Alireza Nazari 1,5, Atlas Mashayekhi Sardoo2, Elnaz Tahmooresi Fard3, Hossein Khorramdelazad4,5, Gholamhossein Hassanshahi5 and Ali Esmaeili Nadimi6,7
1Department of Urology, Faculty of Medicine, University of Medical Science, Rafsanjan, Iran.
2School of Engineering and Design and Physical Sciences, Brunel University London, London, United Kingdom.
3Department of Biology, Faculty of Sciences, University of Guilan, Rasht, Iran.
4Department of Immunology, Faculty of Medicine Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
5Molecular Medicine Research Center, Rafsanjan University of Medical Science, Rafsanjan, Iran.
6Department of Cardiology, Faculty of Medicine, University of Medical Science, Rafsanjan, Iran.
7Occupational Environmental Research Center, Rafsanjan University of Medical Science, Rafsanjan, Iran.
Corresponding Author E-mail: dr_esmaeili_n@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1074
Abstract
Studies have shown that the soluble C-X-C chemokine ligand 16 (CXCL16), a scavenger receptor for oxidized low-density lipoproteins, plays an important role in acute coronary syndrome with atherogenic effects. This study aimed to discover the association between circulating CXCL16 levels in hemodialysis patients (HD patients) with cardiovascular disease (CVD). In this cross-sectional study, we enrolled Two hundred twenty subjects, which included 82 HD patients, 70 HD patients with CVD, and 68 healthy controls, who were referred to the Ali Ebn- Abitaleb Hospital, (Rafsanjan, Iran) from Sep 2014 to Feb 2016. Serum CXCL16 levels were measured by ELISA and other clinical parameters were examined based on standard methods. Our results indicated that serum CXCL16 levels were significantly increased in HD patients with CVD when compared with HD patients without CVD and healthy subjects (p < 0.05). Findings of this study demonstrated that the serum level of CXCL16 can be a potential predictor for the diagnosis of cardiovascular disease in HD patients.
Keywords
Cardiovascular Diseases; CXCL16; chemokine; Hemodialysis
Download this article as:| Copy the following to cite this article: Nazari A, Sardoo A. M, Fard E. T, Khorramdelazad H, Hassanshahi G, Nadimi A. E. Plasma CXCL16 Level is Associated With Cardiovascular Disease in Iranian Hemodialysis Patients. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Nazari A, Sardoo A. M, Fard E. T, Khorramdelazad H, Hassanshahi G, Nadimi A. E. Plasma CXCL16 Level is Associated With Cardiovascular Disease in Iranian Hemodialysis Patients. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=14023 |
Introduction
One of the most significant causes of morbidity and mortality in patients with chronic kidney disease (CKD), primarily among hemodialysis (HD) patients, is atherosclerotic cardiovascular disease (CVD). Mortality risk induced by CVD in end-stage renal disease patients is ten to forty times higher than that for the general population (1, 2). Accordingly, the evaluation and prevention of CVD are major goals for managing patients with end-stage renal disease (ESRD). The most common CVD risk factors: male gender, older age, systolic hypertension, diabetes mellitus, obesity, smoking, dyslipidemia, and positive family history for CVD, occur most frequently in patients with CKD, but these risk factors cannot entirely explain the elevated CVD risk for HD patients. The atherosclerotic process may be accelerated by particular metabolic changes, or other as-yet unknown factors, co-occurring with the development of renal dysfunction (3, 4).
One member of the group known as scavenger receptors is CXCL16. Based on atherogenesis studies, this chemokine appeared to be the primary receptor for oxidized low-density lipoproteins (oxLDLs) (5-7). CXCL16 has been found to be expressed by lipid-laden macrophages in the intima of atherosclerotic plaques harvested from coronary samples or carotid endarterectomies but was not discovered in normal aorta, endothelial, or smooth muscle cells (8). Several studies showed that CXCL16 could have played an essential role in the development of atherogenic effects in vivo, through endocytosis of oxLDL and activation of proinflammatory mechanisms following ligand binding (7, 9, 10). For example, in patients suffering from acute coronary syndrome, CXCL16, as one of the possible risk factors, can be associated with long-term mortality (11). Further, elevated circulating soluble CXCL16 levels are typically regarded as biomarkers for inflammation and atherosclerosis (12) and have been found in acute coronary syndromes, chronic artery disease, and acute stroke (13, 14). This type of CXCL16 (soluble CXCL16) was associated with increased serum levels of triglycerides, C reactive protein (CRP), and with lower HDL-cholesterol.
In addition, CXCL16, when expressed in podocytes, act as a scavenger receptors for oxLDL in the pathology of glomerular kidney diseases, particularly membranous nephropathy (15). For example, oxLDL was related to severe renal injury caused by generation and hypercholesterolemia after unilateral ureteral obstruction, when CXCL16 levels were increased (16, 17). The results of recent studies supported the idea that serum CXCL16 levels were significantly increased in CKD and gout subjects and were independently linked with renal dysfunction (18, 19). These findings indicated that CXCL16 played a crucial role in the pathogenesis of cardiovascular diseases in HD patients. Therefore, the main objective of the present study was to determine the relationship between CXCL16 and CVD in HD patients.
Materials and Methods
Subjects
In this cross-sectional study three groups of subjects, consisting of HD patients (n=82); HD patients suffering from CVD (n=70); and healthy volunteers (n=68) who were referred to the Ali Ebn- Abitaleb Hospital, Rafsanjan, Iran, from Sep 2014 to Feb 2016 were included in the study. The present research was performed in accordance with the guidelines of the Helsinki Declaration on Human Experimentation. This study was approved by the ethics committees on human research at the Rafsanjan University of Medical Sciences, Rafsanjan, Iran, and all of the subjects provided informed consent.
Inclusion Criteria
All patients undergoing HD received conventional dialysis treatment for 3 to 5 hours, three times a week, using a standard bicarbonate dialysis solution. Clinical evidence of subjects showed that none of them had malignant diseases or overt infections. Also, estimated glomerular filtration rate (eGFR) <50 ml/min, having two years of dialysis, no mental illness and ability to communicate were other inclusion criteria for HD patients.
The medical history of all participants were investigated to determine the existence of CVD of several different types on the basis of standard medical diagnosis criteria. First, symptomatic stroke was confirmed by magnetic resonance imaging and/or computed tomography. Second, on the basis of raised levels of cardiac enzymes, ischemic heart disease, including myocardial infarction, was diagnosed. Third, angina pectoris and its typical electrocardiography changes were determined without coronary intervention (coronary artery bypass grafting or percutaneous coronary intervention) along with elevated cardiac enzymes. Last, symptomatic peripheral vascular disease was confirmed by computed tomography angiography and/or lower-limb angiography.
Control group were selected from volunteer donors of Rafsanjan Blood Transfusion Center. None of the control subjects had a history of renal disease, hypertension, diabetes mellitus, or CVD, and none were using any medications 6 weeks before the study. Exclusion criteria for control subjects were eGFR <50 ml/min, hsCRP >1.5 mg/L, or any chronic illness likely to affect markers of inflammation.
Blood Analysis
After blood collection, separated serum was immediately frozen and stored at -80° C until the time of the assay. Serum creatinine, total cholesterol, albumin, and C-reactive protein (hs-CRP) were measured using a Hitachi 912 autoanalyser (Hitachi, Mannheim, Germany).
CXCL16 Assay
The CXCL16 serum levels were measured by ELISA (R&D system, Minneapolis, USA), according to the different groups of patients and healthy controls after blood collection. Assays were conducted according to manufacturer’s guidelines. The sensitivity of the kits was 2 pg/ml, and inter- and intra-assay assessments of reliability of the kit were conducted (CV < 15% and CV < 0.05%, respectively).
Statistical Analyses
The results were presented as the mean (±SD). Statistical analysis of the differences between groups was determined by ANOVA using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL). Furthermore, Associations between CVD and other parameters were analyzed by multivariate logistic regression analysis. Variables included in the analysis were serum CXCL16 (quantitative), level BMI (quantitative), smoking habit (yes or no), serum creatinine level (quantitative), serum hs CRP level (quantitative), and total Cholesterol (quantitative). The multivariate-adjusted odds ratios (ORs) are presented with 95% confidence intervals (CIs). A p-value ˂ 0.05 was considered statistically significant.
Results
Anthropometric parameters and the biochemical indices of HD patients without CVD, HD patients with CVD, and healthy subjects are described in Table 1. Results of this study demonstrated that e GFR and albumin were decreased significantly in patient groups in compare with control group (p<0.01). In addition, hsCRP, Systolic blood pressure were significantly increased in HD patients suffering from CVD in comparison with HD patients without CVD and normal subjects (p<0.01). There were no significant differences in total cholesterol (control: 173.7±15.9, HD patients: 215.4±19.4, and HD patients with CVD: 286.1±24.3 mg/dl),triglyceride (control: 138.2±23.9, HD patients: 142±35.4, and HD patients with CVD: 169.1±57.2 mg/dl), HDL (control: 42±17.4, HD patients: 41±13.8, and HD patients with CVD: 38±19.7 mg/dl), and LDL (control: 99±23.2, HD patients: 104±31.5, and HD patients with CVD: 121±24.3 mg/dl), between the 3 groups (Tabel1).
Table 1: Anthropometric parameters and biochemical index between HD patients, HD patients with CDV, and healthy controls.
| Control n=68 | HD
n=82 |
HD with CVD
N=70 |
P value | |
| Age (years) | 58.1±6.3 | 59.3±5.1 | 61±3.2 | 0.07 |
| Men/women (n) | 44/24 | 48/34 | 40/30 | 0.01 |
| Duration of HD (years) | 0 | 5.3±3.8 | 6.2±4.7 | 0.06 |
| Current smoker/nonsmoker (n) | 21/47 | 23/59 | 25/45 | 0.01 |
| e GFR (ml/min) | 118.3±2.91 | 56.4±2.97 | 61.3±6.81 | 0.01 |
| Creatinine (mg/dl) | 0.8±1.2 | 8.6±2.1 | 8.1±1.9 | 0.01 |
| Total cholesterol (mg/dl) | 173.7±15.9 | 215.4±19.4 | 286.1±24.3 | 0.05 |
| Triglyceride (mg/dl) | 138.2±23.9 | 142±35.4 | 169.1±57.2 | 0.07 |
| HDL (mg/dl) | 42±17.4 | 41±13.8 | 38±19.7 | 0.09 |
| LDL (mg/dl) | 99±23.2 | 104±31.5 | 121±24.3 | 0.05 |
| hsCRP (mg/L) | 1.3±0.68 | 3.9±0.91 | 6.8±2.14 | 0.05 |
| Albumin (g/dL) | 4.7±1.14 | 3.4±0.86 | 3.7±1.25 | 0.01 |
| Systolic BP (mmHg) | 114.6±3.6 | 119.7±1.6 | 142.7±8.1 | 0.01 |
NS: not significant. Results are presented as frequencies, mean±SD, or median (interquartile range) as appropriate.
Also, our data showed that fasting serum CXCL16 levels were significantly increased in HD patients with CVD (2.67 ±0.34 ng/ml) compared with healthy subjects (1.1 ±0.14 ng/ml, p < 0.05). Additionally, circulating CXCL16 in HD patients with CVD were also significantly increased compared with HD patients (1.48 ±0.21 ng/ml) (p < 0.05). (Figure 1). Additionally, as shown in table 2, an increase in CXCL16 serum level (OR, 1.00; 95% CI, 1.09 to 1.39; P 0.01) was identified as an independent factor associated with the prevalence of CVD in HD patients.
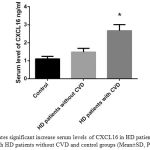 |
Figure 1: Indicates significant increase serum levels of CXCL16 in HD patients with CVD in compare with HD patients without CVD and control groups (Mean±SD, P value<0.05) |
*Significant difference between HD patients and other groups.
Table 2: Multivariate logistic regression models of some clinical parameters and CVD
| Variant | OR | 95% CI | P |
| BMI | 1.03 | 0.99 to 1.91 | 0.39 |
| Current smoker | 0.94 | 0.88 to 2.73 | 0.58 |
| Creatinine | 1.02 | 0.81 to 1.04 | 0.11 |
| hs-CRP | 0.99 | 0.95 to 1.28 | 0.41 |
| Total Cholesterol | 1.05 | 0.82 to 1.46 | 0.27 |
| CXCL16 | 1.00 | 1.09 to 1.39 | 0.04* |
– Dependent variable: CVD (Cardiovascular Disease); OR: Odds Ratio.
*Significant independent determinants.
Discussion
In this study, the aim was to explore the association between CXCL16 levels and CVD in HD patients. Present findings affirmed that the serum CXCL16 levels may reflect chronic inflammation in CVD and HD patients. Moreover, CXCL16 may be considered as a pro-inflammatory factor for diagnosis of CVD in HD patients, as supported by these findings. First, serum CXCL16 levels were significantly increased in HD patients with CVD in comparison with healthy subjects. Second, there was a significant difference in serum CXCL16 levels between HD patients and HD patients with CVD. In addition, our findings showed that the serum level of CXCL16 was identified as an independent factor associated with the prevalence of CVD in HD patients. The pathogenesis of atherosclerosis, which causes CVD, is characterized by the presence of subclinical chronic inflammation (20). Generally, high circulating soluble CXCL16 levels are regarded as an atherosclerotic and inflammatory marker (12) and have been detected in acute stroke, acute coronary syndromes, and chronic artery disease (13, 14). Lehrke et al. reported that patients with acute coronary syndrome and chronic coronary artery disease had higher plasma CXCL16 levels than controls. Soluble CXCL16 correlated with lower HDL-cholesterol and higher CRP (12). Earliest studies in humans have provided conflicting evidence about CXCL16 and its roles in atherosclerosis. For instance, Sheikine and colleagues showed that in post-MI patients, there were no significant associations between CXCL16 levels and different measures of coronary artery disease (CAD) severity determined by quantitative coronary angiography. Also, there were no significant correlations between plasma lipoproteins panel and CXCL16 levels, C-reactive protein inflammatory cytokines in patients and normal subjects (21). Despite conflicting reports, our findings provide support for CXCL16 as a pro-inflammatory factor in patients with CVD. Additionally, chronic inflammation is a frequent characteristic of CKD, and approximately 30% to 50% of predialysis, HD, and peritoneal dialysis patients have serologic indications of an activated inflammatory response (22). Serum levels of CRP, the prototypical acute phase reactant, have been shown to be principally high in patients with renal function failure (23). Recently, findings of cohort research on more than 1,000 patients with CKD followed over 2.5 years showed that the highest CRP quartile was related with a two-fold increase in the risk of cardiac death (24). Thus, chronic inflammation was closely linked to atherosclerosis in HD patients (25).
CXCL16 has been extensively studied in human cardiovascular disease in patients without CKD, but this research program was the first study to compare the serum levels of CXCL16 within different groups of patients suffering from both CVD and HD patients (21, 26, 27). Nevertheless, no co-staining was provided to clearly establish which cells expressed this chemokine, and no image for normal vessels was presented. Consistent with our results, Lehrke et al. showed that patients with acute coronary syndrome had lower plasma CXCL16 than those with chronic coronary artery disease, and both groups had higher levels than healthy subjects. In addition, results of the previously mentioned study indicated that soluble CXCL16 correlated with lower HDL-cholesterol, higher triglycerides, and higher CRP (12).
In another case-controlled study, both stable and unstable angina patients’ levels of CXCL16 were increased compared to healthy controls (28). Plasma CXCL16 steadily increased in the first days after acute ischemic stroke. These findings reemphasized that an increase in CXCL16 from days one to four after a stroke can be a predictor for all-cause and cardiovascular mortality in stroke patients (14). Conversely, few studies reported lower levels of CXCL16 in patients with CVD (21, 27). There was rare data on circulating CXCL16 during human kidney injury, but most studies showed that kidney, urinary, or circulating CXCL16 were amplified in various stages of human kidney injury (29-31). For example, Lin et al. reported that circulating CXCL16 was higher in CKD patients compared with healthy controls. In fact, plasma CXCL16 levels were 2.5 times higher in CKD subjects than in healthy controls. Further, urinary CXCL16 was inversely correlated with creatinine clearance and positively correlated with CRP and CXCL16 plasma levels (32). These findings revealed that CXCL16 levels increased in HD and CKD patients; therefore, checking such patients for heart problems, specifically CVD, should be routinely performed. Main strengths and weaknesses of this cross-sectional study were comparison of the serum levels of CXCL16 in HD patients with and without CVD in comparing to control for the first time and difficulty in interpretation of associations identified, respectively.
Conclusions
Finally, based on previous studies and our findings, CXCL16 can be considered as a pro-inflammatory factor when evaluated in the circulation or in urine in a variety of pathogenic states including kidneys or the vasculature injury. Though, to date, no standardized methodology or cut-off values to measure CXCL16 are existing. This matter should be addressed before any try to testing the biomarker potential of CXCL16 to influence decision-making and outcome in clinical trials. Furthermore, Interventional and prospective studies are needed to further clarify the association between plasma CXCL16 levels and CVD in CKD and HD patients.
Acknowledgments
Authors of the current article take this opportunity to thank all of the patients who warmly co-operated in this research program. This project was financially supported by a grant from Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
References
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108(17):2154-69.
CrossRef - Collins AJ. Cardiovascular mortality in end-stage renal disease. The American journal of the medical sciences. 2003;325(4):163-7.
CrossRef - Kendrick J, Chonchol MB. Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nature Clinical Practice Nephrology. 2008;4(12):672-81.
CrossRef - Wyatt CM, Shineski M, Chertow GM, Bangalore S. ISCHEMIA in chronic kidney disease: improving the representation of patients with chronic kidney disease in cardiovascular trials. Kidney international. 2016;89(6):1178-9.
- Chung AC, Lan HY. Chemokines in renal injury. Journal of the American Society of Nephrology. 2011;22(5):802-9.
- Xia Y, Entman ML, Wang Y. Critical role of CXCL16 in hypertensive kidney injury and fibrosis. Hypertension. 2013;62(6):1129-37.
CrossRef - Moreno JA, Moreno S, Rubio-Navarro A, Sastre C, Blanco-Colio LM, Gómez-Guerrero C, et al. Targeting chemokines in proteinuria-induced renal disease. Expert opinion on therapeutic targets. 2012;16(8):833-45.
- Minami M, Kume N, Shimaoka T, Kataoka H, Hayashida K, Akiyama Y, et al. Expression of SR-PSOX, a novel cell-surface scavenger receptor for phosphatidylserine and oxidized LDL in human atherosclerotic lesions. Arteriosclerosis, thrombosis, and vascular biology. 2001;21(11):1796-800.
- Norlander AE, Saleh MA, Madhur MS. CXCL16 A Chemokine-Causing Chronic Kidney Disease. Hypertension. 2013;62(6):1008-10.
- Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circulation research. 2004;95(9):858-66.
- Shimaoka T, Kume N, Minami M, Hayashida K, Kataoka H, Kita T, et al. Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. Journal of Biological Chemistry. 2000;275(52):40663-6.
CrossRef - Lehrke M, Millington SC, Lefterova M, Cumaranatunge RG, Szapary P, Wilensky R, et al. CXCL16 is a marker of inflammation, atherosclerosis, and acute coronary syndromes in humans. Journal of the American College of Cardiology. 2007;49(4):442-9.
CrossRef - Sun Y, Chang Z, Zhang S. Increased serum CXCL16 level is a marker for acute coronary syndromes. Archives of medical research. 2008;39(3):332-7.
CrossRef - Ueland T, Smedbakken L, Hallen J, Atar D, Januzzi J, Halvorsen B, et al. Soluble CXCL16 and long-term outcome in acute ischemic stroke. Atherosclerosis. 2012;220(1):244-9.
CrossRef - Gough PJ, Garton KJ, Wille PT, Rychlewski M, Dempsey PJ, Raines EW. A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. The Journal of Immunology. 2004;172(6):3678-85.
CrossRef - Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, et al. A new class of membrane-bound chemokine with a CX3C motif. 1997.
- Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo J-A, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387(6633):611-7.
CrossRef - Schulte A, Schulz B, Andrzejewski M, Hundhausen C, Mletzko S, Achilles J, et al. Sequential processing of the transmembrane chemokines CX3CL1 and CXCL16 by α-and γ-secretases. Biochemical and biophysical research communications. 2007;358(1):233-40.
CrossRef - Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-γ and TNF-α and shed by the activity of the disintegrin-like metalloproteinase ADAM10. The Journal of Immunology. 2004;172(10):6362-72.
CrossRef - Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Atherosclerosis as an inflammatory disease. Current pharmaceutical design. 2012;18(28):4266-88.
CrossRef - Sheikine Y, Bang CS, Nilsson L, Samnegård A, Hamsten A, Jonasson L, et al. Decreased plasma CXCL16/SR-PSOX concentration is associated with coronary artery disease. Atherosclerosis. 2006;188(2):462-6.
CrossRef - Stenvinkel P. Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood purification. 2000;19(1):53-61.
CrossRef - Stenvinkel P, Alvestrand A, editors. Review Articles: Inflammation in End‐stage Renal Disease: Sources, Consequences, and Therapy. Seminars in dialysis; 2002: Wiley Online Library.
- Parekh RS, Plantinga LC, Kao WL, Meoni LA, Jaar BG, Fink NE, et al. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney international. 2008;74(10):1335-42.
CrossRef - Hage FG, Venkataraman R, Zoghbi GJ, Perry GJ, DeMattos AM, Iskandrian AE. The scope of coronary heart disease in patients with chronic kidney disease. Journal of the American College of Cardiology. 2009;53(23):2129-40.
CrossRef - Ørn S, Breland UM, Mollnes TE, Manhenke C, Dickstein K, Aukrust P, et al. The chemokine network in relation to infarct size and left ventricular remodeling following acute myocardial infarction. The American journal of cardiology. 2009;104(9):1179-83.
CrossRef - Mitsuoka H, Toyohara M, Kume N, Hayashida K, Jinnai T, Tanaka M, et al. Circulating soluble SR-PSOX/CXCL16 as a biomarker for acute coronary syndrome-comparison with high-sensitivity C-reactive protein. Journal of atherosclerosis and thrombosis. 2009;16(5):586-93.
CrossRef - Smith C, Halvorsen B, Otterdal K, Wæhre T, Yndestad A, Fevang B, et al. High levels and inflammatory effects of soluble CXC ligand 16 (CXCL16) in coronary artery disease: down-regulatory effects of statins. Cardiovascular research. 2008;79(1):195-203.
CrossRef - Schramme A, Abdel-Bakky MS, Gutwein P, Obermüller N, Baer PC, Hauser IA, et al. Characterization of CXCL16 and ADAM10 in the normal and transplanted kidney. Kidney international. 2008;74(3):328-38.
CrossRef - Gutwein P, Abdel-Bakky MS, Schramme A, Doberstein K, Kämpfer-Kolb N, Amann K, et al. CXCL16 is expressed in podocytes and acts as a scavenger receptor for oxidized low-density lipoprotein. The American journal of pathology. 2009;174(6):2061-72.
CrossRef - Gutwein P, Abdel‐Bakky MS, Doberstein K, Schramme A, Beckmann J, Schaefer L, et al. CXCL16 and oxLDL are induced in the onset of diabetic nephropathy. Journal of cellular and molecular medicine. 2009;13(9b):3809-25.
- Lin Z, Gong Q, Zhou Z, Zhang W, Liao S, Liu Y, et al. Increased plasma CXCL16 levels in patients with chronic kidney diseases. European journal of clinical investigation. 2011;41(8):836-45.
CrossRef








