Arnold V. Popkov, Dimitry A. Popkov, Natalia A. Kononovich and Elena N. Gorbach
Russian Ilizarov Scientific Center for Restorative Traumatology and Orthopedics Ministry of Health of the Russian Federation 640014, M.Ulyanova Street 6, Kurgan, Kurgan region, Russian Federation.
DOI : https://dx.doi.org/10.13005/bpj/1078
Abstract
The purpose of the study is providing rationale for the optimal type and structure of an extramedullary implant to achieve its rapid osseointegration and, consequently, to reduce the time required for bone fracture union. In an experimental study on 10 mongrel dogs, extramedullary osteosynthesis of tibia was done with 1.0 mm thick titanium alloy (Ti6Al 4V) plates having either solid or perforated structure, and the osseointegration process was compared in animals with an implant with no bioactive coating with those with hydroxyapatite (HA) on the implant surface. The plates were fixed to the tibia under the periosteum. Radiological, anatomical and histological studies demonstrated that the process of osseointegration of a perforated implant with a bioactive coating actively begins at Days 7-14 first with formation of granulation tissue and then followed by formation of fibrous connective tissue, so by Day 28 the entire implant area is covered with tissue substrate, having the signs of osteogenesis, which connects an extramedullary implant and bone surface into a single implant-tissue segment. Fixation of perforated implants with a bioactive coating under the periosteum stimulates reparative osteogenesis and rapid implant osseointegration to achieve consolidation of bone fragments based on the principle of the primary bone union.
Keywords
Extramedullary osteosynthesis; hydroxyapatite coating; osseointegration; additive technology
Download this article as:| Copy the following to cite this article: Popkov A. V, Popkov D. A, Kononovich N. A, Gorbach E. N. Osseointegration of A Bioactive Implant in Extramedullary Osteosynthesis. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Popkov A. V, Popkov D. A, Kononovich N. A, Gorbach E. N. Osseointegration of A Bioactive Implant in Extramedullary Osteosynthesis. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13829 |
Introduction
The injury rate has been found to be 872±6.3 cases per 10,000 in the Russian Federation over last three decades and has not tended to decline [10, 2]. The severity of traumas is the main cause for failures in restorative processes, results in permanent disability and reduces quality of life in subjects with traumas. Among the causes of primary disability, consequences of traumas rank third after cancer and cardiovascular diseases [1, 3, 8] and rank first in the working-age group demonstrating a steady upward trend [11, 8].
Subjects with limb fractures account for the largest group among people disabled due to traumas – 67.9%. At the Ninth Russian Congress of Trauma Orthopedists, I.V. Shvedovchenko et al named drawbacks of outpatient therapy (33.3%) and inpatient therapy for patients and disabled people (14.6%) as the reasons for post-traumatic disability. The method of extramedullary osteosynthesis is applied in 13.2% of cases.
A large number of various plates to be used in different areas of bones as well as special tools have been designed based on AO/ASIF philosophy. When neutralizing plates are used, the main load falls on a fixation device which shunts the load onto the fracture surface. This leads to a number of negative consequences: osteoporosis develops in an unloaded area of the bone, efficiency of osteoreparation reduces in the fracture area, and fracture risk increases for plates and screws, even though plates may be 2.5 to 8 mm thick [14, 15, 17]. The disadvantages of extramedullary osteosynthesis include excessive pressure which the plate exerts on the periosteum causing necrosis and reducing osteogenic capacity thereof. Even emergence of massive extramedullary plates with a bioactive coating could not help to reduce the time required for fracture union [6].
A hypothesis was proposed to address the said disadvantages of modern extramedullary implants: rapid implant osseointegration is required to ensure maximum stability of osteosynthesis. To achieve osseointegration, an implant should be preferably placed under the periosteum, and thickness of the implant should be reduced to 1 mm. Reduced strength of such implant can be improved with a two-plane plate when inner surfaces completely follow the peculiarities of the outer surface of a broken bone. Production of such implant is possible with an additive technology of laser sintering of titanium powder accompanied with computer modeling of the bone surface after computed tomography (CT) of subject’s damaged limb [5].
The purpose of this Study
is to provide rationale for the optimal type and structure of the extramedullary implant to achieve its rapid osseointegration and, consequently, to reduce the time required for bone fracture union.
Material and Methods
The experiments studied the osseointegration process on 10 mongrel dogs of both sexes aged 1 to 3 years and body mass of 20±2.9 kg. One-millimeter thick titanium alloy (Ti6Al 4V) plates (20×10 mm) were implanted to anesthetized animals under periosteum; some plates had solid structure (3 plates), while the others were perforated (10 plates). The study compared the osseointegration process in animals with implants with no bioactive coating and in those with implants with a hydroxyapatite (HA) coating on the surface of the implant fixed with a microarc oxidation technology (MAO). The plates were fixed onto the tibia with two screws. After the surgery, cefazolin was administered intramuscularly for 7 days (0.5 g b.i.d.). Wounds were monitored on a daily basis.
The animals were observed for 7 to 28 days after the surgery. The osseointegration process was monitored with X-ray and anatomical dissection methods.
The X-ray system PremiumVet (Sedecal, Spain) was used for X-ray studies over time.
Tissue substrate formed on implanted plates at Day 7, 14, and 28 days of the experiment was studied using light and scanning electron microscopy.
Microscopic sections stained with hematoxylin and eosin using Van Gieson and Masson methods were studied with the stereo microscope AxioScope.A1 and the digital camera AxioCam ICc 5 supplied with the software package Zen blue (Carl Zeiss MicroImaging GmbH, Germany).
A tissue-implant segment dried using the original technology [7] was studied with the X-ray electron probe microanalyzer INCA-200 mounted on the scanning electron microscope JSM-840. Calcium and phosphorus were measured in tissue matrix on the surface of the plate.
The study results were processed by the methods of nonparametric statistics. The significance of differences between two samples was assessed using Wilcoxon W-test for independent samples.
The experiments were performed in accordance with the “European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes” (Strasbourg, 1986) and were approved by the Ethics Committee of the federal state budget institution “Academician G.A. Ilizarov Russian Research Center for Restorative Traumatology and Orthopedics”.
Results
The clinical examinations during the experiment showed neither changes in the general condition of animals nor deviations in food and water consumption. No neurological or infectious complication was reported. The supporting function of the test segment retained until the end of the experiment. Implants remained fixed during the experiment, and no implant displacement was observed.
The signs of osseointegration, such as a thin (0.5 mm) layer of mineralized tissue on the outer surface of the plate, were observed by X-ray imaging during a 28-day fixation period. This pattern was observed in animals with perforated plates coated with a bioactive hydroxyapatite using MAO technology. No X-ray signs of osseointegration were found during this observation period in animals with non-perforated plates.
The anatomical dissection around the plate with a solid structure showed no signs of osseointegration on the side of the periosteum (Fig. 1a).
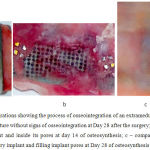 |
Figure 1: Anatomic preparations showing the process of osseointegration of an extramedullary plate
|
a – a titanium implant with a solid structure without signs of osseointegration at Day 28 after the surgery; b – granulation tissue on the surface of an implant and inside its pores at day 14 of osteosynthesis; c – compact substance of the bone covering an extramedullary implant and filling implant pores at Day 28 of osteosynthesis
Anatomical dissection of extramedullary osteosynthesis with perforated plates demonstrated granulation tissue filling pore spaces and restoring direct binding of the periosteum with compact substance of the tibia (Fig. 1b) as early as Day 7-14.
The osseointegration process was most active around perforated plates with a bioactive hydroxyapatite coating (Fig. 1c). A plate was covered with a thin layer of connective tissue at Day 14, and this layer became thicker and denser and covered the entire area of the implant by Day 28.
Histological studies showed that granulation tissue with signs of loose connective tissue on outer areas (Fig. 2) was formed by Day 7. In the panel of experiments with non-perforated solid plate, the tissue was less organized in the contact area (Fig. 2a), while the tissue around an HA-coated plate demonstrated a larger number of capillary vessels with extended openings (Fig. 2b) and inclusions of fragments of hydroxyapatite coating. In the panel of experiments with perforated plates, the connective tissue was better structured and more vascularized, and these signs were even more distinct in the panel of experiments with HA-coated plates (Fig. 2c and d).
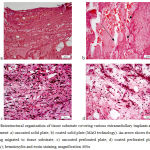 |
Figure 2: Histostructural organization of tissue substrate covering various extramedullary implants at Day 7 of the experiment: a) uncoated solid plate; b) coated solid plate (MAO technology).
|
An arrow shows fragments of HA coating migrated to tissue substrate; c) uncoated perforated plate; d) coated perforated plate (MAO technology); hematoxylin and eosin staining, magnification 400x
At Day 14, the panel of experiments with HA-coated perforated plates showed the presence of single poorly mineralized trabeculae of bone in the area of contact with the surface of the plate (Fig. 3c). The outer layers demonstrated loose fibrous connective tissue having the structure which resembled that of the periosteum. In two other panels of experiments (with non-perforated plates and with uncoated perforated plates) no trabeculae of bone were observed. In the panel with a HA-coated solid plate, a larger number of microvessels were observed.
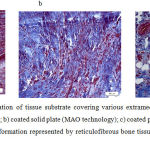 |
Figure 3: Histostructural organization of tissue substrate covering various extramedullary implants at Day 14 of the experiment: a) uncoated solid plate; b) coated solid plate (MAO technology); c) coated perforated plate (MAO technology).
|
An arrow shows trabecula under formation represented by reticulofibrous bone tissue. Hematoxylin and eosin staining, magnification 400x.
At Day 28, the difference between panels of the experiment was more apparent. Histological preparations examined by light microscopy showed that a tissue layer formed on the surface of a solid non-perforated extramedullary plate had loose fibrous vascularized connective tissue. There were no signs of osteogenesis on the surface of the plate; however, the connective tissue layer was more vascularized and thickened in animals with solid non-perforated HA-coated plates.
In studies with perforated plates, reticulofibrous bone tissue (Fig. 4) was observed on the surface thereof in the contact area with tissue substrate and inside perforation openings. HA-coated plates demonstrated larger amounts of this tissue. Loose fibrous vascularized connective tissue was observed in inter-trabecular spaces in all animals, including hydroxyapatite fragments in animals with HA-coated plates.
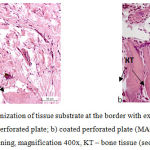 |
Figure 4: Histostructural organization of tissue substrate at the border with extramedullary implants at Day 28 of the experiment: a) uncoated perforated plate; b) coated perforated plate (MAO technology).
|
Hematoxylin and eosin staining, magnification 400x, KT – bone tissue (see arrows).
Mapping of Ca, P and Ti distribution in tissue substrate by X-ray electron microprobe analysis of implant-tissue segments showed that bone tissue was more mineralized on the implant surface in experiments with HA coated plates (coated by MAO technology) (Fig. 5).
 |
Figure 5: Specifics of structural organization and mineralization of tissue substrate covering perforated extramedullary implants at Day 28 of the experiment. The top row shows an uncoated perforated plate, the lower row demonstrates a coated plate (MAO technology).
|
A, e – SEM of the implant material before surgery, magnification 170x. B, f – SEM of the implant material, electronic images with tissue substrate on the implant surface, magnification 1,700x. C, g – electronic images with tissue substrate in implant pores. SEM, magnification 170x. D, h – aligned image of tissue substrate formed on the implant surface and inside implant pores made by the electron X-ray microanalyzer. Images are taken in typical characteristic radiation for Са, Р, and Тi. Са – red, Р – green, Ti – blue, magnification 170x.
The said electron X-ray probe microanalysis showed that the most mineralized bone substrate was formed on the surface of HA-coated perforated plates (MAO technology), while the minimum content of osteotropic elements was observed in tissue substrate on uncoated solid titanium plate (Table 1).
Table 1: Content of Ca and P in tissue matrix on the plate surface at Day 28 of the experiment
| Type of an extramedullary plate | Contents (W), Wt% | ||
| Са | Р | Са/Р | |
| Uncoated solid plate | 0.71±0.024 | 0.99±0.029 | 0.72±0.031 |
| HA-coated solid plate | 0.98±0.035 | 1.24±0.037 | 0.79±0.035 |
| Uncoated perforated plate | 1.2±0.041 | 1.71±0.044 | 0.7±0.019 |
| HA-coated perforated plate (MAO technology) | 4.1±0.191 | 4.27±0.178 | 0.96±0.033 |
All values demonstrate a statistically significant difference from each other (р≤ 0.05).
Discussion
The basic principle of modern extramedullary osteosynthesis is to provide mechanical immobility of bone fragments. That is why implants (biomaterials) are made rigid enough and are produced from a durable metal alloy and of significant thickness. Further solutions to enhance strength of an implant and durability of osteosynthesis, such as increasing implant thickness, using reinforcing ribs, either half, third, or quarter-tubular (varying by bending degree of plate plane along the axis of the fixation device), partially restricted contact with the periosteum, a second row of openings, compressive oval openings, angular stability of a screw etc., do not affect the process of reparative regeneration of a bone, do not guarantee osseointegration of an implant and do not reduce the time required for fracture union.
When assessing implants by their impact on bone reparative ability, it should be noted that there is no bioactive material among metals. Typical bioactive materials include bio glass (the most frequently used composition is 24.5% Na2O, 24.5% CaO, 45.0% SiO2, and 6% P2O5) and hydroxyapatite-based materials, such as Ca10(PO4)6(OH)2 (dense and porous ceramics).
However, such bioactive materials are fragile and, when used in their pure form, are considerably inferior to metal materials in terms of strength. The solution was to produce hydroxyapatite-coated metal implants [4, 9]. Calcium phosphate ceramics is characterized by formation of very close chemical bonding with a bone (binding osteogenesis) [18, 13]. It boosts bone forming reactions starting from the implant surface and induces formation of a continuous binding from tissue to its surface (the osseointegration process).
The significant regenerative potential of bone tissue should be noted here. Restoration of lost bone tissue takes place at organ’s special sites, a kind of regeneration centers, and the periosteum is one of them. Therefore, reduction in its reparative capacity due to compression caused by extramedullary implants is unacceptable, as this leads to blood circulation disorder and necrosis, bone atrophy, early osteoporosis and slows down the consolidation process.
To prevent such negative effect, the implant should be placed under the periosteum. For this purpose, the thickness of the implant should be minimized, and the structure must be perforated, so the blood supply from the periosteum could be quickly restored and its osteoinductive capabilities could be used.
In our opinion, rapid osseointegration of an implant, as described above, is associated with structured nature of its surface, HA induction effect on cells of the cambial layer, the periosteum formed on the surface of the plate and having inducible properties and presence of perforations (500-600 mm in diameter), in which blood vessels grow from both sides: the newly formed periosteum and the bone whereon the plate is mounted. Mineralized bone matrix formed on surfaces and inside perforations of the extramedullary implant shows osseointegration of bone tissue into plate structures thereby contributing to the formation of a single implant-tissue segment. A plate with perforations only (with no hydroxyapatite coating) provided formation of mineralized bone matrix to a lesser extent due to the lack of stimulating effect of hydroxyapatite.
Conclusion
The philosophy of internal (submersible) osteosynthesis, especially today, attempts to uphold the concept of stable fixation, early activation and functionality that may not be fully applicable to the current level of osteosynthesis. Further development of this type of osteosynthesis must meet the principle of customized production of thin implants using an additive technology and chemically inert materials with a bioactive coating and perforated structure. Fixation of such implants under the periosteum should stimulate reparative osteogenesis and rapid osseointegration of the implant to achieve consolidation of bone fragments based on the principle of the primary bone union.
Acknowledgement
The study is prepared with the financial support of the Russian Science Foundation (project No. 16-15-00176).
Disclosure Statement
The authors do not have any conflicts of interest to declare.
References
- Akhmetianov, R.F. 2005. Features of primary disability due to injuries and other external causes in the Russian Federation. Medical-Social Examination and Rehabilitation 1: 37-40.
- Kuvakin, V.I., Black, A.Z., Vorontsov, T.N. 2013. Retrospective analysis of injury and state trauma and orthopedic care to the population at the turn of XX-XXI centuries. Herald of the Russian Military Medical Academy 3(43): 1-5.
- Kupkenov, D.E. 2010. Use of core machines with diaphyseal fractures of the tibia. Traumatology and Orthopedics of Russia 2(56): 39-44.
- Petrovskaya, T.S., Shakhov, V.P., Vereshchagin, V.I., Ignatov, V.P. 2011. Biomaterials and implants for trauma and orthopedics. Tomsk: Publishing house of Tomsk Polytechnic University, 307 p.
- Popkov, A.V., Popkov, D.A. 2016. Extramedullary personalized bioactive implant for long bones. RU№166786 patent (Application №2016109513 (014,997), on 16.03.2016 (The positive decision of 25 July 2016).
- Popov, V.P., Zdrelko, V.P., Trukhachev, I.G., Popov, A.V. 2014. Complications of osteosynthesis in patients with long bone fractures. Genius of Orthopedics 2: 5-9.
- Silantieva, T.A., Gorbach, E.N. 2015. Preparation of samples for the study of biological tkney scanning electron microscope using camphene. Fundamental Research 2-22: 4919-4923.
- Tatarnikov, M.A. 2006. Milestones and prospects of Russian health reforming. Medical Director 12: 29-39.
- Tverdohlebov, S.I., Ignatov, V.P., Stepanov, I.B., Sivin, D.O., Petlin, D.G. 2012. The hybrid method of forming on the surface biocomposite implants made of stainless steel. Biotechnosphere 5-6(23-24): 63-69.
- Tikhilov, R.M. 2012. Status of orthopedic injuries and diseases of the adult population of St. Petersburg in 2009-2011. and the work of city trauma and orthopedic services. Traumatology and Orthopedics of Russia 4: 110-119.
- Khanapiyaev, U.B., Asamov, M.S., Shodiev, V.U. 2000. Influence of immunomodulating on the immune status of patients with open fractures of the tibia. Orthopedics, Travmatolgiya and Prosthetics 3: 82-84.
- Shvedovchenko, I.V., Shestakov, V.P., Lebedeva, N.N., Nikitchenko, I.I., Svintsov, A.A. 2010. Disability due to injuries and diseases of the musculoskeletal system and ways of its prevention in the Russian Federation. Abstracts of IX Congress of Orthopaedic Trauma of Russia 3: 1043-1044.
- Barrere, F., van der Valk, C.M., Dalmeijer, R.A. et al. 2003. Osteogenecity of octacalcium phosphate coatings applied on porous metal implants. Journal of Biomedical Materials Research 66A: 779-788.
CrossRef - Helfet, D.L., Haas, N.P., Schatzker, J., Matter, P., Moser, R., Hanson, B. 2003. AO philosophy and principles of fracture managmentits evolution and evaluation. Journal of Bone and Joint Surgery. American Edition 85: 1156-1160.
- Perren, S.M., Matter, P. 2003. Evolution of AO philosophy. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca 70/4(205-6), 0001-5415.
- Tverdokhlebov, S.I., Bolbasov, E.N., Shesterikov, E.V., Malchikhina, A.I., Novikov, V.A., Anissimov, Y.G. 2012. Research of the surface properties of the thermoplastic copolymer of Vinilidene Fluoride and Tetrafluoroethylene modified with radio-frequency magnetron sputtering for medical application. Applied Surface Science 263: 187-194.
CrossRef - Smith, W.R., Stahel, P.F., Morgan, S.J., Trafton, P.G. 2013. Trauma of lower extremity. Moscow: Panfilov Publishing house.
- Yuan, H., van den Doel, M., Li, S.H. et al. 2002. Comparison of the osteoinductive potential of two calcium phosphate ceramics implanted intramuscularly in goats. Journal of Materials Science: Materials in Medicine 13: 1271-1275.
CrossRef







