Salai B. Magesh1, Rashmi Rajappa1, K. M. Ramkumar2, S. Suryanarayanan1 and SubbaRao V. Madhunapantula3
1Department of Water and Health, Faculty of Life Sciences, Jagadguru Sri Shivarathreeshwara University, Mysuru– 570015, Karnataka, India.
2SRM Research Institute, SRM University, Kattankulatur - 603203, Tamil Nadu, India.
3Centre of Excellence in Molecular Biology and Regenerative Medicine (CEMR), JSS Medical College, Jagadguru Sri Shivarathreeshwara University, Mysuru -570 015, Karnataka, India.
Corresponding Author E-mail: mvsstsubbarao@jssuni.edu.in
DOI : https://dx.doi.org/10.13005/bpj/1143
Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), an organic pollutant, is a potent toxin known to modulate lipid metabolism in mice. Prior studies have demonstrated the ability of Acetyl-L-Carnitine (ALC) to mitigate various diseases associated with abnormal lipid metabolism. Therefore, the efficacy of administration of ALC to protect mice from TCDD-induced abnormal lipid metabolism in mice was tested in this study. Experimentally, mice (n=5) were administered with 100ng/kg body weight TCDD for 30 days, followed by treatment with ALC (Oral, 50mg and 100mg/kg body weight) for additional 30 days. Compared to mice that received vehicle corn oil, mice that received TCDD showed elevated cholesterol, triglycerides, free fatty acids and phospholipids in liver and serum. In addition serum lipoproteins such as LDL and VLDL were also elevated in the TCDD treated mice. Oral administration of ALC (50mg and 100mg/kg b.w) showed a dose dependent health promoting effect against TCDD-induced abnormal lipid metabolism by restoring the lipids and lipoproteins to near normal level. In conclusion, ALC is a potent anti-hyperlipidemic compound, which helps in the treatment of disorders associated with lipid metabolism.
Keywords
TCDD; Triglycerides; ALC; Free fatty acids; hyperlipidemia
Download this article as:| Copy the following to cite this article: Magesh S. B, Rajappa R, Ramkumar K. M, Suryanarayanan S, Madhunapantula S. V. Acetyl-L-Carnitine Restores Abnormal Lipid Metabolism Induced by 2,3,7,8-Tetrachlorodibenzo-P-Dioxin in Mice. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Magesh S. B, Rajappa R, Ramkumar K. M, Suryanarayanan S, Madhunapantula S. V. Acetyl-L-Carnitine Restores Abnormal Lipid Metabolism Induced by 2,3,7,8-Tetrachlorodibenzo-P-Dioxin in Mice. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=15336 |
Introduction
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), a potent toxic environmental pollutant produced as a by-product of various industrial processes 1. It is resistant to degradation and hence tends to accumulate in the environment, in animals, and even in human 2. TCDD has a biological half-life of ≥7 years in humans and is categorized as a Class-I carcinogen by the International Agency for Research on Cancer (IARC) 3. In addition, TCDD is responsible for “Wasting Syndrome”, a disease that involves decrease in the body weight and adipose tissue 4. Furthermore, a significant increase in serum lipids and lipid metabolites was also reported in Wasting Syndrome5. For example, TCDD affects the cholesterol metabolism and biosynthesis of bile acids 6. In addition, TCDD also reduces the expression of peroxisome proliferator activated receptor (PPAR) and lipogenesis-related factors sterol regulatory element binding proteins (SREBP 1 & 2) 7. Taken together, TCDD disrupts lipid metabolism causing wasting syndrome and dyslipidemia associated disorders 8.
Since TCCD exposure to the body is mainly through diet, attempts have been made to identify effective compound(s) that can suppress TCDD-induced toxicity 9. Acetyl-L-Carnitine (ALC) is an acetyl ester of the L-carnitine, produced in mammalian brain, liver and kidney by the enzyme, ALC-transferase 10. Key functions of ALC include, accelerating the uptake of acetyl CoA, increasing acetyl CoA production and membrane phospholipid synthesis 11. A wide range of clinical and preclinical applications of ALC have been reported and are primarily associated with neurologic disorders, in particular, Alzheimer’s disease, Parkinsons disease, diabetic neuropathy, ischemia and reperfusion of the brain impairment 12. Consumption of ALC for 4 weeks reversed the age related weakening of mitochondrial membrane potential in the hepatocytes of old rats 13. In addition, ALC elevated the cardiolipin level and cellular oxygen utilization in the old rats to the level similar to young rats 14. However, not much is known about its antihyperlipidemic activity 15. Only few studies have demonstrated that administration of ALC effectively normalizes the concentration of plasma cholesterol and triglycerides in an experimental diabetic model and in hyper-cholesterolemic rabbits 16. Hence in this study, the efficacy of ALC on lipid metabolism was investigated by measuring and comparing the changes in serum and hepatocyte lipids of control and TCDD administered mice.
Materials and Methods
Acetyl L Carnitine, TCDD, and silymarin were procured from Sigma Chemical Company (St Louis, MO). All the other chemicals used are of analytical grade.
Animal Experiments
JSS Medical College Animal Ethics Committee (JSSMC/IAEC/18/5675/DEC2013), Mysuru, India, approved the in vivo study protocol. Male Swiss albino mice weighing about 20-25g were procured from the Central Animal Facility, JSS Medical College. The mice were kept at standard laboratory conditions of controlled environment with the temperature 25±2ºC and 12h light- dark cycle. The mice were allowed to eat commercially available standard diet and allowed free access of water.
Experimental Design
Mice were divided into nine groups (n=5) and treated with respective compounds as mentioned below,
Group 1: Vehicle corn oil control; Control
Group 2: Normal mice treated with ALC (50mg/kg bw); C+ALC (L)
Group 3: Normal mice treated with ALC (100mg/kg bw); C+ALC (H)
Group 4: TCDD (100ng/kg bw) for 30 days; TCDD
Group 5: TCDD for 30 days + Saline for 30 days; TCDD+ C
Group 6: TCDD for 30 days + ALC (50mg/kg bw) for 30 days; TCDD+ALC (L)
Group 7: TCDD for 30 days + ALC (100mg/kg bw) for 30 days; TCDD+ALC (H)
Group 8: TCDD + ALC (100mg/kg bw) Co treated; TCDD+ALC (Co)
Group 9: TCDD + Silymarin (50mg/kg bw); TCDD+SIL
TCDD (100 ng/kg bw) was dissolved in 0.15ml corn oil and administered intra-peritonially 17. ALC was dissolved in the aqueous suspension (50 and 100mg/kg bw) and administered orally 18.
Corn oil alone is administered to the control group. After treatment period, mice were deprived of food overnight, anesthetized and sacrificed 19. Serum was separated from the collected blood by centrifugation and stored at -20οC. Liver was quickly excised from the experimental animals and rinsed in ice-cold phosphate buffered saline, blotted and stored at -80οC. The extraction of lipids from liver tissues is done by the method of Folch et al., 1957 20. Figure 1 show the schematic representation of experimental design.
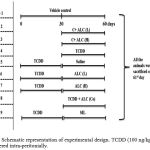 |
Figure 1: Schematic representation of experimental design. TCDD (100 ng/kg bw) was administered intra-peritonially. Click here to View figure |
ALC was dissolved in the aqueous suspension (50 and 100mg/kg bw) and administered orally. Control group was administered with the corn oil alone. The treatment period of the study is 60 days. On 61st day mice were deprived of food for overnight, anesthetized and sacrificed. Blood and liver tissues were collected to determine the lipid profile.
Hematological and Biochemical Analysis
Blood collected from the control and experimental animals were used for the analysis of serum lipids. Total Cholesterol, Triglycerides and High density lipoprotein-cholesterol (HDL) were determined using kits from Coral Clinical System, India. Phospholipids and Free fatty acid (FFA) levels were assessed as described by Zilversmit et al., 1950 21 and Falholt et al., 1973 22. Very low density lipoprotein-cholesterol (VLDL) and Low-density lipoprotein-cholesterol (LDL) were calculated by Friedwald formula: VLDL=TG/5; LDL=TC-(HDL+VLDL).
Analysis of lipid profile in liver Tissue
Estimation of Total Cholesterol
The total cholesterol levels were assessed by the method of Zlatkis et al., 1953 23. Cholesterol was first oxidized by Ferric chloride – acetic acid solution and, next, the color formed measured at 560 nm. Briefly, to 0.1 ml of lipid extract 9.9ml of ferric chloride – acetic acid reagent was added and the reaction mixture was permitted to stand for 15 minutes. Next, the reaction mixture was centrifuged, and to 5.0ml supernatant 3.0ml of sulfuric acid added. The contents were kept at 37º C for 20 min and the color developed measured at 560nm. Results were expressed as mg total cholesterol/100g tissue.
Estimation of Triglycerides
Triglycerides were determined by the method of Foster and Dunn 24. First, the glycerol moiety is oxidized to formaldehyde. Next, the oxidized glycerol was allowed to condense with acetyl acetone to yield 3,5-Dicarbethoxy-1,4-dihydrocollidine which is read at 405nm. Briefly, 0.1ml of lipid extract was vaporized to dryness. Then 0.1ml of methanol, 4 ml of isoproponal and 0.4ml of neutral alumina were added. The tubes are well shaken for 20 min, centrifuged and the supernatant collected. To 2.0ml of supernatant added 600µl of saponification reagent and incubated at 65ºC for 20.0 min and allowed to cool. Then, added 1.0 and 0.5ml of sodium metaperiodate and acetyl acetone reagent, respectively. The reaction mixture was heated at 65ºC for 15.0 min using water bath, cooled and yellow color formed was read at 405 nm. The levels of triglyceride is expressed as mg/100g tissue.
Estimation of free fatty Acid (FFA)
The FFA levels were assessed by the method of Falhot et al., 1968 22. Free fatty acids react with copper reagent and form a complex. The complex thus formed was made soluble in chloroform, and allowed to react with diphenyl carbazide (a color developer). The developed color was read at 550nm. Briefly, to 0.1 ml lipid extract, 6.0ml chloroform: heptane: methanol mixture (5:5:1), 2.5 ml copper reagent and 200mg of activated silicic acid were added. Mixed well, centrifuged and copper layer collected. To 3.0ml of copper layer added 0.5ml of diphenyl carbazide and the color developed was read at 550nm. The results were expressed as mg/100g tissue.
Estimation of Phospholipids
The inorganic phosphorus, phosphomolybdic acid reacts with ANSA solution to form a blue color which is measured at 660 nm 21. Briefly, to 0.1 ml of lipid extract, was added 1.0ml of 5N H2SO4 and 2-3 drops of conc. nitric acid. Then, added 2.5ml of 3% ammonium molybdate and 0.1 ml of ANSA solution, mixed well and heated in water bath for 6.0min. The blue color developed was read at 660 nm and the inorganic phosphorous content expressed as g/100g tissue.
Statistical Analysis
The statistical significance among nine groups was evaluated by one way ANOVA using SPSS version 20 followed by Tukey post hoc test. P<0.05 was considered significant.
Results and Discussion
Hyperlipidemia is one of the known consequences of TCDD 25. Exposure to TCDD has been shown to promote the accumulation of lipids, which leads to wasting syndrome and diabetes like symptoms 26. TCDD induced hyperlipidemia is associated with decreased expression of peroxisome proliferator activated receptor gamma (PPAR- γ) and lipogenesis factors such as sterol regulatory element binding proteins (SREBP) 27. Earlier studies carried out with ALC showed a significant anti-hyperlipidemic effect in experimental models 28, 29. In the present study, the effect of ALC on lipid metabolism in TCDD exposed mice was evaluated. Mice exposed to TCDD showed an about one fold increase in the serum cholesterol, LDL, VLDL, triglycerides, free fatty acids and phospholipids compared to vehicle control group (Table 1). However, surprisingly, no significant change in the HDL content was noticed in the TCDD treated group compared to the vehicle control. In addition, level of cholesterol in liver tissues was significantly higher in TCDD induced mice compared to vehicle control (Figure 2A). Increase in the concentration of serum lipids with TCDD exposure was attributed to an increase in the mobilization of FFA from the peripheral fat depots due to the action of hormone-sensitive lipases 30. Further the observed increase in the cholesterol in TCCD induced mice could be due to high HMG CoA reductase (a rate-limiting enzyme involved in cholesterol biosynthesis) activity 31. Oral administration of ALC (50 and 100 mg/kg bw) and SIL significantly reduced these elevated lipids in a dose dependent manner (Table 1 and Figure 2A).
Table 1: ALC administration mitigate the changes in serum lipids induced by TCDD administration in mice
|
Groups and treatment |
Serum Lipid Profile | ||||||
| Cholesterol
(mg/dl) |
Triglycerides (mg/dl) | HDL
(mg/dl)
|
LDL
(mg/dl)
|
VLDL
(mg/dl) |
Phospholipids (mg/dl) | FFA
(mg/dl) |
|
| Control | 69.9±3.7 | 42.3±2.4 | 41.4±0.9 | 18.8±0.8 | 8.4±0.4 | 68.6±1.6 | 3.2±0.3 |
| C+ALC (L) | 68.4±2.8 | 33±2.3 | 39.3±1.2 | 17.6±0.5 | 6.6±0.4 | 60.8±1.1 | 2.2±0.2 |
| C+ALC (H) | 68.2±2.4 | 31.3±2.3 | 39±1.8 | 19.9±0.6 | 6.2±0.4 | 63.5±3.1 | 2.9±0.2 |
| TCDD | 101.3±2.6* | 92±3.2* | 39.4±1.0 | 36±1.8* | 18.4±0.6* | 95.4±1.2* | 6.8±0.3* |
| TCDD+C | 85.9±2.3* | 76.6±7.2* | 44.8±1.8 | 28.5±2.2* | 16±0.4* | 83.8±2.5* | 5.8±0.2* |
| TCDD+ALC (L) | 75.9±1.5** | 67.6±2.4** | 49.0±1.1 | 21.7±1.2** | 13.5±0.4** | 71.8±1.2** | 4.1±0.1** |
| TCDD+ALC (H) | 71.2±1.6** | 48±5.5** | 44.2±1.5 | 18.9±1.5** | 9.6±1.1** | 70.3±1.2** | 3.6±0.2** |
| TCDD+ALC (Co) | 87.6±2.4** | 73±4.6** | 46.2±0.9 | 23.2±1.3** | 14.6±0.9** | 79.9±4.3** | 4.2±0.3** |
| TCDD+SIL | 66.2±3.3** | 42.3±2.4** | 47.4±1.1 | 18.5±1.1** | 8.4±0.4** | 61.5±1.4** | 2.1±0.1** |
Table 1. Analysis of serum free fatty acids, lipids and lipoproteins showed a significant increase in TC, LDL, VLDL, Phospholipids and free fatty acids upon TCDD administration. Treating TCDD administered mice with ALC reduced these elevated lipids and lipoproteins to the levels that are close to normal control mice Co-administration of ALC with TCDD had much lesser effect compared to treating mice after TCDD administration. * Indicate groups compared with vehicle control group. ** Indicate groups compared with TCDD administered mice.
TCDD exposed animal models are susceptible to hypertriglyceridemia, which might be due to the elevated VLDL secretion or decreased VLDL clearance. Elevated VLDL and LDL in the serum of TCDD injected mice (Table 1) was related to the reduction in the activity of lipoprotein lipases as evidenced by Brewster et al., 1984 32. Oral administration of ALC restored the VLDL and LDL suggesting that ALC promotes the release and triggers the lipoprotein lipases thus resulting in the reduced lipid content 33. In addition, lipoprotein lipases play a key role in the hydrolysis of triglycerides and uptake of FFA. Deficiency/reduced activity of lipoprotein lipases enhances the release of FFA from adipose tissue 34. Excess free fatty acids in the plasma promotes the conversion in to phospholipids and cholesterol in the liver 35. In the current study, concentration of triglycerides in serum and liver tissues of TCDD induced mice was increased (~ 1 fold) compared to the vehicle control (Table 1 and Figure 2B). Oral administration of ALC significantly decreased the levels of triglycerides in serum and liver tissues (Table 1 and Figure 2B). In addition, TCDD induced mice showed higher levels of phospholipids and free fatty acids in serum and liver tissue (Table 1 and Figure 3A& B) which is in agreement with the earlier studies 36. However, ALC treatment significantly decreased the levels of free fatty acids and phospholipids in the serum and tissues thus improving lipid metabolism in TCDD induced mice.
 |
Figure 2: The effect of ALC on cholesterol and triglycerides levels. Click here to View figure |
The total cholesterol was estimated by the method of Zlatkis et al., 1953. Cholesterol was oxidized by Ferric chloride – acetic acid solution. The colour thus formed was measured at 560nm. Triglycerides were estimated by the method of Foster and Dunn, 1973. The glycerol moiety was oxidized to formaldehyde and further condensed by acetyl acetone to yield 3,5-Dicarbethoxy-1,4-dihydrocollidine which was read at 405nm. A significant 1 fold increase in liver cholesterol and triglycerides levels was observed upon TCDD administration. However, oral treatment of ALC (50 mg and 100 mg/ kg body weight) and silymarin for 30 days significantly reduced the total cholesterol and triglycerides content to near normal.
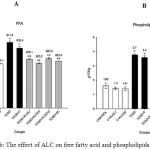 |
Figure 3a,b: The effect of ALC on free fatty acid and phospholipids. Click here to View figure |
Free fatty acids were estimated by the method of Falhot et al., 1968. Free fatty acids react with copper reagent, and form a colored complex with cupric ions. The complex thus formed was made soluble in chloroform, and a color developing agent diphenyl carbazide added. The colour developed was read at 550 nm. Phospholipid content was estimated by the method of Zilversmit et al., 1950. The inorganic phosphorus, phosphomolybdic acid reaction with ANSA solution forms a blue color which was measured at 660nm. A significant elevation in liver free fatty acids and phospholipids levels (~ 1 fold) was observed when mice were exposed to TCDD. However, oral treatment with ALC (50 mg and 100 mg/ kg body weight) and silymarin for 30 days significantly reduced the values to near normal.
Conclusion
In conclusion, oral administration of ALC is an effective lipid lowering agent to protect mice from TCDD induced toxicity, hence, can be a potential therapeutic agent in managing hyperlipidemia related diseases.
Acknowledgements
The authors, Salai B. Magesh and Rashmi Rajappa gratefully acknowledge JSS University, Mysuru for the award of JSS University Research Fellowship.
Conflict of Interest
None
References
- Nakashima, K. I., Tanabe, H., Fujii-Kuriyama, Y., Hayashi, H., & Inoue, M. Atranorin and lecanoric acid antagonize TCDD-induced xenobiotic response element-driven activity, but not xenobiotic response element-independent activity. Journal of natural medicines. 2016; 70: 476-482.
CrossRef - Kao, C. M., Chen, S. C., Liu, J. K., & Wu, M. J. Evaluation of TCDD biodegradability under different redox conditions. Chemosphere. 2001; 44: 1447-1454.
CrossRef - Steenland, K., Bertazzi, P., Baccarelli, A., & Kogevinas, M. Dioxin revisited: developments since the 1997 IARC classification of dioxin as a human carcinogen. Environmental health perspectives. 2004: 1265-1268.
CrossRef - Seefeld, M. D., Corbett, S. W., Keesey, R. E., & Peterson, R. E. Characterization of the wasting syndrome in rats treated with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Toxicology and applied pharmacology. 1984; 73: 311-322.
CrossRef - Uno, S., Dalton, T. P., Sinclair, P. R., Gorman, N., Wang, B., Smith, A. G.,& Nebert, D. W. Cyp1a1 (−/−) male mice: protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicology and applied pharmacology. 2004; 196: 410-421.
CrossRef - Fletcher, N., Wahlström, D., Lundberg, R., Nilsson, C. B., Nilsson, K. C., Stockling, K., & Håkansson, H. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the mRNA expression of critical genes associated with cholesterol metabolism, bile acid biosynthesis, and bile transport in rat liver: a microarray study. Toxicology and applied pharmacology. 2005; 207: 1-24.
CrossRef - Nishiumi, S., Yabushita, Y., Furuyashiki, T., Fukuda, I., & Ashida, H. Involvement of SREBPs in 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced disruption of lipid metabolism in male guinea pig. Toxicology and applied pharmacology. 2008; 229: 281-289.
CrossRef - Cranmer, M., Louie, S., Kennedy, R. H., Kern, P. A., & Fonseca, V. A. Exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) is associated with hyperinsulinemia and insulin resistance. Toxicological Sciences. 2000; 56: 431-436.
CrossRef - Ciftci, O., Tanyildizi, S., & Godekmerdan, A. Protective effect of curcumin on immune system and body weight gain on rats intoxicated with 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD). Immunopharmacology and Immunotoxicology. 2010; 32: 99-104.
CrossRef - Veronese, N., Sergi, G., Stubbs, B., Bourdel-Marchasson, I., Tessier, D., Sieber, C., & Maggi, S. Effect of acetyl-l-carnitine in the treatment of diabetic peripheral neuropathy: A systematic review and meta-analysis. European Geriatric Medicine. 2017; 8: 117-122.
CrossRef - Guo, X., Dumas, M., Robinson, B. L., Ali, S. F., Paule, M. G., Gu, Q., & Kanungo, J. Acetyl L‐carnitine targets adenosine triphosphate synthase in protecting zebrafish embryos from toxicities induced by verapamil and ketamine: An in vivo assessment. Journal of Applied Toxicology. 2017; 37: 192-200.
CrossRef - Skvarc, D. R., Dean, O. M., Byrne, L. K., Gray, L., Lane, S., Lewis, M., & Marriott, A. The effect of N-acetylcysteine (NAC) on human cognition–a systematic review. Neuroscience & Biobehavioral Reviews. 2017; 78:44-56
CrossRef - Mollica, M. P., Iossa, S., Soboll, S., & Liverini, G. Acetyl-L-carnitine treatment stimulates oxygen consumption and biosynthetic function in perfused liver of young and old rats. Cellular and Molecular Life Sciences. 2001; 58: 477-484.
CrossRef - Hagen, T. M., Ingersoll, R. T., Wehr, C. M., Lykkesfeldt, J., Vinarsky, V., Bartholomew, J. C., & Ames, B. N. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proceedings of the National Academy of Sciences. 1998; 95: 9562-9566.
CrossRef - Tanaka, Y., Sasaki, R., Fukui, F., Waki, H., Kawabata, T., Okazaki, M., & Ando, S.. Acetyl-L-carnitine supplementation restores decreased tissue carnitine levels and impaired lipid metabolism in aged rats. Journal of lipid research. 2004; 45: 729-735.
CrossRef - Ido, Y., McHowat, J., Chang, K. C., Arrigoni-Martelli, E., Orfalian, Z., Kilo, C., & Williamson, J. R. Neural dysfunction and metabolic imbalances in diabetic rats: prevention by acetyl-L-carnitine. Diabetes. 1994; 43: 1469-1477.
CrossRef - Kalaiselvan, I., Samuthirapandi, M., Govindaraju, A., Sheeja Malar, D., & Kasi, P. D. Olive oil and its phenolic compounds (hydroxytyrosol and tyrosol) ameliorated TCDD-induced heptotoxicity in rats via inhibition of oxidative stress and apoptosis. Pharmaceutical biology. 2016; 54: 338-346.
CrossRef - Flatters, S. J., Xiao, W. H., & Bennett, G. J. Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful peripheral neuropathy. Neuroscience letters. 2006; 397: 219-223.
CrossRef - Miller, J. M., Jope, R. S., Ferraro, T. N., & Hare, T. A. Brain amino acid concentrations in rats killed by decapitation and microwave irradiation. Journal of neuroscience methods. 1990; 31: 187-192.
CrossRef - Folch, J., Lees, M., & Sloane-Stanley, G. H. A simple method for the isolation and purification of total lipids from animal tissues. J biol Chem. 1957; 226: 497-509.
- Zilversmit, D. B., & DAVIS, A. K. Microdeter mination of plasma phospholipids by trichloro acetic acid precipitation. Journal of Laboratory and Clinical Medicine. 1950; 35: 155-160.
- Falholt, K., Lund, B., & Falholt, W. An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clinica Chimica Acta. 1973; 46: 105-111.
CrossRef - Zlatkis, A., Zak, B., & Boyle, A. J. A new method for the direct determination of serum cholesterol. Journal of Laboratory and Clinical Medicine. 1953; 41: 486-492.
- Foster, L. B., & Dunn, R. T. Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clinical Chemistry. 1973; 19: 338-340.
- Angrish, M. M., Dominici, C. Y., & Zacharewski, T. R. TCDD-elicited effects on liver, serum, and adipose lipid composition in C57BL/6 mice. Toxicological Sciences. 2013; 131: 108-115.
CrossRef - Tuomisto, J. T., Pohjanvirta, R., Unkila, M., & Tuomisto, J. TCDD-induced anorexia and wasting syndrome in rats: effects of diet-induced obesity and nutrition. Pharmacology Biochemistry and Behavior. 1999; 62: 735-742.
CrossRef - Nishiumi, S., Yabushita, Y., Furuyashiki, T., Fukuda, I., & Ashida, H. Involvement of SREBPs in 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced disruption of lipid metabolism in male guinea pig. Toxicology and applied pharmacology. 2008; 229: 281-289.
CrossRef - Tanaka, Y., Sasaki, R., Fukui, F., Waki, H., Kawabata, T., Okazaki, M., & Ando, S. Acetyl-L-carnitine supplementation restores decreased tissue carnitine levels and impaired lipid metabolism in aged rats. Journal of lipid research. 2004; 45(4): 729-735.
CrossRef - Malaguarnera, M., Vacante, M., Avitabile, T., Malaguarnera, M., Cammalleri, L., & Motta, M. L-Carnitine supplementation reduces oxidized LDL cholesterol in patients with diabetes. The American journal of clinical nutrition. 2009; 89: 71-76.
CrossRef - Boverhof, D. R., Burgoon, L. D., Tashiro, C., Chittim, B., Harkema, J. R., Jump, D. B., & Zacharewski, T. R. Temporal and dose-dependent hepatic gene expression patterns in mice provide new insights into TCDD-Mediated hepatotoxicity. Toxicological Sciences. 2005; 85: 1048-1063.
CrossRef - Shertzer, H. G., Genter, M. B., Shen, D., Nebert, D. W., Chen, Y., & Dalton, T. P. TCDD decreases ATP levels and increases reactive oxygen production through changes in mitochondrial F 0 F 1-ATP synthase and ubiquinone. Toxicology and applied pharmacology. 2006; 217: 363-374.
CrossRef - Brewster, D. W., & Matsumura, F. TCDD (2, 3, 7, 8-tetrachlorodibenzo-p-dioxin) reduces lipoprotein lipase activity in the adipose tissue of the guinea pig. Biochemical and biophysical research communications. 1984; 122: 810-817.
CrossRef - Paradies, G., Ruggiero, F. M., Petrosillo, G., Gadaleta, M. N., & Quagliariello, E. Carnitine-acylcarnitine translocase activity in cardiac mitochondria from aged rats: the effect of acetyl-L-carnitine. Mechanisms of ageing and development. 1995; 84: 103-112.
CrossRef - Herrera, E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. European journal of clinical nutrition. 2000; 54: 47-51.
CrossRef - Pari, L., & Latha, M. Effect of Cassia auriculata flowers on blood sugar levels, serum and tissue lipids in streptozotocin diabetic rats. Singapore Med J. 2002; 43: 617-621.
- Lakshman, M. R., Campbell, B. S., Chirtel, S. J., & Ekarohita, N. Effects of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) on de novo fatty acid and cholesterol synthesis in the rat. Lipids. 1988; 23: 904-906.
CrossRef








