Aghil Rahmani1,Valiollah Arash2, Reza Ghorbanipour2, Sayed Mahmood Rabiee3, Shima Nafarzadeh4 and Ali Bijani5
1Department of Orthodontics, School of Dentistry, Babol University of Medical Sciences, Babol, Iran.
2Dental Materials Reaserch Center, Institute of health, Babol University of Medical Sciences , Babol, I.R.Iran.
3Faculty of Material Engineering, Babol Noshirvani University of Technology, Babol, Iran
4Department of Pathology, School of Dentistry, Babol University of Medical Sciences, Babol, Iran.
5Social Determinants of Health Research Center, Babol University of Medical Sciences, Babol, Iran.
Corresponding Author E-mail: Vali_arash1344@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1138
Abstract
Many orthodontists believe that the only way to maintain perfect alignment after treatment is some form of permanent retention. Considering the antibacterial properties of silver ions, this in vivo study aimed to investigate the effects of silver ion coating of orthodontic fixed retainers on gingival health in rabbits. In this experimental study 24 adult male New Zealand rabbits, weighing approximately 1.8‒3 kg at 9 months of age, were examined. Twenty-four pairs of maxillary teeth in these rabbits were randomly divided into 4 groups (n=6): group 1: twist wire without silver ion coating (control); group 2: twist wire with silver ion coating; group 3: prefabricated fixed retainers without silver ion coating (control); and group 4: prefabricated fixed retainers with silver ion coating. Then the samples were evaluated in terms of clinical, histological, histomorphometrical and free ions. Data were analyzed with SPSS. There were statistically significant differences between the four groups in terms of the amount and frequency of lymphocytic infiltration and capillaries (P=0.001). However, there were no statistically significant differences in the thickness of parakeratinized epithelium between the four study groups (P=0.09). The release of silver ions into artificial saliva during the first 24 hours did not exceed the limit set by WHO. In the surrounding area, fixer retainers coated with silver ions were resistant to bacterial growth and reduced gingival inflammation.
Keywords
Silver ions; fixed retainers; gingiva; orthodontics
Download this article as:| Copy the following to cite this article: Rahmani A, Arash V, Ghorbanipour R, Rabiee S. M, Nafarzadeh S, Bijani A. Clinical, Histological and Histomorphometric Evaluation of Effects of Silver Ion Coating of Orthodontic fixed Retainers on Gingival Health in Rabbits. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Rahmani A, Arash V, Ghorbanipour R, Rabiee S. M, Nafarzadeh S, Bijani A. Clinical, Histological and Histomorphometric Evaluation of Effects of Silver Ion Coating of Orthodontic fixed Retainers on Gingival Health in Rabbits. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=15305 |
Introduction
One of the major challenges of orthodontists is long-term stability of orthodontic treatment. This has prompted orthodontists to look for ways to enhance the stability of changes during treatment. Various researchers have proposed various methods to improve the stability of treatment, ultimately leading to the use of fixed and removable retainers (1‒8). Many orthodontists believe that the only way to maintain perfect alignment after treatment is permanent use of retainers (8‒10). Therefore, now fixed bonded retainers are being left in the mouth for a long period of time (8). The major advantage of the bonded retainers, compared to the removable ones, is that they are compliant free, except for their difficult oral hygiene maintenance (11). The initial adhesion of bacteria to the wire is a key step to induce a biofilm (12‒15). The next growth of bacteria makes it difficult to completely remove it with a brush, ultimately leading to the formation of pathogenic oral biofilms, which are key factors in dental caries and periodontal diseases. Thus, it is important to prevent pathogenic bacteria from adhering to the wire in the first place (19‒16). Silver is known as a strong disinfectant. It is an important property and the bacteria are not able to mount strong resistance against silver. Even at low concentrations, silver ions in the aqueous medium exhibit strong antifungal and antibacterial effect (26). Also it has been shown that wires coated with silver ions are resistant to bacterial growth in the surrounding area within a radius of more than 2 mm. Recently, Ag ions and silver nanoparticles have been used to prevent the initial bacterial adhesion to dental materials by incorporating them into the materials. This has reduced biofilm formation (27). Yusuke Morita (2014) conducted a study of the effects of silver ion coating of orthodontic fixed retainers on oral pathogenic bacteria. In this study stainless steel and titanium wires were used to fabricate retainers. The study showed that incorporation of simple pure silver ions into stainless steel and titanium wire surfaces could limit growth and pathological activity of pathogenic oral bacteria (27). In 2013 Kaji et al conducted a study on the effects of mandibular fixed retainers on periodontal health. The results showed no differences between the two groups in terms of periodontal conditions. In addition, the occlusogingival position of the retainers had no effect on periodontal health. However, the study was conducted on patients who had good oral hygiene (29). Despite several studies on the use of silver in dentistry, we did not find any study on the clinical, histological and histomorphometric impacts of silver ion coating of orthodontic fixed retainers on gingival health. Therefore, the present in vivo study was undertaken to investigate the effect of silver ion coating of orthodontic fixed retainers on gingival inflammation in rabbits.
Materials and Methods
In this experimental study, 24 adult male rabbits (New Zealand), weighing 1.8‒3 kg and 9 months of age, were procured from the Pasteur Institute, Karaj, Iran. All the rabbits were kept in separate cages at a constant temperature of 23°C and in 12-hour cycles of simulasted day and night. All the samples underwent general anesthesia by intramuscular injection of a combination of ketamine (30 mg/kg) and thiopental (20 mg/kg). Before starting the tests, in order to control their weight and health status, the rabbits were evaluated on a weekly basis (30). Two types of retainers, twisted wire T(DENTARUM, Germany 0.45 mm/18) and mandibular prefabricated retainers (Yamei Co, China), were bonded to the buccal surfaces of maxillary teeth using Z250 restorative composite resin (3M ESPE, St Paul, MN, USA). Silver ion coating was conducted by physical vapor deposition (PVD) to a thickness of 200 µm by EDS-160 at Sharif University of Technology. The device power was 1000 W and the rate of deposition was 2 Å/second. After the coating process, to determine the adequacy of the coating, AFM analysis was carried out with Easyscan 2 Flex AFM (Switzerland) in Babol Noshirvani University of Technology. To study the surface morphology of the samples, SEM photomicrographs were used at ×20000. In this study the device used to perform SEM analyses was KYKY, EM3200 model.
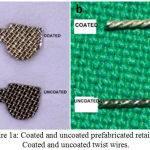 |
Figure 1a: Coated and uncoated prefabricated retainers; b) Coated and uncoated twist wires. |
Twenty-four pairs of upper teeth in 24 rabbits were randomly divided into 4 groups (n=6) (31) as follows: uncoated twist wire in group 1 (control); coated twist wire in group 2; uncoated prefabricated retainer (control) in group 3; and coated prefabricated retainer in group 4. To bond the retainers, after cleaning the incisors with sterile gauze and humidity control of rabbit oral cavity by a saliva ejector, 37% phosphoric acid gel (M Unitek, USA) was applied to etch the enamel for 15‒20 seconds. The acid was rinsed off the tooth surfaces and the teeth were dried. Then a thin layer of bonding agent was applied. Then without any force, the retainers were placed on gingival one-third of buccal surfaces of the teeth. Both ends of the retainers were covered with Z250 composite resin (3M ESPE, St Paul, MN, USA), measuring 2 mm in width and in thickness. To this end, a special stent was used. Then composite resin was light-cured using a light-curing unit (Dentsply, USA) for 40 seconds (30). To match the sizes of rabbit teeth, prefabricated retainers were formed to 2.5 mm of dimension using a high-speed diamond bur and a digital caliper (30). Plaque and food debris accumulating around the buccal surfaces of retainers were cleaned daily with a soft toothbrush in each rabbit (30). In addition, to minimize the rate of tooth growth, a soft diet (lettuce, grated carrots) was served (32). Furthermore, after 15 days since the start of the study all the retainers were rebonded in their initial position. All these steps were performed by the same orthodontist using the same technique (30).
Before the start of the experiment and after its completion, the left and right base of papilla of all the rabbits underwent probing by a gentle force of 25 g and bleeding on probing was reported as – or +.
Histological Examinations
Thirty days after the start of the experiment, all the rabbits were anesthetized and total facial tissue of maxillary incisor papillae of all the rabbits were isolated by scalpel and forceps and kept in 10% formalin. Two sections were prepared from the central part of each sample and then placed on glass slides, which were observed by an experienced pathologist in order to report infiltration of lymphocytes in the gingival tissue.
Infiltration of lymphocytes in five separate areas of each slide (high power) without any overlap were reported in five different grades:
Grade 0: absence of inflammatory cells
Grade 1: a few scattered inflammatory cells (mild)
Grade 2: 5‒10 inflammatory cells (focal)
Grade 3: 11‒50 inflammatory cells (focal)
Grade 4: more than 50 inflammatory cells (severe inflammation) (33)
Histomorphometric review of all the biopsies was performed by the digital camera of an Olympus microscope at ×40 and all the photos were imported to ANALYSIS LS Starter software program in JPEG format. The number of blood vessels was evaluated in three microscopic fields at a magnification of ×10 and reported as follows:
Score 0: less than three vessels
Score 1: 3‒5 vessels
Score 2: more than 5 vessels (33)
In addition, the thickness of the facial gingival epithelium was measured at ×40 perpendicular to the outer surface of the border of the connective tissue (34).
Measurement of Released Ag Ions
The measurement of silver ions released from the surface of coated wires was made by inductively coupled plasma optical emission spectrometer (ICP-OES, System, varian-730, USA). After 24 hours of immersion in 10 mL of artificial saliva, the amount of silver ions released from the samples (all length of 29 mm), was measured in ppm. The amounts of silver ions released were compared with the control groups. Three samples from each group were measured (27).
Results
The chronic inflammatory cell counts in gingival tissue after one month is presented in Table 1.
Table 1: infiltration of chronic inflammatory cells in the presence of coated and uncoated wires and coated and uncoated prefabricated retainer after one month
| Group | Infiltration of inflammatory cells | Total | ||||
| Severe inflammation (more than 50 inflammatory cells) (grade 4) |
11 to 50 the number of inflammatory cells (grade 3) |
5 to 10 inflammatory cells) (grade 2) |
Mild (small and scattered inflammatory cells) (grade 1) | The absence of inflammatory cells (grade 0) |
||
| Uncoated wire | 6 (100%) | 4 (0%) | 3 (50%) | 3 (50%) | 0 (0%) | 0 (0%) |
| Coated wire
|
6 (100%) | 0 (0%) | 0 (0%) | 3 (50%) | 3 (50%) | 0 (0%) |
| Uncoated
prefabricated retainer (control)
|
6 (100%) | 0 (0%) | 1 (16.7%) | 4 (66.6%) | 1 (16.7%) | 0 (0%) |
| Uncoated prefabricated retainer | 6 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (50%) | 3 (50%) |
| Total | 24 (100%) | 0 (0%) | 4 (16.7%) | 19 (41.7%) | 7 (29.2%) | 3 (12.5%) |
Kruskal-Wallis test showed significant differences between the four study groups in term of chronic inflammatory cell infiltration (χ2=15.42, df=3, P=0.001).
The number of vessels in the presence of coated and uncoated wires and coated and uncoated prefabricated retainer after one month is presented in Table 2.
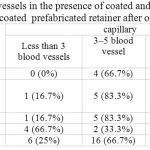 |
Table 2: The number of vessels in the presence of coated and uncoated wires and coated and uncoated prefabricated retainer after one month
|
Capillaries
Kruskal-Wallis test revealed statistically significant differences between the four groups in terms of the number of capillaries (χ2=9.32, df=3, P=0.025).
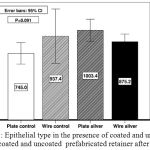 |
Figure 1: Epithelial type in the presence of coated and uncoated wires and coated and uncoated prefabricated retainer after one month
|
ANOVA showed no significant difference between the 4 study groups in terms of the epithelial thickness and type (P=0.09).
Table 4 presents the results of bleeding on probing of the study groups.
Table 3: The results of bleeding on probing of the study groups
| BOP | Total | ||
| Group | + | – | |
| Uncoated wire | 6 (100%) | 0 (0%) | 6 (100%) |
| Coated wire | 3 (50%) | 3 (50%) | 6 (100%) |
| Uncoated prefabricated retainer | 6 (100%) | 0 (0%) | 6 (100%) |
| Coated prefabricated retainer | 2 (33.3%) | 4 (66.7%) | 6 (100%) |
| Total | 17 (70.8%) | 7 (29.2%) | 24 (100%) |
Chi-square test showed a significant correlation between the 4 study groups in terms of bleeding on probing (χ2=10.28, df=3, P=0.021).
As shown in AFM (scale: 20×20 μm), Ag nanoparticles were present on the surface of the samples.
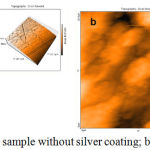 |
Figure 2: AFM analysis. a) A sample without silver coating; b) a sample with silver coating.
|
SEM images comparing coated and uncoated samples in the presence of silver atoms on the surface are presented in Figure 3.
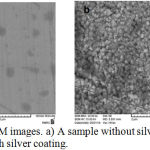 |
Figure 3: SEM images. a) A sample without silver coating; b) a surface with silver coating.
|
Post Hoc Findings Were as Follows
Comparison of the amount of lymphocyte infiltration between coated and uncoated prefabricated retainers was performed using Mann-Whitney test and the difference was statistically significant (P=0.006, Z= -2.76). In addition, Mann-Whitney test revealed a statistically significant difference between coated and uncoated wires (P=0.019, Z= -2.34). Therefore, silver ion coating of orthodontic fixed retainers can reduce the gingival inflammation caused by plaque accumulation. In terms of the capillaries, Mann-Whitney test showed no statistically significant difference between coated and uncoated prefabricated retainers (P=0.093, Z= -1.68). In addition, no significant difference was found between coated and uncoated wires (P= 0.092, Z= -1.68).
Silver Ion Release
Silver ions released from the samples were measured by ICP-OES. After 24 hours of immersion in 10 mL of artificial saliva the mean concentrations of silver ions were as follows: 0 ppm for uncoated wires and uncoated prefabricated retainers; 0.029 ± 0.005 ppm for coated wires; and 0.040 ± 0.005 ppm for coated prefabricated retainers.
Discussion and Conclusion
This study aimed to investigate the effect of silver ion coating of orthodontic fixed retainer on gingival inflammation in rabbits. Based on the results of this study, there were statistically significant differences in lymphocytic infiltration between the four study groups. Therefore, silver ions on the surface of fixed orthodontic retainers can reduce the gingival inflammation caused by the accumulation of plaque. Morita et al (2014) in an in vitro study reported that simple incorporation of silver ions into stainless steel and titanium wires can inhibit the growth of pathogenic oral bacteria as well their pathologic activity. (27). Our results are consistent with those of that study. In our study it was found that the addition of a thin layer of silver to two different types of orthodontic fixed retainers can reduce initial adhesion and growth of bacteria in the oral cavity, resulting in a significant reduction in infiltration of lymphocytes. The exact mechanism of antibacterial effect of silver is not known. One of the mechanisms suggested for the antibacterial activities of silver is interaction with thiol groups (sulfhydryl). Silver ions result in the release of K+ ions from bacteria. Therefore, the bacterial plasma or cytoplasmic membrane that is associated with many important enzymes is an important target site of silver ions. Finally, silver ions interact with nucleic acids. They preferentially interact with DNA bases. However, the primary mechanism of its bactericidal activity is uncertain (6). Using radial diffusion test, Yusuke Morita also showed that all the fixed retainers coated with silver ions were resistant to bacterial growth in the surrounding area within a radius of more than 2 mm. The findings showed that despite the fact that silver ions prevent bacterial growth on the surface of the retainers, they can also reduce bacterial activity adjacent to the wire, creating a favorable environment for gingival health. In this regard, in our study, despite the problem of continuing eruption of teeth in rabbits, the anti-inflammatory effect of silver ions was retained because of this antibacterial margin. Azarsina (2013) et al investigated the effect of silver-containing composite resins on Streptococcus mutans and Lactobacillus and concluded that incorporation of silver nanoparticles with 0.5 an 1 wt% into Z250 composite resin had a significant impact on reducing the number of these bacteria. The antibacterial properties of composite resins were different, depending on the concentration of added silver ions, and it was shown that increasing the silver concentration resulted in increased antibacterial properties of composite resin (35). This study showed that adding silver to other materials might also have antibacterial effects, consistent with our results. The problem with this method is that adding silver to the composite resin structure makes inner ions out of reach when polymerization is completed. In the present study, silver was applied to the surface of retainers. The coating process was carried out using physical vapor deposition technique with a thickness of 200 µm. In the physical vapor deposition technique, or PVD, coatings on solid surfaces are produced through condensation of elemental and composition of the gas phase. Evaporation is the most common method of preparation of highly pure layers under relatively controlled conditions. The principles of this method are generally based on pure physical effects, but PVD might in some cases be associated with chemical reactions (36).
Hwang-Sog Ryu et al (37) investigated the antimicrobial effect of adding a hard coating of silver ‒platinum alloy with a thickness of approximately 1.03‒2.34 µm to the surface of stainless steel orthodontic brackets, created by PVD, and reported that Ag‒Pt coating on the surface of brackets resulted in favorable antimicrobial effects during active orthodontic treatment, consistent with the results of the present study. In this study we used a thickness of 200 µm for coating because of a longer period of time for placement of fixed retainers and being more susceptible to the effects of possible frictional movements in comparison to brackets.
Stormann and Ehmer reported that plaque accumulation increase with time in all types of retainers (36). In addition, Liran Levin (38) found that the presence of a fixed retainer is associated with an increase in the incidence of localized lingual gingival recession, an increase in the amount of plaque retention and an increase in bleeding on probing, consistent with our findings. In the present study, presence of a fixed retainer was associated with an increased incidence of bleeding on probing. Zachinsky showed that the epithelium thickness of the gingiva, except for ulcers or severe hypertrophy, was not related to oral health (39). Similarly, in our study it was found that in terms of the epithelial thickness and type (parakeratinized orthokeratinized) in 4 cases, there was no significant difference. In this study, the amount of silver ions released in artificial saliva was 0.029 ± 0.005 ppm for coated wire and 0.040 ± 0.005 ppm with coated prefabricated retainer, which are less than the maximum permissible levels set by the WHO (0.1 mg/L) (40). Yusuke Morita also showed that this rate of silver ion release cannot be toxic to human gingival fibroblasts cells (27).
Conclusion
The results of this animal study showed that two different types of orthodontic fixed retainers coated with silver ions were resistant to bacterial growth in the surrounding area and reduced gingivitis.
References
- Andrews LF. The six keys to normal occlusion. Am J Orthod. 1972;62:296–309.
CrossRef - Edwards JG. A long-term prospective evaluation of the circumferential supracrestal fiberotomy in alleviating orthodontic relapse. Am J Orthod Dentofacial Orthop. 1988;93:380–387.
CrossRef - Boese LR. Fiberotomy and reproximation without lower retention 9 years in retrospect: part II. Angle Orthod. 1980;50:169–178.
- Boese LR. Fiberotomy and reproximation without lower retention, nine years in retrospect: part I. Angle Orthod. 1980;50:88–97.
CrossRef - Blake M, Bibby K. Retention and stability: a review of the literature. Am J Orthod Dentofacial Orthop. 1998;114:299–306.
- Little RM, Wallen TR, Riedel RA. Stability and relapse of mandibular anterior alignment-first premolar extraction cases treated by traditional edgewise orthodontics. Am J Orthod. 1981;80:349–365.
CrossRef - Shah AA. Postretention changes in mandibular crowding: a review of the literature. Am J Orthod Dentofacial Orthop. 2003; 124: 298–308.
CrossRef - Booth FA, Edelman JM, Proffit WR. Twenty-year follow-up of patients with permanently bonded mandibular canine-to-canine retainers. Am J Orthod Dentofacial Orthop. 2008;133:70–76.
CrossRef - Durbin DD. Relapse and the need for permanent fixed retention. J Clin Orthod. 2001;35:723–727.
- Cerny R. Permanent fixed lingual retention. J Clin Orthod. 2001;35:728–732.
- Artun J, Spadafora AT, Shapiro PA. A 3-year follow-up study of various types of orthodontic canine-to-canine retainers. Eur J Orthod. 1997;19:501–509.
CrossRef - Lim BS, Lee SJ, Lee JW, Ahn SJ. Quantitative analysis of adhesion of cariogenic streptococci to orthodontic raw materials. Am J Orthod Dentofacial Orthop 2008;133:882‒888.
CrossRef - Papaioannou W, Gizani S, Nassika M, Kontou E, Nakou M. Adhesion of Streptococcus mutans to different types of brackets. Angle Orthod 2007;77:1090‒1095.
CrossRef - De Siate A, Milano V, Laforgia A. A bacteriological study of supragingival bacterial plaque in subjects undergoing orthodontic therapy. Minerva Stomatol 1991;40:101‒105.
- Mitchell L. Decalcification during orthodontic treatment with fixed appliances —an overview. Br J Orthod 1992;19:199‒205.
CrossRef - Gwinnett AJ, Ceen RF. Plaque distribution on bonded brackets: a scanning microscope study. Am J Orthod 1979;75:667‒677.
CrossRef - Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev 1986;50:353‒380.
- Hamada S, Slade HD. Biology immunology and cariogenicity of Streptococus mutans. Microbiol Rev 1980;44:331‒384.
- Kuribayashi M, Kitasako Y, Matin K, Sadr A, Shida K, Tagami J. Intraoral pH measurement of carious lesions with Knierim RW. Invisible lower cuspid to cuspid retainer. Angle Orthod. 1973;43:218–220.
- Pandis N, Vlahopoulos K, Madianos P, Eliades T. Long-term periodontal status of patients with mandibular lingual fixed retention. Eur J Orthod. 2007;29:471–476.
CrossRef - Zachrisson BU. Clinical experience with direct-bonded orthodontic retainers. Am J Orthod. 1977;71:440–448.
CrossRef - Zachrisson BU. The bonded lingual retainer and multiple spacing of anterior teeth. Swed Dent J Suppl. 1982;15:247–255.
- Artun J. Caries and periodontal reactions associated with long-term use of different types of bonded lingual retainers. Am J Orthod. 1984;86:112–118.
CrossRef - Rose E, Frucht S, Jonas IE. Clinical comparison of a multistranded wire and a direct-bonded polyethylene ribbon-reinforced resin composite used for lingual retention. Quintessence Int. 2002;33:579–583.
- Tanaka Y, Matin K, Gyo M, Okada A, Tsutsumi Y, Doi H, Nomura N, Tagami J, Hanawa T. Effects of electrodeposited poly(ethylene glycol) on biofilm adherence to titanium. Biomed Mater Res A 2010;95:1105-1113.
CrossRef - Morita, Yusuke, et al. “Effect of silver ion coating of fixed orthodontic retainers on the growth of oral pathogenic bacteria.” Dental materials journal 33.2 (2014): 268-274.
CrossRef - Yamasaki A, Nikai H, Niitani K, Ijuhin N. Ultrastructure of the junctional epithelium of germfree rat gingiva. J Periodontol. 1979;50(12):641–8.
CrossRef - Kaji, Akihiko, et al. “Influence of a mandibular fixed orthodontic retainer on periodontal health.” Australian orthodontic journal 29.1 (2013):76-85.
- Oshagh, Morteza, et al. “Evaluation of Histological Impacts of Three Types of Orthodontic Fixed Retainers on Periodontium of Rabbits.” Journal of Dentistry 15.3 (2014):104.
- Oshagh, Morteza, et al. “Evaluation of Histological Impacts of Three Types of Orthodontic Fixed Retainers on Periodontium of Rabbits.” Journal of Dentistry15.3 (2014):104.
- Taylor, A. C., and Earl O. Butcher. “The regulation of eruption rate in the incisor teeth of the white rat.” Journal of Experimental Zoology 117.1 (1951):165-188.
CrossRef - Hasheminia S.M, Feizi G, Razavi S.M, Feizianfard M, Gutknecht N, Mir M. A comparative study of three treatment methods of direct pulp capping in canine teeth of rats: histologic evaluation. Laser Med Sci, 2008; DOI 10.1007/s 10103-008-0584-90.
- Ayanoglou, C. M., and C. Lesty. “Cyclosporin A?induced gingival overgrowth in the rat: a histological, ultrastructural and histomorphometric evaluation.” Journal of periodontal research 34.1 (1999):7-15.
CrossRef - Azarsina, Mohadese, et al. “The Antibacterial Properties of Composite Resin Containing Nanosilver against Streptococcus mutans and Lactobacillus.” The journal of contemporary dental practice 14.6 (2013):1014-1018.
CrossRef - Störmann, Ilka, and Ulrike Ehmer. “A prospective randomized study of different retainer types.” Journal of Orofacial Orthopedics/Fortschritte der Kieferorthopädie 63.1 (2002):42-50.
CrossRef - Ryu, Hwang-Sog, et al. “Antibacterial effect of silver-platinum coating for orthodontic appliances.” The Angle Orthodontist 82.1 (2011):151-157.
CrossRef - Levin, Liran, Gili R. Samorodnitzky-Naveh, and Eli E. Machtei. “The association of orthodontic treatment and fixed retainers with gingival health.” Journal of periodontology 79.11 (2008):2087-2092.
CrossRef - Zachinsky, Leo. “Range of histologic variation in clinically normal gingiva.”Journal of dental research4(1954):580-589.
CrossRef - Arango S, Peláez-Vargas A, García C. Coating and Surface Treatments on Orthodontic Metallic Materials. Coatings. 2013;3(1):1.
CrossRef








