Canadian contraception consensus-update on depot medroxyprogesterone acetate (DMPA). SOGC. 2006;174:305-309.
CrossRef - Moeloek N and Sutyarso. Kombinasi progestogen dengan androgen untuk kontrasepsi hormonal pada pria. Majalah Kedokteran Indonesia, (Indonesian) [Combination of progestogen and androgen for male hormonal contraception. Journal of the Indonesian Medical Association]. 1995; 11:737-740.
- Meriggiola C. M and Bremner W. J. Progestin-androgen combination regimens for male contraception. Androl. 1997;18(3):240-243.
- Kusmana D. Pengaruh penyuntikan kombinasi hormon testosteron enantat (TE) dan depot medroksiprogesteron asetat (DMPA) terhadap spermatogenesis beruk jantan yang diberi pakan berkadar protein, lemak dan karbohidrat berbeda. Dissertation, Doctoral Program Biomedial Science Faculty of Medicine Universitas Indonesia, Jakarta (Indonesian) [Influence of the injection of a hormone combination of testosterone enantate (TE) and depot medroxyprogesterone acetate (DMPA) on the spermatogenesis of male macaques fed diets differing in protein, fat and carbohydrate content]. 2001.
- Pengaruh pemberian pakan berkadar protein, lemak, dan karbohidrat berbeda terhadap timbulnya azoospermia pada monyet jantan (Macaca fascicularis) yang disuntik kombinasi testosteron enantat (TE) dan depot medroksiprogesteron asetat (DMPA). Dissertation, Doctoral Program Biomedial Science Faculty of Medicine Universitas Indonesia, Jakarta. (Indonesian) [Influence of feeding diets with different protein, fat, and carbohydrate content on the emergence of azoospermia in male monkeys (Macaca fascicularis) after injection of a combination of testosterone enantate (TE) and depot medroxyprogesterone acetate (DMPA)]. 1997.
- McLachlan R. I., O’Donnell L., Stanton P. G., Balourdos G., Frydenberg M., deKretser D. M and Robertson D. M. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. Clin. Endocrinol. Metab. 2002;87(2):546-556.
CrossRef - Ericson R. J and Dutt R. H. Progesterone and 6α-methyl-17-OH-progesterone acetate as inhibitors of spermatogenesis and accessory gland function in the ram. Endocrinology. 1965;77:203-208.
CrossRef - Nuraini A. Mengenal etnobotani beberapa tanaman yang berkhasiat sebagai aprodisiaka. Info POM, Badan Pengawas Obat dan Makanan Republik Indonesia. (Indonesian) [Ethnobotanical knowledge of several plants with special use as aphrodisiac. Information POM, Committee of the Indonesian Republic Supervising Drugs and Food]. 2003;4(10):1-4.
- Sa’roni and Pudjiastuti A. Penelitian efek androgenik dan anabolik buah cabe jawa. Cermin Dunia Kedokteran, (Indonesian) [Research on the androgenic and anabolic effects of Javanese long pepper fruit. Mirror of Medical World ]. 1989;59:22-24.
- Isnawati A., Endreswari S., Pudjiastuti and Murhandini. Efek mutagen ekstrak etanol buah cabe jawa (Piper retrofractum Vahl). Jurnal Bahan Alam Indonesia, (Indonesian) [Mutagenic effect of ethanolic extract of Javanese long pepper fruit (Piper retrofractum Vahl). Journal of Indonesian Natural Materials]. 2002;1(2):63-67.
- Wahjoedi B.,Adjirni P., Nuratmi B and Astuti Y. Efek androgenik ekstrak etanol cabe jawa (Piper retrofractum Vahl) pada anak ayam. Jurnal Bahan Alam Indonesia.(Indonesian) [Androgenic effect of ethanolic extract of Javanese long pepper (Piper retrofractum Vahl) on baby chicks. Journal of Indonesian Natural Materials]. 2004;3(2):201-204.
- Moeloek N., Yurnadi., Lestari S. W and Wahjoedi B. Uji Klinik Ekstrak Cabe Jawa sebagai fitofarmaka androgenik pada pria hipogonad. Majalah Kedokteran Indonesia. (Indonesian) [Clinical study of Javanese long pepper extract as androgenic phytopharmacon for hypogonadal men. Journal of the Indonesian Medical Association]. 2010;60(6);255-262.
- Yurnadi., Asmida Y., Suryandari D. A., Wahjoedi B and Moeloek N. Penentuan Dosis Minimal Depot Medroksi Progesteron Asetat serta Pengaruhnya terhadap Viabilitas Spermatozoa dan Kadar Hormon Testosteron Tikus. Kedokt. Indon. (Indonesian) [Determination of minimal doses of depot medroxy progesterone acetate and its Eeffect on sperms viability and testosterone level in Rrat. Journal of the Indonesian Medical Association]. 2008;58(6):192-199.
- Yurnadi., Suryandari D. A and Moeloek N. Pengaruh Penyuntikan Dosis Minimal Depot Medroksiprogesteron Asetat (DMPA) Terhadap Berat Badan Dan Kimia Darah Tikus Jantan Galur Sprague-Dawley. Makara Sains, (Indonesian) [Effect of injection minimal dosages of depot medroxyprogesterone acetate (DMPA) to body weight and blood chemistry of male rat strain Sprague-Dawley. Makara Science. Journal of University Indonesia]. 2009;13(2):189-194.
- Yurnadi., Asmida Y., Suryandari D. A and Moeloek N. Pengaruh Kombinasi Dosis Minimal Depot Medroksiprogesteron Asetat dan Ekstrak Cabe Jawa terhadap Konsentrasi dan Viabilitas Spermatozoa serta Kadar Hormon Testosteron Tikus. Kedokt. Indon. (Indonesian) [The effect of a combination of minimal dosage of depot medroxyprogesterone acetate and Javanese Long Pepper extract toward sperms concentration and viability and testosterone level in rat. Journal of the Indonesian Medical Association]. 2010;60(9):393-400.
- Yurnadi., Asmida Y., Suryandari D. A and Moeloek N. Combination of Depot Medroxy Progesterone Acetate and Javanese Long Pepper Extract on Body Weight, Hematology, and Blood Biochemistry as a Safe Contraception Model. Makara Sains. Journal of the University Indonesia. 2011;15(2):155-162.
- Abercrombie M. Estimation of nuclear population from microtome sections. Rec. 1946;94:239-247.
CrossRef - Steel R. G. D and Torrie J. H. Prinsip dan prosedur statistika (Suatu Pendekatan Biometrik, translator Sumantri B). Jakarta: PT Gramedia Pustaka Utama,(Indonesian) [Principles and procedures of statistics]. 1993;559-649.
- Dahlan M. S. Statistika untuk kedokteran dan kesehatan. Jakarta: PT Arkans, (Indonesian) [Statistics for Medicine and Health]. 2004;85.
- Turner L., Conway A. J., Jimenez M., Liu P. Y., Forbes E., McLachlan R. I and Handelsman D. J. Contraceptive efficacy of a depot progestin and androgen combination in men. Clin. Endocrinol. Metab. 2003;88(10):4659-4667.
CrossRef - Katzung B. G. Farmakologi dasar dan klinik. (6th, translator pharmacology team FM UNSRI). Jakarta: Penerbit EGC.(Indonesian) [Basic and Clinical Pharmacology]. 1997;641-651.
- Ilyas S. Azoospermia dan pemulihannya melalui regulasi apoptosis sel spermatogenik tikus (Rattus sp.) pada penyuntikan kombinasi testosteron undekanoat (TU) dan depot medroxyprogesteron asetat (DMPA). Dissertation, Doctoral Program Biomedial Science Faculty of Medicine Universitas Indonesia, Jakarta (Indonesian) [Azoospermia and recovery through regulation of apoptosis of spermatogenic cells in rats (Rattus sp.) after injection of a combination of testosterone undecanoate (TU) and depot medroxyprogesterone acetate (DMPA)]. 1997.
- Moeloek N., Pujianto D. A., Agustin R., Arsyad K. M., Waluyo P. Y and Bizvo M. T. Achieving azoospermia by injections of testosterone undecanoate alone or combined with depot medroxyprogesterone acetate in Indonesian men (Jakarta Center Study). In: Robaire B., Chemes H., Morales C. R. (eds.) Proceedings of the VIIth International Congress of Andrology: plenaries, symposia and workshops. Montreal, Canada: Medimond Publishing Company Inc. 2001;545-550.
- Berndston W. E and Thompson L. T. Changing relationship between testis size, Sertoli cell number and spermatogenesis in Sprague Dawley. J. Androl. 1990;11:429-435.
- Amatayakul K., Ryan R., Vozumi T and Albert A. A reinvestigation of testicular-anterior pituitary relationships in the rat: effects of castration and cryptorchidism. Endocrinology. 1971;88:872-880.
CrossRef - Marson J., Meuris., Cooper R. W and Jouannet P. Puberty in the male Chimpanzee: progressive maturation of semen characteristics. Reprod. 1991;44:446-455.
CrossRef - Bercovitch F. B and Rodriguez J. F. Testis size, epididymis weight and sperm competition in Rhesus macaques. J. Primatol. 1993;30:163-168.
CrossRef - Histologik testis tikus putih yang diberi suntikan medroksiprogesteron asetat. Research Center of Demography and Development Airlangga University Surabaya, 1999; p. 413 (Indonesian) [Histology of the testis of white rats after injection of medroxyprogesterone acetate].
- Nistal M and Paniagua R. Testicular and epididymal pathology. New York: Thieme-Stratton Inc. 1984.
- McLachlan R. I., O’Donnell L., Meachem S. J., Stanton P. G., deKretser D. M., Pratis K and Robertson, D.M. Hormonal regulation of spermatogenesis in primates and man: insight for development of the male hormonal contraceptive. Androl. 2002;23(2):149-162.
- Patton P. E and Battaglia D. E. Office Andrology. New Jersey: Humana Press Inc. 2005;11-18.
CrossRef - Lui W. Y., Lee W. M and Cheng C. Y. Sertoli cell tight junction dynamics their regulation during spermatogenesis. Reprod. 2003;68:1087-1097.
CrossRef - Lue Y., Sinha H. A. P., Wang C., Leung A and Swerdloff R. S. Functional role of inducible nitric oxide synthase in the induction of male germ cell apoptosis, regulation of sperm number and determination of testis size: evidence from null mutant mice. Endocrinology. 2003;144(7):3092-3100.
CrossRef - Lei Z. M., Mishra S., Ponnuru P., Li X., Yang Z. W and Rao C. V. Testicular phenotype in luteinizing hormone receptor knockout animals and the effect of testosteron replacement therapy. Reprod. 2004;71(5):1605-1613.
CrossRef - Meistrich M. L and Shetty G. Inhibition of spermatogonial differentiation by testosterone. Androl. 2003;24(2):135-148.
CrossRef - Kierszenbaum A. L. Apoptosis during spermatogenesis: The thrill of being alive. Reprod. Dev. 2001;58:1-3.
- Huleihel M and Lunenfeld E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J. Androl. 2004;6:259-268.
CrossRef - Federman D. D. The biology of human sex differences. Engl. J. Med. 2006;354(14):1507-1514.
CrossRef - Zhang F. P., Pakarainen T., Zhu F., Poutanen M and Huhtaniemi I. Molecular characterization of postnatal development of testicular steroidogenesis in luteinizing hormone receptor knockout mice. Endocrinology. 2004;145(3):1453-1463.
CrossRef - Morris A. J., Taylor M. F and Morris I. D. Leydig cell apoptosis in response to ethane dimethanesulphonate after both in vivo and in vitro treatment. Androl. 1997;18(3):274-280.
- Prajogo B., Pramesti D and Musta’ina S. Study on contraceptive effect of ethanol extracted Justicia gendarussaF. leaves in fertile men: Phase II clinical trial. Abstracts from the 39th American Society of Andrology Annual Meeting 5 – 8 April 2014, Atlanta, GA. Androl. 2014;2(1):40 (Poster No. 25).
- Waller D., Bolick D., Lissner E., Premanandan C and Gamerman G. Azoospermia in rabbits following an intravas injection of Vasalgel™. Basic and Clinical Andrology. 2016;26(6). DOI: 10.1186/s12610-016-0033-8.
- Behre H. M., Zitzmann M., Anderson R. A., Handelsman D. J., Lestari S. W., McLachlan R. I., Meriggiola M. C., Misro M. M., Noe G., Wu F. C. W., Festin M. P. R., Habib N. A., Vogelsong K. M., Callahan M. M., Linton K. A and Colvard D. S. Efficacy and Safety of an Injectable Combination Hormonal Contraceptive for Men. Clin. Endocrinol. Metab. 2016;101. DOI: 10.1210/jc.2016-2141.
CrossRef








