Sima Rassai and Ziba Mehrjui
Department of Dermatology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Corresponding Author E-mail: Drziba2013@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1091
Abstract
Melasma is an acquired hyper melanosis disease and its treatment is not often satisfactory. Given the role of hormones in pathogenesis of melasma, sex hormone related drugs may be effective in the treatment of melasma. Hence, this study is carried out to compare the effects of finasteride tablets along with topical medication with topical medicine and placebo for the treatment of melasma in women visiting Imam Khomeini Hospital in Ahwaz. This is a randomized, double-blind clinical trial conducted on 30 women suffering melasma in 2015. Patients were randomly divided into two groups. A group was treated with 5mg finasteride with topical hydroquinone 4%, and the other group was treated with placebo as well as topical hydroquinone 4%. Demographic and clinical characteristics as well as the melasma area and severity index (MASI) and satisfaction of patients were also evaluated. Finally, the collected data were analyzed by SPSS. The age range of the participating patients was 20 to 50 and most patients (80%) were between 30-40 years of age. No significant difference was observed in both groups regarding age, marital status, duration of melasma, melasma area, type of melasma, melasma history, pregnancy, and OCP use (P>0.05). Evaluation results of MASI index showed a decreasing trend during treatment (P<0.05) but no significant difference was observed between MASI index and satisfaction of patients in two treatment groups. As well, hirsutism, androgenic alopecia, and acne were respectively seen in 20%, 63.3% and 13.3% of patients. Results of this study showed that although the degree of satisfaction was higher in patients receiving finasteride and the difference between the first and second MASI was significantly higher in the group receiving finasteride, treatment outcomes at the end of the study were not statistically different and the difference in first and fourth MASI showed no significant difference in the two groups. This difference may become statistically significant with greater sample size and increased duration of treatment. Since this study is probably the first clinical experience in the use of finasteride for melasma treatment, definitive conclusions about its use or non-use is not possible before doing further studies in the future.
Keywords
melasma; finasteride; hydroquinone
Download this article as:| Copy the following to cite this article: Rassai S, Mehrjui Z. The Comparison of Efficacy of oral Finastride and Topical Hydroquinone Versus Placebo and Topical Hydroquinone in Treatment of Melasma: A Randomized Double-Blind Clinical Trial. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Rassai S, Mehrjui Z. The Comparison of Efficacy of oral Finastride and Topical Hydroquinone Versus Placebo and Topical Hydroquinone in Treatment of Melasma: A Randomized Double-Blind Clinical Trial. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=14045 |
Introduction
Melasma is a common acquired illness with outbreak of brown and often symmetrical hypersegmented patches on frequently sun-exposed areas of the face (1 and 2). Melasma accounts for about 4 to 10 percent of the complaints of those visiting skin clinics and is more common among Hispanic and Asian races as well as Hispanic and Asian young to middle-aged women and dark-skinned people (1 and 3).
The pathogenesis of melasma is not completely known. However, factors such as pregnancy, use of oral combination contraceptives (OCP), stress, exposure to ultraviolet radiation, endocrine factors, genetic and racial factors, and autoimmune disorders like thyroid disease are known as effective on its outbreak or development (1&4-6).
Although accurate protection against sun like using sun hats and sunscreens are essential in melasma treatment, other treatments are also necessary. Topical treatments include skin whitening agents (hydroquinone, kojic acid), tretinoin, azelaic acid, and chemical peels (retinol-trichloroacetic). There are also different types of laser treatments for melasma (1, 7 and 8). In general, melasma treatment is often not satisfactory and treatment with topical medications is followed by numerous side effects such as contact dermatitis, inflammation, and ulcers (3, 9 and 10).
Results obtained in connection with the concurrence of melasma with androgenic disorders like alopecia, hirsutism, and acne and the high incidence of ovarian cysts in patients with melasma as well as the effectiveness of anti-androgenic therapies for the treatment of acne and hirsutism have raised the use of androgenic drugs such as flutamide and finasteride for the treatment of melasma (11 and 12). Recently, a clinical study showed that the anti-androgen flutamide is as effective as hydroquinone in melasma treatment (13).
Finasteride is an inhibitor of the 5-alpha reductase enzyme. Since 5-alpha reductase enzyme is responsible for converting testosterone into dihydrotestosterone (DHT), which is much more active than testosterone, finasteride has anti-androgenic effects (14).
There are reports of the benefits of finasteride in women with hair loss due to hyperandrogenism (15) or androgen therapy (16). It is also reported that finasteride is effective for the treatment of hirsutism in women (17 and 18). The results of some studies have shown that acne is significantly common in women with melasma. It is also possible that androgenic underlying disorders causing acne play an etiologic role in the development of melasma (12).
Melasma treatment is an important issue because it affects the patients’ appearance and topical treatments, despite their use of several times a day and several months, are not completely able to eliminate the lesions and ablative treatments like derm abrasion and lasers have dual results and many complications. Accordingly, oral medications that can fade melasma might be a better and easier solution to treat it. In addition, various studies have shown that hormones also play a role in the pathogenesis of melasma (19 and 20). So finasteride as an anti-androgen factor may be effective in the treatment of melasma and the present study aimed to compare the efficacy of topical hydroquinone and finasteride tablet with topical hydroquinone and placebo in the treatment of melasma.
Methodology
This randomized clinical trial (RCT) is a double-blind pilot study that aimed to compare the effect of 5-mg oral finasteride in combination with topical drugs containing hydroquinone with placebo and topical hydroquinone on melasma lesions. The study population consisted of 30 women with melasma who visited the dermatology clinic of Imam Khomeini educational therapeutic hospital in 2015 and were willing to cooperate. Patients with liver diseases, pregnant and lactating women, patients who used oral contraceptives, patients treated with anti-androgenic drugs, and patients receiving topical treatment had not the inclusion criteria from a month earlier. Also, before entering the study, all patients were BHCG tested and sufficient information about finasteride side effects on the fetus was given to them.
Patients were randomly were divided into 2 A and B groups (simple computer randomization). Group A was treated with 5-mg finasteride (Suha Pharmaceutical Co., Iran) with 4% topical hydroquinone (cream eldoquin fort 4% hydroquinone cream containing sulfites made by Valeant Pharmaceuticals.inc.) and group B was treated with placebo (a combination of starch and sweetening matter quite similar to the 5-mg finasteride tablets produced by the School of Pharmacy) and 4% topical hydroquinone (cream eldoquin fort 4% hydroquinone cream containing sulfites made by Valeant pharmaceuticals.inc).
Melasma treatment was carried out for 3 months and the subjects took 5 mg finasteride tablets every night half an hour before going to bed and sufficient amount of 4% hydroquinone cream was rubbed onto the facial spots (the patients had been advised not to rub cream around the eyes and mouth to avoid medicine contact with these areas), and they also used sunscreen during the next day (MY sunscreen with SPF = 60 produced by Iran Kimia Arman Co.).
Follow-ups were done once every four weeks by measuring the MASI index and photography (using 8-megapixel IXUS 950Canon camera) (21). These follow-ups were conducted 3 times at the end of the first, second and third months. MASI index of the patients was also measured and recorded on admission to the study.
To ensure compliance with requirements of the double-blind study, medications were randomly prescribed for patients and the patients, hospital staff, and researchers did not know the type of treatment until the end.
All participating patients were examined regarding hirsutism, acne and androgenic alopecia; and demographic information (such as age and marital status) and clinical data of each patient including skin type, type of melasma lesions (epidermal, dermal and combined), melasma area, family history of melasma, duration of melasma, history of using OCP and pregnancy were also recorded.
Since there was no laboratory test for evaluation of lesions in patients, the response to treatment in various follow-ups was evaluated by dermatologist examinations. Thus, the recovery of all lesions meant a complete response, MASI reduction of more than 50% meant partial response, MASI reduction of less than 50% meant low response and non-recovery of lesions meant no response to treatment. At the last follow-up of patients, their satisfaction with the treatment was also asked and recorded.
To analyze the data and investigate the relationship between the studied variables, non-parametric tests including Mann-Whitney and Wilcoxon were used. In order to do statistical analysis, SPSS version 22 was used. The level of significance in the above tests was considered as 0.05.
Findings
The results showed that the average age of participants in the study was 33.66 ± 5.85 years (ranging from 25 to 51) and the average ages in the finasteride and hydroquinone groups were respectively 32.78 ± 5.46 years and 34.37 ± 6.36. Also, most participants were aged between30 to 40 (80%).
The results of statistical analysis showed no significant difference regarding age, marital status, skin type, family history of melasma, pregnancy history, oral contraceptive use, type of melasma, melasma area, duration of melasma, and hair loss between the two groups (P=0.261 ). Frequency and characteristics of each of the variables studied in both groups are shown in Table 1.
Table 1: Profile of patients
| Demographic and clinical characteristics | Finastride | Hydroquinone | Total |
| Frequency (%) | Frequency (%) | Frequency (%) | |
| Marital status | Single: 3 (21.4) | Single: 5 (31.2) | Single: 8 (26.7) |
| Married: 11 (78.6) | Married: 11 (68.8) | Married: 22 (73.3) | |
| Duration of melasma | 1-5 y: 9 (64.3) | 1-5 y: 10 (62.50 | 1-5 y: 19 (63.3) |
| 5-10 y: 2 (14.3) | 5-10 y: 3 (18.8) | 5-10 y: 5 (16.7) | |
| >10 y: 3 (21.4) | >10 y: 3 (18.8) | >10 y: 6 (20) | |
| Melasma location | Cheek: 6 (42.9) | Cheek: 6 (37.4) | Cheek: 12 (40) |
| Forehead: 0 | Forehead: 5 (31.3) | Forehead: 5 (16.7) | |
| Chin: 2 (14.2) | Chin: 0 | Chin: 2 (6.6) | |
| All: 6 (42.9) | All: 5 (31.3) | All: 11 (36.7) | |
| Type of melasma | Epidermal: 4 (28.6) | Epidermal: 7 (43.8) | Epidermal: 11 (36.7) |
| Dermal: 2 (14.3) | Dermal: 4 (25) | Dermal: 6 (20) | |
| Mix: 8 (57.1) | Mix: 5 (31.2) | Mix: 13 (43.3) | |
| Skin Type | II: 0 | II: 2 (12.5) | II: 2 (6.6) |
| III: 9 (64.3) | III: 5 (31.2) | III: 14 (46.7) | |
| IV: 5 (35.7) | IV: 9 (56.3) | IV: 14 (46.7) | |
| History of Pregnancy | Yes: 10 (71.4) | Yes: 10 (62.5) | Yes: 20 (67.7) |
| No: 14 (28.6) | No: 6 (37.5) | No: 10 (33.3) | |
| History of OCP usage | Yes: 7 (50) | Yes: 5 (31.2) | Yes: 12 (40) |
| No: 7 (50) | No: 11 (68.8) | No: 18 (60) | |
| Melasma history | Yes: 11(78.6) | Yes: 9 (56.3) | Yes: 20 (67.7) |
| No: 3 (21.4) | No: 7 (43.7) | No: 10 (33.3) | |
| Hirsutism | Yes: 5 (35.7) | Yes: 1(6.2) | Yes: 6 (20) |
| No: 9 (64.3) | No: 15 (93.8) | No: 24 (80) | |
| Hair loss | Yes: 7 (50) | Yes: 4 (25) | Yes: 11 (36.7) |
| No:7 (50) | No: 12 (75) | No: 19 (63.3) | |
| Acne | Yes: 4 (28.6) | Yes: 0 | Yes: 4 (13.3) |
| No:10 (71.4) | No:16 (100) | No: 26 (86.7) |
Abbreviation: OCP, oral contraceptive pill.
Study results showed that 23.3%, 40%, and 36.7% of the patients had respectively low, average, and high satisfaction with the treatment (Figure 1). The results of statistical analyses showed no significant difference between satisfaction of the two groups regarding treatment (P= 0.338).
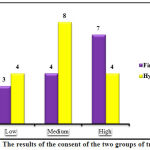 |
Figure 1: The results of the consent of the two groups of treatment. |
Studying the patients’ MASI results showed a decreasing trend in level of this index (Figure 2). MASI index in the beginning of study was the minimum of 3.6 and maximum of 18 and after the treatment, it was ranging from 1.8 to 12.9. However, no significant statistical differences was observed between the two groups regarding MASI reduction (Table 2). The results also showed that the difference between the first and second MASI in the two study groups was the highest and this difference in the finasteride and placebo groups were respectively 2.9 and 1.19 (Table 2).
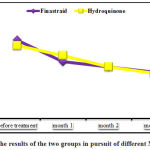 |
Figure 2: The results of the two groups in pursuit of different MASI index |
Table 2: Results of MASI index tracking different in the two groups
| p-value | S.D. ± Mean | Group | Follow up |
| 699/0 | 52/5±84/9 | Finastride | MASI 1 |
| 89/3±16/9 | Hydroquinone | ||
| 592/0 | 39/4±10/7 | Finastride | MASI 2 |
| 67/3±90/7 | Hydroquinone | ||
| 997/0 | 29/4±55/6 | Finastride | MASI 3 |
| 82/3±46/6 | Hydroquinone | ||
| 919/0 | 22/4±80/5 | Finastride | MASI 4 |
| 14/3±66/5 | Hydroquinone | ||
| 073/0 | 77/0±90/2 | Finastride | dMASI 1&2 |
| 29/0±19/1 | Hydroquinone | ||
| 070/0 | 28/0±07/1 | Finastride | dMASI 2&3 |
| 30/0±21/1 | Hydroquinone | ||
| 674/0 | 19/0±71/0 | Finastride | dMASI 3&4 |
| 26/0±07/1 | Hydroquinone |
Evaluation of the responses to treatment in the present study showed that 42.85% of the finasteride group and 25% of the hydroquinone group experienced more than 50% reduction in MASI index at the end of the treatment period (Figure 3). As well, complete response and non-response to treatment were not observed in any of the two groups.
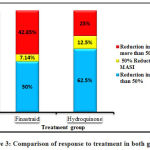 |
Figure 3: Comparison of response to treatment in both groups |
Discussion
The results showed that the mean age of the participants in this study was 33.6 (ranging from 25 to 51). In the study conducted by Edalatkhah and Sadeghi (2015), the aveerage age of patients with melasma was 33.8 (ranging from 20 to 52) (13). In the present study, the skin type of most patients suffering melasma was IV and III which is similar to other studies including the study of Haj Heidari et al. (2014) as well as Kodali et al. (2010) (22 and 23). As well, cheek was the most common area of melasma in the studied patients which is consistent with the results of other studies (24, 25). But Moy’s study results showed that centrofacial area is the most melasma-affected area (26).
Haj Heydari (2014) showed that the most common area for the incidence of melasma was the forehead and cheeks (44 cases) and the least affected area was chin (4 cases) (22). In the study conducted by Espahbodi et al. (2009) the most common areas of melasma were respectively nose, cheeks, and forehead (33%), nose and cheeks (31%), both cheeks (19%), nose, cheeks, forehead, chin, and upper lip (17%) (27). Differences in melisma area can be due to differences in age, race, and other factors affecting the development of melasma.
Mix Melasma (43.3%) and epidermal melasma (36.7%) were most frequent in the studied subjects which is consistent with the results obtained by Mahmood et al. (2011) (24).
The results of melasma concurrence with androgenic features showed that 63.3% and 13.3% of patients respectively suffered androgenetic alopecia and acne. The prevalence of hirsutism in this study was 20%. The study conducted by Edalatkhah and Amini (2007) showed that 23.8% of the patients suffered melasma and hirsutism together (11).
MASI score in the beginning of study was much less than other studies (9.48). MASI score in the beginning of studies conducted by Haj Heidari et al. (22), Edalatkhah and Sadeghi (13), and Sarkar et al. (28) (India) were respectively 27, 19, and 19. In the study conducted by Haj Heidari et al. (2014), patients were used whose disease had not responded to other treatments which is one of the reasons for the high MASI Score at the beginning of that study. As well, the duration of affection with this disease in this study was much less than the study conducted by Haj Heydari (7.76 ± 4.62) such that only 20% of the studied patients mentioned their disease duration over 10 years and the disease duration of most patients was 1-5 years (63.3%).
In the present study, MASI results showed a decreasing trend in the index. However, no significant difference was observed between MASI reductions in two groups which indicates the similarity of the effectiveness of two drug protocols used for melasma treatment. In the study of Edalatkhah and Sadeghi (2015), there was a significant difference between the levels of MASI index of the two groups treated with finasteride and hydroquinone and the efficiency of treatment was higher in the flutamide group (13). Different results of the current study and the results obtained by Edalatkhah and Sadeghi (13) can be due to the differences in the type of medication (oral and topical), duration of treatment, duration of sunlight exposure, type of melasma (dermal or epidermal), inheritance, and different hormonal characteristics of the two groups.
In the study conducted by Haj Heidari et al (2014), MASI Score changes from the beginning the study until the end of sixth months showed a decreasing trend (p < 0.001) and the most effective treatment was observed at the end of the first and second months (22). In this study, the results showed that the first and second MASI (MASI decline during the first month of study) had the greatest difference in the two studied groups which was statistically significant (P <0.05). In other cases, no significant difference was observed.
Patients receiving finasteride showed a greater reduction in MASI at the beginning of treatment and this difference was statistically significant. However, at the end of study, MASI decrease was similar in both groups and no significant difference was observed. This indicated the faster response to treatment in patients receiving finasteride. More reductions at the beginning of treatment can be due to full observation of the therapeutic principles by patients. Also, the patients might not have observed the treatment principles in the following two month due to the successful treatment effect during the first month and their temporary treatment.
Several studies have mentioned the treatment duration of melasma disease as 4 to 6 months (7 and 29). It can be said that because the treatment period in this study was 3 months, no complete recovery was observed in any patient. Also, due to the declining MASI Score, full recovery can be expected in case of continued treatment.
Results of this study showed no significant differences in satisfaction of the two study groups with treatments. But it seemed that patients were more satisfied with finasteride. This is while in the study conducted by Edalatkhah and Sadeghi, satisfaction of patients with flutamide treatment was significantly higher than the control group (treatment with topical hydroquinone) (13).
In this study, use of oral finasteride in combination with hydroquinone was safe and had no side-effects. Although the present study is the first study on the impact of finasteride on improvement of melasma, this drug is already studied in the form of topical and oral medications for the treatment of acne, hirsutism, and hair loss (18, 30-32). Results obtained by Edalatkhah and Amini (2007) also indicated the role of androgenic disorders in incidence of melasma (11). But understanding the exact mechanism and the effect of finasteride on melasma is hard. Edalatkhah and Sadeghi (2015) showed in a study that the anti-androgen flutamide is effective in the treatment of melasma and acne (13). A case study is also reported on the effect of topical flutamide on pigmentation disorders (33).
There are theoretical evidences on the relationship between melasma and other androgenic disorders such as acne, hirsutism, and polycystic ovary. The mechanism of this action may lie in the changes in the hormones stimulating alpha-melanocyte or agents increasing cyclic adenosine monophosphate which affect the synthesis of melanin. Therefore, further studies are recommended to discover the details of this mechanism (34 and 35).
Conclusion
The results showed that the degree of satisfaction was higher in patients receiving finasteride and the difference in first and second MASI was significantly higher in the group receiving finasteride. Nevertheless, there was no significant difference in treatment results at the end of the study and the first and fourth MASI in the two groups showed no significant difference. This difference may become statistically significant with greater sample size and increased duration of treatment.
Since studies on the effects of androgenic drugs such as finasteride on treatment of melasma are very limited and this study is probably the first clinical experience on the use of finasteride in melasma treatment, definitive conclusions about its use or non-use is not possible before conducting further studies in the future.
Refrences
- Bolognia JL, Jorizza JL, Schaffer JV. Dermatology; 2012:1052-1054.
- Hernández-Barrera R, Torres-Alvarez B, Castanedo-Cázares JP, Oros-Ovalle C, Moncada B. Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clinical and Experimental Dermatology. 2008; 33(3):305-308.
- Salim A, Rengifo-Pardo M, Vincent S, Cuervo-Amore LG. Melasma. In: Williams H, editor. Evidence-based Dermatology. 2nd ed. Malden. Blackwell Publishing; 2008:497–510.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005; 27(2):96-101.
- Kang WH, Yoon KH, Lee E-S, et al. Melasma: histopathological characteristics in 56 Korean patients. Br J Dermatol. 2005:146:228-237.
- Mosher DB, Fitzpatric TB, Hori Y, Ortonne JP. Disorder of melanocyte. In: Fitzpatrick TB, Freedberg IM, Editors. Fitzpatricks dermatology in general medicine. New York, NY: McGraw-Hill Medical Publishing Division; 2003. p. 903-87.
- Rendon M, Berneburg M, Arellanom I, Picardo M. Treatment of melasma. J Am Acad Dermatol. 2006; 54(5): 272-282.
- Nanda S, Grove Ch, Reddy BS. Efficacy of hydroquinone (2%) versus tretinoin (0.025%) as adjuant topical agents for chemical peelin in patients of melasma. Dermatol Surg. 2004; 30(3): 385-388.
- Sardesai VR, Kolte JN, Srinivas BN. A clinical study of melasma and a comparison of the therapeutic effect of certain currently available topical modalities for its treatment. Indian J Dermatol. 2013; 58(3):239.
- Rivas S, Pandya AG. Treatment of melasma with topical agents, peels and lasers: an evidence-based review. Am J Clin Dermatol. 2013; 14(5): 359–376.
- Adalatkhah H, Amani F. The Correlation between Melasma, Ovarian Cysts and Androgenic Hormones (A Case-Control Study). Research Journal of Biological Sciences. 2007; 2:593-596.
- Adalatkhah H, Sadeghiyeh Ahari S. The possible role of androgenic disorders in the development of melasma (a case-control study). The second Congress of Dermatology North West of Iran, Tabriz. Iran. 1393.
- Adalatkhah H, Sadeghi-Bazargani H. The first clinical experience on efficacy of topical flutamide on melasma compared with topical hydroquinone: a randomized clinical trial. Drug Design, Development and Therapy. 2015:9 4219–4225.
- Laurance L, Bruce A, Bjorn C. The pharmalogical basis of therapeutics. 2012; 2392-2395.
- Shum KH, et al. Hair loss in women with hyperandrogenism: four cases responding to finastride. JAM Acad Dermatol. 2002; 47:733-90
- Hong JB, et al. A woman with iatrogenic androgenic alopecia responding to finastride. Br J Dermatol. 2007; 156:754-50.
- Moghetti P, et al. Comparision of spirenolacton, flutamid, and finastride efficacy in the treatment of hirsutism: a randomized, double blind, placebo controlled trial. J Clin Endocrinol Metab. 2000; 85:89-94.
- Heydari I, Amiri a, Razmjou S, Seyfodin M.The Efficacy of Topical Finasteride in the Treatment of Idiopathic Hirsutism. J Turk Acad Dermatol. 2008; 2(2):1-4.
- Passeron T. Melasma pathogenesis and influencing factors – an overview of the latest research. J Eur Acad Dermatol Venereol. 2013; 27(1):5-6.
- Ortonne JP, Arellano I, Berneburg M, et al. A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J Eur Acad Dermatol Venereol. 2009; 23(11):1254-1262.
- Pandya AG, Hynan LS, Bhore R, Riley FC, Guevara IL, Grimes P, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011; 64(1): 78-83.
- Haj Heydari z, bahari m, Golpour M, Yazdani charati j, baes M, Mohammad Pour Ra. Effects of triple combination therapy (hydroquinone topical 5%, 4% salicylic acid, fluocinolone 01/0%) in patients with melasma. Journal of Mazandaran University of Medical Sciences, 1393; 24 (122): 361-366.
- Kodali S, Guevara I, Carrigan CR, Daulat S, Blanco G, Boker A, et al. A prospective, randomized, split-face, controlled trial of salicylic acid peels in the treatment of melasma in Latin American women. J Am Acad Dermatol. 2010; 63(6): 1030-1035.
- Mahmood K, Nadeem N, Aman S, Hameed A, Kazmi AH. Role of estrogen, progesterone and prolactin in the etiopathogenesis of melasma in females. Journal of Pakistan Association of Dermatologists. 2011; 21 (4): 241-247.
- Victor FC, Gelber J, Rao B. Melasma: a review. J Cutan Med Surg. 2004; 8: 97-102.
- Lieberman R, Moy L. Estrogen receptor expression in melasma: results from facial skin of affected patients. J Drug Dermatol. 2008; 12: 27-30.
- Espahbodi N, Shariati A, Abbasi A, Feyzi R. Compare the effect of kojic acid cream and hydroquinone in melasma. University of Medical Sciences 1387; 10 (7): 45-51
- Sarkar R, Kaur C, Bhalla M, Kanwar AJ. The Combination of Glycolic Acid Peels with a Topical Regimen in the Treatment of Melasma in Dark-Skinned Patients: A Comparative Study. Dermatol Surg. 2002; 28(9): 828-832.
- Ortonne JP, Arellano I, Berneburg M, Cestari T, Chan H, Grimes P, et al. A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J Eur Acad Dermatol Venereol. 2009; 23(11): 1254-1262.
- Kohler C, Tschumi K, Bodmer C, Schneiter M, Birkhaeuser M. Effect of finasteride 5 mg (Proscar) on acne and alopecia in female patients with normal serum levels of free testosterone. Gynecol Endocrinol. 2007; 23(3):142-5.
- Price TM, Allen S, Pegram GV. Lack of effect of topical finasteride suggests an endocrine role for dihydrotestosterone. Fertil Steril. 2000; 74: 414-415.
- Lucas KJ. Finasteride cream in hirsutism. Endocr Pract. 2001; 7: 5-10.
- Taheri A, Mansoori P, Sandoval LF, Feldman SR. Treatment of Becker nevus with topical flutamide. J Am Acad Dermatol. 2013; 69(3):147-148.
- Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009; 35(2):193-199.
- Yamaguchi Y, Morita A, Maeda A, Hearing VJ. Regulation of skin pigmentation and thickness by Dickkopf 1 (DKK1). J Investig Dermatol Symp Proc. 2009; 14(1):73–75.








