Wejdan R. Taj Al-daan, Shahad Aabed Al-Redha Ali and Anwar kadhim Al-Saffar
Biology department, college of science, University of Babylon.
DOI : https://dx.doi.org/10.13005/bpj/1088
Abstract
Thirty five E .coli isolates were collected from patient suffering from long term urinary tract catheters and urinary tract infection for both genera with age ranging between (11-60) years. The isolates were identified according to cultural , microbiological, biochemical testes.Detection bacteriocin production by E. coli were showed only (5) isolates of E coli produced bacteriocin. To esteemed effect of P. cubeba fruits extract on bacteriocin production from E. coli exposed to this extract, the results showed did not changing in bacteriocin production but the extract caused changes in the antimicrobial activity between bacteriocin produced before and after treated bacteria with P. cubeba fruits extract.In the case of bacteria cultured without the extract, protein concentration of partially purified bacteriocin (by ammonium sulphat) was (9.21mg/ml) while bacteriocin protein concentration of bacteria grown with the extract was(10.6mg/ml). The statistical analysis of result did not show significant differences and "p value " was 0.177. SDS PAGE reveal that bacteriocin have molecular weight (25KDa) and identified as Colicin.
Keywords
Escherichia coli; Piper cubeba; Bacteriocin; plant extract
Download this article as:| Copy the following to cite this article: Al-daan W. R. T, Ali S. A. AR, Al-Saffar A. K. Effect of Piper Cubeba Fruits Extract on Bacteriocin Production of E. Coli Isolated from Patient With urinary Tract Infection. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Al-daan W. R. T, Ali S. A. AR, Al-Saffar A. K. Effect of Piper Cubeba Fruits Extract on Bacteriocin Production of E. Coli Isolated from Patient With urinary Tract Infection. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13874 |
Introduction
Virulent bacteria are capable to create molecules that dynamically reduce the immune response of the host, so increased bacterial persistence and tissue damage. The virulence factors encoding genes of uropathogenic E. coli are localized on chromosomal gene clusters called “pathogenicity islands” [1; 2]. Virulence factors have a vital role in determining the invading of organism to the urinary tract and the level of infection. Uropathogenic E.coli (UPEC) infect the urinary tract via expressing specific virulence factors that allow adherence and colonization of the lower urinary tract [3 ; 4]. Bacteriocin is one of these virulence factors.which is Proteinaceous toxins that produced via bacteria and have the ability to inhibit the growth of similar or closely related bacterial strain [5].Bacteriocins, a member of the narrow-spectrum toxins are described as the “microbial weapon of choice”, due to their abundance and diversity among producing bacteria[6]. They are similar to killing factors of yeast and paramecium but are structurally, functionally, and ecologically different. Applications of bacteriocins are tested to evaluate its application as a narrow-spectrum antibiotics [5]. They are classified in different ways, such as producing strain, mechanism of resistance, mechanism of killing.There are large class of bacteriocin that are only phenomenologically related. These classes are the bacteriocins from gram-positive bacteria, colicins, [7] microcins, and the bacteriocins from Archaea.[8].E . coli produce two kind of bacteriocins, classified in dependent on their molecular weight into colicins (25-80 kDa) and microcins (10 kDa). Colicins and microcins are alike in numerous ways, but microcin synthesis is not lethal to the producing strain additional, and all colicins are encoded by plasmid, while genes encoding to microcin found on the chromosome.[7]
Recently, attention to medicinal plant studies that focusing on inhibition of as a target activity is increase, several bioassays to assess virulence factors have develop for number of microorganisms, especially bacteria and yeast. This is an important source of molecules to investigate new anti-virulence factors mechanisms of microbes.many medicinal plant metabolites have antimicrobial activity [9; 10]. Traditional medicine Practitioners think that the components of plants are unique because of they contain both active ingredients and “non active” components that are play a role in enhancing the well being of their patients.[11]. numerous virulence factors can be neutralized via plant compounds. A broad field of studies on this subject is further on, science advances in phytochemistry and molecular microbiology providing new features that will end in virulence factors based new therapy strategies. [12].Piper cubeba (cubeba) or the tailed pepper is a member of genus Piper mostly known as, tailed pepper (because of the stalks attached), Java pepper (in Java) and kemukus (in Indonesia),[13]. Cubeba is a perpetual plant, with climbing stem, round branches, ash colored and it leaves are from 4 to 6, so soft Flowers have a spikes shape at the end of the branches with ovate-oblong, acuminate.Cubeba is one of the popular medicinal plants [14]. It is used to treat genitourinary diseaseKidney and Bladder calculi [15]. gonorrhea dysentery, syphilis, abdominal pain and asthma[16]. also it use as gastroprotactive [17]. The effect of Piper cubeba extract on Bacteriocin of uropathogenic isolates have notbeen compared before so in this study, we have been detection the effect of P. cubeba extracts on Bacteriocin of uropathogenic Escherichia coli.
Material and Methods
Collection and Drying of Piper Cubeba
Piper cubeba fruits were collected from the local markets of Babylon Province then it was washed three times by D.W. P. cubeba furits were dried by using oven at 55°C for 5 hours. The powdered samples were stored in a clean container until the time of the extraction.
Extract Preparation
Hot water were used to prepare the extract of P. cubeba fruits. An amount of 30g. of fruitso in100ml of hot water (100 c◦) and adjust to magnetic stirrer for 5h .Then filtered through a sterilized whatman No.1 filter paper [18, 19 ,20] Filtered extracts were air dried at 40˚C for 48 h. then stored in labeled sterile container in a deep freeze at -18˚C until further use[21].
Phytochemical Analysis of P. Cubeba
Hot aqueous extract were tested chemically to identify its chemical compounds according to[22 ]
Bacterial strain
In the present study 35 E.coli isolates were collected from patient suffering from long term urinary tract catheters and urinary tract infection for both genera with age ranging between (11-60) years in Babylon Province, Iraq during a period from September 2015 to February 2016. isolates were identified according to morphology , microscopic examination and biochemical tests. Bacterial cultures were maintained on nutrient broth as a basal medium, supplemented with 15% glycerol. and kept at 4˚C until used[23].
Escherichia. coli was grown with (25 mg/ml) MIC concentration of P. cubeba hot aqueous extract at 37c for 24h in tests of detection Bacteriocin production (Antibacterial activity and MIC concentration of hot aqueous extract of P. cubeba fruits was published in other research)
Production and Extraction of Crude Bacteriocin
To determine bacteriocin production, E.coli grown in 400ml m63 broth at 37C for 24 h in triplicates The, cultures were centrifuged at 4,400 rpm for 15 min at 4°C and filtrated through 0.22μm Millipore filter [24], The crude bacteriocin were then assayed using well diffusion method.
Precipitation of Bacteriocin
Bacteriocin precipitate from crud extraction by ammonium sulphat with saturated ratio 80%. This method was done according to method of [25] as the followed:
Ammonium sulphat 51.6g was gradually added to 100ml of crud extraction
(CE) of bacteriocin protein with continuous mixing by magnetic stirrer at 150m for 10 min at 4c .
After that the solution was centrifuged at 10000rpm for 15min, the supernatant was removed and the sediment was used.
Phosphate buffer saline 5ml was added to the sediment (bacteriocin).
Measurement of Bacteriocin Protein Concentration
Bradford method[26] was used to measure bacteriocin protein concentration
Antimicrobial Assay of Bacteriocin
The antibacterial action of bacteriocin isolated from E coli determined by using the well diffusion method. Amount of 50 μL of the supernatant were placed in wells with 4mm diameter on Mueller-Hinton agar plates that cultured previously with the indicator bacteria. Diameters of the zones of growth inhibition were measured after 12-18 h of incubation.[27].
Electrophoretic Separation of Bacteriocin
Bacteriocin extracts were electrophoresed on SDS-PAGE according to [28].
Result
In the present study 35 E. coli isolates were collected from patient suffering from long term urinary tract catheters and urinary tract infection whose did not take any drug and for both genera with age ranging between (11-60) years during a period from September 2015 to February 2016 All samples were cultured on nutrient MacConkey and Blood agar plates then it was incubated at 37 for18- 24 hours. Identification of pure isolate was done by observing morphological, cultural and biochemical characters according to[29]. phytochemical screening of hot aqueous P. cubeba extract showed it was containing glycosides, phenols, Flavonoids and tannin. coli isolates incubated with the MIC concentration (25mg/ml) of hot aqueous extract at 37 for 24h to detect effect of hot aqueous extract of P. cubeba on Bacteriocin production.
Bacteriocin Production
Bacteriocins are peptides that ribosomally synthesized have antimicrobial activity broadly distributed in nature. This peptide biodiversity is supported via many differences in their structures. All synthesized peptides, regardless of sub-classification, share a net positive charge that causes them to fold into an amphiphilic conformation on interaction with bacterial membranes.[30].
Production of Bacterocins are a main characteristic of E. coli and several species Enterobacteriaceae of family [31]. in the present study detection of bacteriocin production by E.coli isolates studies and was found out of 35 E. coli isolates only 5 (14.2%) isolates produced bacteriocin and then antimicrobial of this bacteriocin was tested.[32] found an relationship among bacteriocin and virulence factors, in A number of virulence factors adhesins ability, cytotoxins, siderophores, etc.) was identified, that occur more frequently.In Many studies were done to detect bacteriocin production, one of these, study of [33] was found among 30 E. coli isolates 17(56.7%)were produced bacteriocin ,while 13(43.3%) were found no ability to produce bacteriocin.[34] have been found 102 (38%) of isolates was bacteriocin producing among 266 human E. coli strains. similar result by [35] reported that 195 (54%) bacteriocin producing of UTI E. coli strains, were identified among 361 tested .An even lower frequency (32.3%) it was show that among 440 E. coli UTI strains [36]. and this result were similar to our findings in present study . Bacteriocin was proposed as a replacement for antibiotics to which pathogenic bacteria was become resistant. Potentially, the bacteriocin could be produced by bacteria intentionally introduced into the patient to combat infection.
In current study, (5) isolates of E. coli were produced bacteriocin and then bacteria grown with P. cubeba extract the result showed no changes in bacteriocin production between before and after treated bacteria with P. cubeba extract.
Bacteriocin was precipitated by using ammonium sulphat (80%) method. The ammonium sulphat used because of its being safe, don’t interfere with products and easily to dissolve, so, this salt is useful in salting out. It is separating proteins method based upon that the proteins are less soluble at high concentrations of salt. This process is as well used to concentrate dilute proteins solutions. Dialysis can be used to remove the salt if needed [37]. and then bacteriocin protein concentration was measured by Bradford method[26] because It is simple and very fast method used to measure the same amount of protein that was measured by Lowey assay, commonly used to determine the total protein concentration of sample, especially for protein of cell fraction and for gel electrophoresis. It was used for measure bacteriocin concentration for both bacteria grown with and without P. cubeba extract and then was compared between it. In the case of bacteria cultured without extract protein concentration of partially purified (by ammonium sulphat) was (9.21mg/ml) while bacteriocin protein concentration of bacteria grown with the extract was(10.6mg/ml). The statistical analysis of result did not show significant differences and”p” value was 0.177. Purified of bacteriocin decreased portion concentration but enhance it is activity fractionation by Ammonium sulphat may increase the amount of bacteriocin activity against tested bacteria.
Assessment of antimicrobial activity of bacteriocin, P. cubeba extract, and bacteriocin of bacteria grown with P.cubeba extract via agar well diffusion method at 37 for 24h against S. saprophyticus isolated from vaginal swap and urine sample as indicator bacteria. As show previously the extract haven’t any antimicrobial activity against S. saprophyticus from urine sample but it was exhibited antimicrobial effect against S. saprophyticus from vaginal swap with inhibition zone (10mm). In case of bacteriocin only it was show ability to inhibition growth of S. saprophyticus with inhibition zone (40mm) but indicator bacteria return to grow again and resist bacteriocin as shown in figure (1), whereas the Bacteriocin derived from E. coli grown with P. cubeba extract inhibited S. saprophyticus growth with inhibition zone(30mm) and bacteria didn’t returned to grow again. P. cubeba extract may be effect on bacteriocin production when bacteria grown with the extract so give good inhibition to indicator bacteria, As illustrated previously activity of bacteriocin produced by bacteria were grown with the extract give inhibition zone less than bacteriocin of bacteria grown without the extract but the extract increased the activity of killing or inhibition so that indicator bacteria didn’t returned to grow again. [38] reported that ethanolic extracts of neem (Azadirachta indica) leaf produced antibacterial action on Gram positive and negative bacteria and they observed a positive synergism in terms of antibacterial potential of the extract upon combination with bacteriocin from lactic acid bacterium.
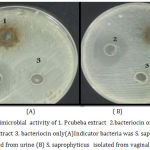 |
Figure 1: Antimicrobial activity of 1. Pcubeba extract 2. bacteriocin of E.coli grown with the extract 3. bacteriocin only(A)Indicator bacteria was S. saprophyticus isolated from urine (B) S. saprophyticus isolated from vaginal swap.
|
As showed in the present study bacteriocin inhibit bacterial growth that may be due to variety of mechanisms like inhibition synthesis of macromolecular, stopping protein synthesis, breakdown of DNA, or killing its targets via membrane permeabilization or degradation of nucleic acid [39; 40; 41; 6].
According to result of this study that antimicrobial activity of bacteriocin in presence of P. cubeba was more than that in absence the extract.and bacteriocin protein concentration in presence the extract were higher than the bacteriocin concentration of E coli without the extract.
Determination the Molecular Size of Bacteriocin by using SDS-PAGE Method
In order to determine the molecular size of the bacteriocin isolated from E. coli isolate (grownwith and without P. cubeba), the bacteriocin was subjected to SDS-PAGE analysis. the estimated molecular mass of bacteriocin as described by [28], Bacteriocin (for E. coli grown with and without P. cubeba extract) was found to be (25 KD) as evidenced in SDS-PAGE. This molecular weight within the range of colicin molecular weight 25KDa-80KDa [40]. Colicins are proteins with high molecular weight that kill the target cells via a several mechanisms. A colicin is including three domains functionally distinct; receptor recognition, protein translocation, and killing [42]. Possession of E coli isolate for bacteriocin considered an indication that bacteria may be used for producing and developing of antibiotics.
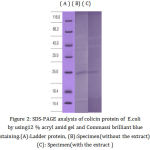 |
Figure 2: SDS-PAGE analysis of colicin protein of E. coli by using12 % acryl amid gel and Commassi brilliant blue staining. (A):Ladder protein, (B):Specimen (without the extract), (C): Specimen (with the extract)
|
The activity spectrum of colicin against other bacterial species may help in the possibility of using colicin for used as epidemiological marker by studding typing of E. coli or other bacterial species according to their sensitivity to the colicin [43]. The molecular Wight of colicin show in figure(2)
Conclusion
Hot aqueous extract of Piper cubeba have activity on antimicrobial activity of bacteriocin of E. coli. Piper cubeba may serve as auxiliary agents that can enhance standard conventional antibacterial therapy in UTIs. Activity of Piper cubeba on bacteria is still not completely known and needed more studies .
References
- Hacker ,J .; Bender, L .; Ott ,M .; Wingender, J .; Lund, B .; Marre, R .;and Goebel, W. (1990). Deletions of chromosomal regions coding for fimbriae and hemolysins occur in vitro and in vivo in various extraintestinal Escherichia coli isolates. Microb Pathog. 8(3) : 213 25.
- Schneider, G .; Dobrindt ,U .; Middendorf ,B .; Hochhut,B.; Szijártó,V.; Emődy,L. and Hacker;J, et al.(2011). Mobilisation and remobilisation of a large archetypal pathogenicity island of uropathogenic Escherichia coli in vitro support the role of conjugation for horizontal transfer of genomic islands. BMC microbiology. 11:210.
- Yamamoto, S.; Tsukamoto, T.; Terai, A.; Kurazono, H.; Takeda, Y. and Yoshida ,0.( 1997) Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli’ J Urol, 157(3), 1127 – 9 .
- Schlager, T. A .; . Hendley,J.O.; Bell,A.L. and Whittam ,T. S. (2002) Clonal diversity of Escherichia coli colonizing stools and urinary tracts of young girls Infect Immun, 70(3), 1225-9.
- Cotter, P. D.; Ross, R. P.; Hill, C. (2012). “Bacteriocins a viable alternative to antibiotics?”. Nature Reviews Microbiology 11 (2): 95–105.
- Riley ,MA. and Wertz, JE.(2002). Bacteriocins: evolution, ecology, and application. Annu Rev Microbiol 56:117–137.
- Cascales, E.; Buchanan, SK.; Duche´,D.; Kleanthous, C.; Lloube`s ,R .; Postle ,K.; Riley, M.; Slatin, S. and Cavard,D. (2007) Colicin Biology. Microbiol Mol Biol Rev 71: 158–229.
- Prema, P.; Bharathy, S.; Palavesam, A.; Sivasubramanian, M. and Immanuel,G. (2006). “Detection, purification and efficacy of warnerin produced by Staphylococcus warneri”. World Journal of Microbiology and Biotechnology 22 (8): 865–72.
- Abreu, O. A. and Barreto,G. (2012).Antiadhesive Effectof Plant Compounds in Bacteria. Phytochemicals as Nutraceuticals-Global Approaches to Their Role in Nutrition and Health Edited by Dr Venketeshwer Rao (Ed.), ISBN: 978-953-51-0203-8,
- Mahady, G. B. (2005). Medicinal Plants for the Prevention and Treatment of Bacterial Infections. Current Pharmaceutical Design, 11, 2405-2427
- Owhe-Ureghe, UB.; Ehwarieme, D.A. and Eboh D.O (2010).Antibacterial activity of garlic and lime on isolates of extracted carious teeth. Afri. J. Biotechnol .9(21):3163-3166.
- Abreu, O.A. & Cuéllar, A. (2008). Strategies for the selection of medicinal plants to be studied. Revista Cubana de Plantas Medicinales,13(3).On-line ISSN 1028-4796
- Koul, JL.; Koul. SK.; Taneja. SC.and Dhar KL.(1996). Oxygenated cyclohexanes from Piper cubeba. Phytochem. 41: 1097-9
- Usia, T.; Watabe, T.; Kadaota, S.; Tezuka, Y. (2005). Potent CYP3A4 inhibitory constituents of Piper cubeba. J. Nat Prod., 68: 64-68.
- Ahmad Q.Z. (2008).Evaluation of nephroprotective effect of Kabab Chini (Piper cubeba) in chemically induced nephrotoxicity, Deptt. Of Ilmul Advia, NIUM, Bangalore, MD Thesis. ; 53-54.
- Eisai, P.T. (1995). Medicinal herb index in Indonesia. 2nd Jakarta: Dian Rakyat.
- Morikawa, T.; Matsuda, H.; Yamaguchi, I.; Pongipiriyadacha, Y. and Yoshikawa, M. (2004). New amides and gastroprotective constituents from the fruit of Pipper cubeba. Planta Med., 70(2): 152-159.
- Lokhande, P.D.; Gawai, K.R.; Kodam, K. M.; and Kuchekar, B. S. (2007). Antibacterial activity of extract of Piper longum. J. Pharmacol. ToxicLol.2(6): 574- 579.
- Bag, A.; Bhattacharya, S. K.; Bharati, P. N. K. and Chattopadhyay, R. R. (2009). Chebulic myrobalan (fruit of Teminalia chebula Retz) extracts against methicillin resistant Staphylococcus aureus and trimethoprim-suphamethoxazole resistant uropathogenic Escherichia coli. Afr.J.Plant Sci., 3:25-29
- Ogundiya, M.O.; Okunade, M. B. and Kolapo, A. L. (2006). Antimicrobial activity of some Nigerian chewing sticks. Ethnobot Leaflts., 10:265-271.
- Ganesh.P.; Kumar ,R. S. and Saranraj,p. (2014). Phytochemical analysis and antibacterial activity of Pepper (Piper nigrum L.) against some human pathogensCent. Euro. J. Exp. Bio., 2014, 3 (2):36-41
- Aneja, K.R. and Joshi, R. (2009). Antimicrobial activity of Amonum subulatum and Elettaria cardamomum against dental caries causing microorganisms. Ethnobot Leaflts. 13: 840-849
- Swadhini, S.P.; Santhosh,R.; Uma,C.; Mythili,S. and Sathiavelu,A.(2011). Phytochemical screening and antimicrobial activity of five medicinal plants against Myrothecium sp International Journal of Pharma and BioSciences. 2(1): 115–120.
- Rajaram, G.; Manivasagan, P.; Thilagavathi, B. and Saravanakumar ,A.(2010). Purification and Characterization of a Bacteriocin produced by Lactobacillus lactis isolated from marine environment. Adv. J. Food Sci. Technol. 2(2):138-144.
- Pascual, L.M.; Daniele, M.B .; Giordano, W .; Pájaro, M.C .and Barberis, IL. (2008).Purification and partial characterization of novel bacteriocin L23 produced by Lactobacillus fermentum L23. J. Curr. Microbiol. 56:397-402
- Bradford, M. M. (1970). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of dye binding. Analytical biochemistry.72(1-2):248-254
- Ivanova, I.; Kabadjova, P.; A Pantev, A.; Danova, S. and Dousset, X .(2000). Biocatalysis-2000: Fundamentals and Applications 41, 47.
- Laemmli, UK (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680 .
- Brooks , G. F. ; Butel , J. S.; and Morse, S. A. : Jawetz, Melnick and Adelberg s (2004). Medical Microbioloy. 21 ed. Appelton and Lange.
- Drider, d. ; Fimland, G. ; Hechard, Y. ; McMullen, L. M. and Prevost. H. (2006). The continuing story of class IIa bacteriocins. Microbiol Mol Biol Rev. ;70(2):564-82.
- Smarda, J. ;Smajs , D. and Lhotova , H. (2002). Three recently acknowledged Escherichia species strikingly differ in the incidence of bacteriocinogenic and lysogenic strains. J. Basic Microbiol. 42: 429-433.
- Budič, M .; Rijavec, M. ; Petkovšek, Ž.; Žgur-Bertok, D. (2011). Escherichia coli Bacteriocins: Antimicrobial Efficacy and Prevalence among Isolates from Patients with Bacteraemia PLoS ONE. 6(12): e28769.
- Ali, J. (20).Hemolysin and bacteriocin production of E coli isolated from urinary tract infection Journal of Babylon university /Pure and applied science (5) (20).1448-1451
- Gordon, DM. and O’Brien, CL. (2006). Bactreiocin diversity and the frequency of multiple bacteriocin production in E. coli. Microbiology. 152: 3239-3244.
- Smajs, D. Micenková,L.; Šmarda,J.; Vrba,M.; Ševčíková,A.; Vališová, Z. and Woznicová,V. (2010). Bacteriocin synthesis in uropathogenic and commensal Escherichia coli: Colicin E1 is a potential virulence factor. BMC Microbiology. 10: 288.
- Smarda, J.and Obdrzalek, V. (2001). Incidence of colicinogenic strains among human E. coli. J. Basic Microbiol. 41: 367-374.
- Berg, JM .( 2007). Biochemistry. Sixth Ed. New York: W.H. Freeman. 68-69, 78
- Das, S. ; Chatterjee, S. and Mandal , NC. (2014). Enhanced antibacterial potential of ethanolic extracts of neem leaf(Azadiracta indica A. Juss.) upon combination with bacteriocin. Int.J.Curr. Microbiol. App. Sci. 3(9) 617-621
- Nomura, M. (1967).Colicins and related bacteriocins. Annu Rev Microbiol 21:257–284.
- Braun ,V.;Pilsl, H.; and Gross, P (1994) Colicins: structures, modes of action, transfer through membranes, and evolution. Arch Microbiol 161:199–206
- Smarda, J,and Smajs, D.(1998). Colicins-exocellular lethal proteins of Escherichia coli. Folia Microbiol 43:563–582
- Cao, Z .and Klebba,P. E. (2002).Mechanisms of colicin binding and transport through outer membrane porins. Biochimie 84:399–412.
- AL- Dhumaina, T.F (2009). Extraction and purification of colicin produed by Eshrecia coli isolated from urinary tract infection. J AL-TAQANI 22:(4)81-73.








