Amir Shamshirian1, Shayan Alikhani2, Reza Alipoor3, Hamed Jafarpour4, Fatemeh Espahbodi5 and Soheil Azizi6
1Department of Laboratory Sciences, Faculty of Paramedicine, Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran.
2Hematology, Department of Hematology, Tarbiat Modares University, Tehran, Iran.
3Research Committee, Fasa University of Medical Sciences, Fasa, Iran.
4Faculty of Medicine, Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran.
5Endocrine research center, Imam Khomeini hospital, Mazandaran University of Medical Sciences, Sari, Iran.
6Department of Laboratory Sciences, Faculty of Paramedicine, Mazandaran University of Medical Sciences, Sari, Iran.
Corresponding Author E-mail: soheil_azizi@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1141
Abstract
Hepatitis B Virus (HBV) infection is one of the most common issue worldwide. It is more common in hemodialysis (HD) patients which is sensitive to blood borne viral agents such as HBV more than other people and in terms of vaccine protection, immunogenicity of HBV vaccine in HD patients is still open for discussion. Therefore, aim of this study is to evaluating the immunogenicity of HBV vaccine in HD patients who admitted to the Mazandaran Heart Center hemodialysis unit in 2015. This cross-sectional study performed by using available data and Census method on all chronic HD patients in Mazandaran Heart Center in 2015. Patient’s information was extracted from their records with ethical points including age, gender, HBS Ab level and the number of dialysis per week. Data was analyzed using chi-square test and SPSS 16 software. Of all patients with a mean age of 54.7 ± 13.76 years, Positive anti-HCV was observed in 7(7%) of them. For immune response to HBV vaccine, 35% with no response, 32% with poor response and 33% with complete response were obtained. Also did not observed significant relationship between sex, age and the number of dialysis per week with immunization of HBV vaccine. In conclusion based on the observed information, the immunogenicity of hepatitis B vaccine in hemodialysis patients of Mazandaran Heart Center was moderate to low that the rate of response to vaccine were reasonable according to the previous studies.
Keywords
Hemodialysis patients; Hepatitis B virus; immune response
Download this article as:| Copy the following to cite this article: Shamshirian A, Alikhani S, Alipoor R, Jafarpour H, Espahbodi F, Azizi S. Evaluation of Immunogenicity of Hepatitis B Vaccine in Hemodialysis Patients at Mazandaran Heart Center, Iran. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Shamshirian A, Alikhani S, Alipoor R, Jafarpour H, Espahbodi F, Azizi S. Evaluation of Immunogenicity of Hepatitis B Vaccine in Hemodialysis Patients at Mazandaran Heart Center, Iran. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=15385 |
Introduction
Hepatitis B (HB) is DNA virus of the Hepadnaviridae Family that is the greatest prevalent cause of viremia and one of the main causes of cirrhosis and liver disease. Its transmission occurs from different ways such as percutaneous or mucosal exposition by contaminated blood products, multiple sexual contact, needle stick injury, unsafe drug injections and perinatal transmission. In total; Infected blood products and Contaminated equipment of hemodialysis (HD) unit are the most important factors in transmission of the virus in HD patients(1)(1, 2). Acute and chronic hepatitis, cirrhosis, hepatocellular carcinoma and decreasing the likelihood of transplantation are the problems that virus can cause (3).
Studies have indicated that about 200 billion people worldwide have been infected with this virus and according to 2010 data is estimated that 350 million people are chronic carriers (1, 4, 5). In terms of prevalence, Iran is among the countries with moderate prevalence of the virus that 2-3% of the population are chronic carriers Hepatitis B Virus (HBV)(6). Universal vaccination against the virus from 1980 till now was accomplished and Serologic tests for safety assessment is The most important preventive measures against the virus, which greatly reduced the prevalence, but the prevalence of HB in HD patients on dialysis unit of less developed countries is still high and it is variable in different society (5, 7). For example, previous studies show the prevalence of HBV through HD unit in western countries 0.6-6.6%, in Asia-Pacific region between 1.3% and 14.6% and in China the prevalence is 11.9% that shows the high range of prevalence in less developed countries and it depend on prevalence of HB in general population in each countries(8).
Proper level of Hepatitis B surface antibody (HBs Ab) after vaccination against HB shows the individual Immune that the higher level of antibody will follow the higher immunogenicity. Individuals who carry HBs antibodies above 10 mIU/mL are considered to be protected (1, 3, 9). The effect of hepatitis B vaccine in healthy individuals is above 90%, While in chronic hemodialysis patients for different reasons, such as immunosuppression because of uremia, malnutrition, loss of CD4 T cells, hepatitis C virus infection and the patient’s age, Have a lower response rate about 50 to 80 percent(4, 10, 11). Thus, the first function for protection of patients on dialysis is a programmed vaccination strategy against HBV. And annually serological control test it is necessary to performed on patients who respond to vaccine and Antibody titers less than 10 mIU/mL require booster doses and re-vaccination(1). However, the prevalence of the problem in different areas due to various factors is different and high.
As regards such a study has not been conducted at Mazandaran Heart Center recently, and the specific conditions of patients referred to the center, we decided to perform this study with the aim of determining the immunogenicity of hepatitis B vaccine in chronic HD patients in Mazandaran Heart Center in Iran, to be aware of the results of this study and take the necessary actions to prevent the disease and increase the safety in these patients with the cooperation of staff working in this center.
Methods
This cross-sectional study performed by using available data and Census method on all chronic HD patients in Mazandaran Heart Center in 2015 and at the end of the study the number of patients reached to one hundred. Inclusion criteria included medical records of patients who received all 3 doses of hepatitis B Vaccine and exclusion criteria of the study was the lack of vaccination and HIV patients. For ethics and confidentiality, all information contained in the records which studied will be kept confidential and after reviewing were delivered to hospital’s archive.
A checklist used to collect the data in this study, Including age, sex, Hepatitis B Surface Antibody (HBs Ab) and the number of dialysis per week. The results of the HBs Ab titers extracted 6 months after the last dose of the vaccination for HB that the test mentioned was measured by STAT Fax Machine Made in the United States of America and HBs Ab Kits of Total Quick Company Made in France by ELISA method. According to instructions of the used kit, Patients were separated into three groups based on antibody titer: More than 100 mIU/mL as strong response, 20-100 mIU/mL as poor response and less than 20 mIU/mL as Non-Responder.
After collecting the information, the data examined by use of descriptive statistics such as mean and standard deviation. To analyze the data, the normal distribution of data was evaluated by Kolmogorov-Smirnov test and due to the significance of the above test, chi-square test was used to assess the relationship between independent variables. The P-value less than 0.05 was considered as a minimum value for statistical significance. All data were analyzed using the Statistical Package for the Social Sciences 16.0 (SPSS Inc., Chicago, IL, USA) software.
Results
Of 100 hemodialysis patient’s follow-up, 59 (59%) were males and (41%) were female (Fig 1). Their age ranged were 13 to 89 years and it’s the mean and SD were about 54.7 ± 13.76 years (Fig 2). In terms of hemodialysis duration, 72% of patients had Hemodialysis 3 times a week, 18% of them 2 times a week, 7% of patients once a week and 3% of them 4 times a week. Just 7 (7%) patients were positive for anti-HCV and the rest of the 93 (93%) patients were anti-HCV negative. range of patient’s anti-HBS were from 0 to 1046 mlU/ml with a mean (SD) 124.67 ± 204.74 (Fig 3). In terms of immunogenicity response, 35 (35%) patients had no response, 32 (32%) patients had poorly response and 33 (33%) patients had a complete response (Table1).
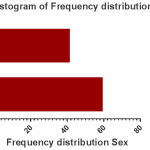 |
Figure 1: Histogram of Frequency distribution Sex
|
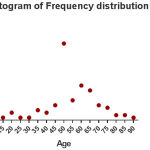 |
Figure 2: Histogram of Frequency distribution Age
|
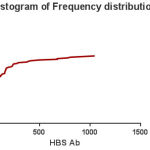 |
Figure 3: Histogram of Frequency distribution HBS-Ag
|
Table 1. Frequency distribution of the hepatitis B vaccine immunization by sex, age and anti-HCV
| P-value | Number & Percentage (%) of Vaccination | Variables | |||
| Strong response | Poor response | Non-responder | |||
| 19(57.6) | 21(65.6) | 19(54.3) | Male | ||
| 0.793 | |||||
| 14(42.4) | 11(34.4) | 16(45.7) | Female | Sex | |
| 3(9.1) | 2(6.3) | 2(5.7) | Positive | ||
| Anti-HCV | |||||
| 0.251 | 30(90.9) | 30(93.8) | 33(94.3) | Negative | |
| 6(18.2) | 4(12.5) | 7(20) | < 45 | ||
| 0.399 | 22(66.7) | 21(65.6) | 20(57.1) | 45-65 | |
| 5(15.2) | 7(21.9) | 8(22.9) | < 65 | Age | |
| 33(33%) | 32(32%) | 35(35%) | Total | ||
According to Table 1 for complete immunization response, in men with 19 (6.57%) patients were more than women with 14 (4.42%) that it shows the poor coverage of vaccination for HB in hemodialysis patients of this study. Based on the results of Table 1, did not exist A significant relationship between sex and immunization of HBV vaccine (P=0.793) and also there was no significant relationship between anti-HCV and HBV vaccine immunization (P=0.251) Based on the results, in terms of anti-HCV, 6 (6%) males and 1 (1%) female were positive for anti-HCV, which is a significant relationship between sex and anti-HCV antibodies was not observed. In terms of age distribution, a significant relationship between age and immunization of HBV vaccine (P=0.399) and also between age and anti-HCV (P=0.703) was not observed. Likewise, the number of dialysis per week had no significant difference with the hepatitis vaccine Immunization (P=0.887).
Discussion
Hepatitis B is one of the remarkable world problems and Individuals undergoing dialysis because of exposure to blood products, Hemodialysis equipment that is shared between different people, defects in the skin and immune deficiency, are at risk of developing the disease more than other people and with all prevention and vaccination and reducing the incidence, prevalence of HBV in HD patients unacceptably remains high (5).
According to the study conducted on 100 cases, in this study, there was not a significant relationship between gender and immunization of Hepatitis Vaccine, also significant relationship between anti-HCV and HBV vaccine immunization and between gender and anti-HCV was not observed. Of all 100 cases of hemodialysis patients studied, 33% had an adequate response to the vaccine, 32% had poor response and 35% of patients did not respond to vaccine, so 65 percent did not adequate response to vaccine. In a study that performed by Andrew I. Chin, aimed at Hepatitis B virus vaccine response in hemodialysis: baseline patient characteristics, has shown that 50 to 80% of patients have shown poor response to vaccine(12), So The results of our study which states that 65% of patients had not a good response to the vaccine is consistent with the current study. Although, several studies in the world showing the relationship between age and vaccine response and states that by age increasing the response will be reduced(7), In this study there was no significant relationship between age and vaccine response. There are different results about the relationship between these two variables in different studies around the world. In a study performed by Parmar, Z and et al(6), in the year 2011, As well as study was done by Al Saran, K. and colleagues (13), in the year 2014, Was not a significant relationship between the age and immune response. But in the study that conducted by Bahri, F et al(14). That was in the year 2016 a significant relationship between the age and immune response to hepatitis C vaccine was observed. Some other studies in the world showing the relationship between age and vaccine response and suggests the response is reduced with age (7, 14). These differences can be explained due to the small sample size or the age distribution in this study or some local factors that may effect on. The meta-analysis study conducted by Khedmat, H. et al(15), showed in most studies, it was found that Male had a significantly lower response to hepatitis B vaccine than the Female. The present study shows; our results are inconsistent with results of other studies and this may also be due to the small sample size or could point to poor vaccination coverage of hepatitis in hemodialysis patients in this study.
In our study, the duration of dialysis had no significant difference with hepatitis vaccine immunization that is in agreement with results reported by Khalid Al Saran et al and Hasanjani Roushan MR et al (13, 16). One of the interesting factors were investigated in previous studies that we did not study on, showed that; the intradermal injection of hepatitis vaccine, as well as increasing the dose used for patients on dialysis can be significantly keep reduce the prevalence of hepatitis in these patients(4, 8, 13, 17).
This study includes some limitations. For example, small sample size could be responsible for significant fail in relationship between variables which was completely significant in most previous studies. Also entering patients with HIV which considered as exclusion criteria into study, could be a significant increase in population and variable changes. Another limitation that could be effected the results, was age distribution that ranged from 13 years to 89 years. Therefore, it is recommended that conducting a study on larger population and entering the patients with HIV, will be able to coverage the gaps that became apparent in this study.
In conclusion based on the observed information, the immunogenicity of hepatitis B vaccine in Mazandaran Heart Center HD patients is moderate to low that the rate of response to vaccine were reasonable according to the previous studies. So given that hepatitis B is a major problem for dialysis patients and by all measures which is done to reduce the prevalence of it, the risk of the disease, still extremely limiting the patients on dialysis. It is recommended to comply with quality control issues in the hemodialysis unit to reduce transmission of the HBV and reduce the incidence of that in Hemodialysis Patients, moreover by conducting comprehensive researches, take a step to find more effective methods of prevention and treatment.
Acknowledgment
The authors would like to express their thanks to the student research committee of Mazandaran University of Medical Sciences to support this research project with the code 352 adopted on December 25, 2015 and as well as cooperation of staff working at Mazandaran Heart Center laboratory.
References
- Ayub MA, Bacci MR, Fonseca FL, Chehter EZ. Hemodialysis and hepatitis B vaccination: a challenge to physicians. Int J Gen Med. 2014;7:109-14.
- Lee WM. Hepatitis B Virus Infection. New England Journal of Medicine. 1997;337(24):1733-45.
CrossRef - Cordova E, Miglia I, Festuccia F, Sarlo MG, Scornavacca G, Punzo G, et al. Hepatitis B vaccination in haemodialysis patients: an underestimated problem. Factors influencing immune responses in ten years of observation in an Italian haemodialysis centre and literature review. Annali di igiene : medicina preventiva e di comunita. 2017;29(1):27-37.
- Sit D, Esen B, Atay AE, Kayabaşı H. Is hemodialysis a reason for unresponsiveness to hepatitis B vaccine? Hepatitis B virus and dialysis therapy. World Journal of Hepatology. 2015;7(5):761-8.
CrossRef - Edey M, Barraclough K, Johnson DW. Review article: Hepatitis B and dialysis. Nephrology (Carlton, Vic). 2010;15(2):137-45.
CrossRef - Parmar z, Khadivi r, Sadeghi b, Rahimi-Madiseh m. Immunization following hepatitis B mass vaccination in the 18 years old students in Chaharmahal va Bakhtyari province in Iran. Journal of Shahrekord Uuniversity of Medical Sciences. 2011;13(4):35-41.
- Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: the effect of age on immunological response to hepatitis B vaccine in end-stage renal disease. Alimentary pharmacology & therapeutics. 2004;20(10):1053-62.
CrossRef - Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Stefanidis I. Factors affecting effectiveness of vaccination against hepatitis B virus in hemodialysis patients. World journal of gastroenterology. 2014;20(34):12018-25.
CrossRef - Al Ghamdi SS, Fallatah HI, Fetyani DM, Al-Mughales JA, Gelaidan AT. Long-term efficacy of the hepatitis B vaccine in a high-risk group. Journal of medical virology. 2013;85(9):1518-22.
CrossRef - Soni R, Horowitz B, Unruh M. Immunization in end-stage renal disease: opportunity to improve outcomes. Seminars in dialysis. 2013;26(4):416-26.
CrossRef - A Ramezani AEf, F Ahmadi, S Maziar, E Razeghi, E Kalantar. Is Any Factor Influence On Hepatitis B Vaccination Response In Hemodialysis Patients? The Internet Journal of Nephrology. 2005;3(2).
- Chin AI. Hepatitis B virus vaccine response in hemodialysis: baseline patient characteristics. Hemodialysis International. 2003;7(4):296-303.
CrossRef - Al Saran K, Sabry A, Al Halawany Z, Ismail M. Factors affecting response to hepatitis B vaccine among hemodialysis patients in a large Saudi Hemodialysis Center. Saudi journal of kidney diseases and transplantation : an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2014;25(1):185-91.
CrossRef - Bahri F, Kheirabad AK, Ghasemzadeh I, Shoja S, Gouklani H. Hepatitis Viruses B and D and Human Immunodeficiency Virus Infections in Hemodialysis Patients in the South of Iran: Prevalence and Genotypes. Hepatitis monthly. 2016;16(1).
CrossRef - Khedmat H, Aghaei A, Ghamar-Chehreh ME, Agah S. Sex bias in response to hepatitis B vaccination in end-stage renal disease patients: Meta-analysis. World journal of nephrology. 2016;5(1):115-24.
CrossRef - Hasanjani Roushan MR, Farokhtabar S, Bayani M, Siadati S. Epidemiological Aspects of Hepatitis B and C and Human Immunodeficiency Viruses Among Hemodialysis Patients in Mazandaran Province, Iran. Nephro-urology monthly. 2016;8(3):e37878.
CrossRef - Gasim GI, Bella A, Adam I. Immune response to hepatitis B vaccine among patients on hemodialysis. World J Hepatol. 2015;7(2):270-5.
CrossRef








