Manuscript accepted on :March 03, 2017
Published online on: --
Plagiarism Check: Yes
A. L. Urakov1, A. V. Samorodov1, F. Kh. Kamilov1, F. A. Khaliullin2 and A. R. Khalimov3
1Department of Pharmacology, Izhevsk State Medical Academy, Izhevsk, Russia.
2Department of Biochemistry, Bashkirian State Medical University, Ufa, Russia.
3Department of Pharmaceutical Chemistry, Bashkirian State Medical University, Ufa, Russia.
Corresponding Author E-mail: AVSamorodov@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1103
Abstract
This paper presents the results of the assessment of the adequacy of the inferior vena cava thrombosis model in rats with the definition of the main markers that can be used to study the efficacy of antithrombotic agents means at the stage of preclinical studies. The first stage of the study examined the processes of clottage and hemostasis system in rats exposed to total occlusion of inferior vena cava on the 1st and 7th days after the operation. The second phase of the experimental work through the example of pentoxifylline rated adequacy of inferior vena cava thrombosis of rats as a model for preclinical studies. The research work covered the functional activity of platelets, coagulation component of hemostasis, thrombosis markers, thromboelastograms and histological material of rats on the 1st and 7th days of acute thrombosis inferior vena cava. It is established that hemostasis system during modeling of the inferior vena cava thrombosis in rats is characterised by natural changes that are platelets hyperaggregation, the emergence of circulating platelet aggregates and hypercoagulation on coagulative component of hemostasis system. These indicators are recorded quite efficiently by method of classical aggregatometry, thromboelastometry and histological studies. The study of preventive effect of pentoxifylline demonstrates that inferior vena cava thrombosis model can be used in preclinical studies.
Keywords
Hemostasis; inferior vena cava thrombosis; preclinical studies
Download this article as:| Copy the following to cite this article: Urakov A. L, Samorodov A. V, Kamilov F. K, Khaliullin F. A, Khalimov A. R. Dynamics of Thrombosis and Hemostasis System Indicators in Rats With Thrombosis of Inferior Vena Cava in Experiment as A Model for Preclinical Studies. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Urakov A. L, Samorodov A. V, Kamilov F. K, Khaliullin F. A, Khalimov A. R. Dynamics of Thrombosis and Hemostasis System Indicators in Rats With Thrombosis of Inferior Vena Cava in Experiment as A Model for Preclinical Studies. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13738 |
Introduction
The most formidable thromboembolitic complication is pulmonary artery thromboembolia (PATE). Pulmonary embolism shows low probability of prediction, a high rate of mortality, mortality and disability and, as a consequence, high economic cost (European Society of Cardiology, 2014). According to the literature, based on the results of the MRI studies usually the source of embolism was thrombosis in inferior vena cava system, with predominant localization in the femoral, iliac, superficial veins of the lower extremities (Langevelde et al., 2013). Equivalent of deep vein thrombosis, mesenteric ischemia and inferior vena cava thrombosis in rats is thrombosis of caudal vena cava. Today there are many model states that are regulated by the FDA and various national guidelines for preclinical evaluation of potential drugs. However, FDA guidelines and national guides on preclinical research (including Russia) require preliminary adaptation studies. This paper presents the results of the assessment of the adequacy of the inferior vena cava thrombosis model in rats with the definition of the main markers that can be used to study the efficacy of antithrombotic agents means at the stage of preclinical studies.
Materials and Methods
Experimental studies performed in vivo are carried out in compliance with international recommendations of the European Convention to protect vertebrate animals for experimental animals, laboratory practice regulations when conducting preclinical studies in Russia (GOST 51000.3-96 3 and 51000.4-96, GOSTR 50258-92) and the order of the Ministry of Public Health and Social Development of Russia № 708n dated from 23/08/2010 “On approval of the rules for laboratory practice” (GLP). The animals were kept in standard conditions with natural vivarium lighting mode, air temperature was 20 ± 2° c and humidity of 55-60% in plastic cages with bedding from sawdust. Twenty four hours prior to the research the feeding was stopped without limiting the access to water.
Design
The first stage of the study examined the processes of clottage and hemostasis system in rats exposed to total occlusion of inferior vena cava on the 1st and 7th days after the operation. The second phase of the experimental work through the example of pentoxifylline rated adequacy of inferior vena cava thrombosis of rats as a model for preclinical studies.
Group of the Study
All the laboratory animals were divided into four large groups:
Intact rats (10 rats).
Group of sham-operated rats (10 rats).
Group of thrombosis without treatment (10 rats).
Group of thrombosis + pentoxifylline (10 rats).
As an object of study to assess the adequacy of the inferior vena cava thrombosis model in rats for preclinical studies was selected pentoxifylline (“Trental” 100 mg-5 ml (Aventis Pharma Ltd., Mumbai, India), 08/2006, series: 236086). Pentoxifylline was injected intravenously into rats in a dose of 40 mg/kg 1hour before simulation of thrombosis. Surgery in sham operated rats was restricted by anaesthetic and midline laparatomy. Sampling of histological material and blood for the second phase of the study was conducted in 24 hours after modeling of thrombosis.
Technique of thrombosis Modeling
Experimental animals under anesthesia (thiopental sodium 50 mg/kg intraperitoneally) underwent midline laparotomy. A part of inferior vena cava was liberated from the connective tissue in length of 3 cm caudally from the confluence of the left renal vein, then ligature was applied under the lower hollow vein and the latter was tied, completely blocking the vessel. The wound was closed (Zhou et al., 2009).
Blood Collection and Centrifugation
Anesthetized animals gave blood from jugular vein by venesection. Venous blood was stabilized by 3.8% sodium citrate solution in a ratio of 9:1. All tests were carried out on enriched and platelet depleted dry blood. Samples of platelet-rich plasma were obtained by centrifuging the citrated blood with 100 g during 10 minutes, plateletless plasma-with 300 g during 15 minutes. The work included centrifuge OPN-3.02 (TNC “DASTAN”, Kyrgyzstan).
Platelet Aggregation
The functional activity of platelets was studied with the laser analyzer of platelets aggregation “Biola 230LA” (“BIOLA”, Russia) (Born, 1962). The aggregation was induced by adenosine diphosphate (ADP) in a concentration of 20 μg/ml, collagen of 5 mg/ml, adrenaline of 5 µg/ml and ristocetin of 10 mg/ml produced by “Technology-Standard” (Barnaul, Russia).
Coagulation Component of Hemostasis
The changes of coagulation component of hemostasis system were studied with the automated selective hemostasis Analyzer STA-Compact (F. Hoffmann-La Roche Ltd., France) using the original reagents kit produced by Roche Diagnostics (F. Hoffmann-La Roche Ltd, France). The research included the indicators of activated partial thromboplastin time (APTT), thrombin time (TT), prothrombin time (PT), and A. Clauss fibrinogen concentration.
Markers of Thrombosis and Fibrinolysis Processes
The number of D-dimers was defined by immunoturbidimetric method and antithrombin III activity was determined by method of chromogenic substrates on the automated selective hemostasis Analyzer STA-Compact (F. Hoffmann-La Roche Ltd., France) using the original reagents kit produced by Roche Diagnostics (F. Hoffmann-La Roche Ltd, France). The soluble fibrin-monomeric complexes (SFMC) were detected by orthophenanthrolin test produced by “Technology-Standard” (Barnaul, Russia).
Thromboelastography
Thromboelastography was carried out with apparatus TEG 5000 (Haemoscope Corporation, United States). Analysis of the thromboelastograms defined general tendency of coagulation (R), functional activity of platelets and fibrinogen (MA, Angle), activity of fibrinolysis (CLT) and the physico-mechanical properties of the formed clots (G). For TEG activator was used 0.2 M CaCl2 (‘Technology-Standard’, Russia).
Definition of Circulating Aggregates
Definition of circulating aggregates (CTA) was conducted by the method of Wu & Hoak modified by FH Kohanna. Venous blood were drawn into two tubes, one of which contains a solution of EDTA and the other contains a mixture of EDTA solution with 4% solution of formalin. After mixing the contents of the tubes were allowed to stand 30 min at room temperature. While standing the platelet aggregates sedimentate, and the individual platelets remain in the supernatant layer. Then the number of platelets in the supernatant layer in each of the test tubes was counted (Kohanna et al., 1984).
Definition of the Blood Clots Mass
In conditions of general anaesthesia in rats there was a surgical access to the abdominal cavity, longitudinal incisions dissected the lumen of the inferior vena cava, the formed clot was extracted. The weight of wet blood clot was defined immediately after the sampling, the weight of dry blood clot was defined through 7 days of drying at 37°C in dry-air sterilizer GP 20 SPM (OJSC “Smolenskoe SCTB SPM”, Russia). The research work used analytical balance BP 221 S (LLC “Sartogosm”, Russia).
Histologic Study
For light microscopy material was fixed in 10% formalin solution. The standard histologic diagnosis was followed by paraffin sections with microtome LEICA RM 2145 (Leica Biosystems, Germany) which were stained with hematoxiline-eosin. Evaluation of morphological changes took place in 10 visual fields at optical light level with increase by x 40 and x 100 using binocular microscope LEICA CME (Leica Biosystems, Germany).
Statistical Processing
The results of the study are processed using the statistical package Statistica 10.0 (StatSoft Inc, USA). The normality of the distribution of actual data was checked by using the criterion of Shapiro-Wilka. The groups were described using the median and interquartile range. Variance analysis was performed using the criterion of Kraskel-Wallis test (for independent observations) and Friedman (for repeated observations). Differences were considered significant at p<0.05.
Results
The first stage of the study examined the processes of clottage and hemostasis system in rats exposed to total occlusion of inferior vena cava on the 1 and 7 days after the operation. According to the results of histological studies it was discovered that the first day of bandaging the lower vena cava already leads to thrombosis (Figure 1). The vein lumen is expanded, it was determined by obstructive thrombus of mixed nature, consisting of fibrin, a large number of erythrocytes, small accumulations of platelets and single leukocytes. Some areas expressed dystrophic changes of endothelium and some lack of endothelial cells. The weights of fresh and dry thrombus in rats in 1 day of inferior vena cava thrombosis amounted to 12.4 g and 5.1 g, respectively.
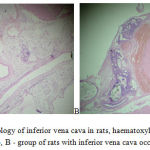 |
Figure 1: Histology of inferior vena cava in rats, haematoxylin-eosin x 100: A -control group, B – group of rats with inferior vena cava occlusion for 1 day. |
Indicators of thromboelastogram of the rats showed changes in the direction of the hemostatic system through activation of coagulation system and fibrinolysis (table 1). The main indicator, which characterizes the functional activity of platelets, MA, increased by 1.3 times (p = 0.0001) in comparison with control, indicating a expressed hyperaggregation of platelets. Figure R, describing the enzymanic part of coagulation till the moment of fibrin thread formation, declined by 2.1 times (p = 0.0007). The indicator K and Angle increased compared to intact rats by 27.4% (p = 0.001) and 35.9% (p = 0.003), respectively. This led to the shift of the common coagulation potential into the direction of hypercoagulation: TMA options reduced by 17.2% (p = 0.001), and TPI increased by 27.4% (p = 0.004). General coagulation potential index, CI, averaged 3.7 (p = 0.0001), suggesting expressed hypercoagulation. It is worth noting the activation of the fibrinolytic system under completed thrombosis-CL30 settings and LY30 were 100.0% (p = 0.003) and 87% (p = 0.0001) for acute thrombosis group. Complete clot lysis time (CLT) averaged 27.4 m (p = 0.0001), which shows more efficacy in reference values in intact rats by 1.7 times (p = 0.0004). In these circumstances, the fibrinolysis is a secondary effect in relation to a hypercoagulation and is associated with the activation of compensatory mechanisms, as a component of the effective protection of thrombosis generalization. Indicators of the clot strength remained on the level of control values.
Table 1: The indicators of thromboelastography of rats on the 1st and 7th day of acute thrombosis of the IVC, Me (25-75)
| Indicator | Control
(intact) |
Thrombosis the 1st day | p1 | Thrombosis the 7th day | p2 | p3 |
| R, min | 11.6
(9,7-13,2) |
5.4
(4,4-7,2) |
0.007 | 9.4
(8,7-11,3) |
0.3 | 0.001 |
| K, min | 4.3
(4,1-5,7) |
5.6
(3,4-6,3) |
0.001 | 4.7
(4,5-5,2) |
0.3 | 0.003 |
| Angle, deg | 43.7
(42,4-44,3) |
73.2
(71,4-75,3) |
0.003 | 54.7
(51,2-57,3) |
0.001 | 0.0006 |
| MA, mm | 55.9
(51,2-57,8) |
71.4
(69,3-73,1) |
0.0001 | 63.2
(61,5-69,4) |
0.0002 | 0.001 |
| TMA, min | 35.4
(32,4-38,5) |
29.4
(24,2-31,3) |
0.001 | 34.2
(31,3-37,2) |
0.4 | 0.001 |
| G, dyne/cm2 | 6,3 (5,7-6,4) | 6,1 (5,4-7,3) | 0.8 | 5,7 (4,3-6,2) | 0.1 | 0.1 |
| E, dyne/cm2 | 127.3
(115,4-142,3) |
135.2
(124,2-139,7) |
0.6 | 131.3
(129,7-137,3) |
0.4 | 0.5 |
| TPI/sec | 15.1
(13,2-17,1) |
19.3
(17,9-22,4) |
0.004 | 17.3
(15,2-19,4) |
0.3 | 0.8 |
| CL30,% | 91.6
(87,3-94,8) |
100.0
(100,0-100,0) |
0.003 | 98,7
(94,5-99,2) |
0.01 | 0.2 |
| LY30,% | 29.6
(27,2-31,9) |
85.9
(82,1-94,7) |
0.001 | 47.4
(45,2-50,3) |
0.007 | 0.001 |
| CLT, min | 47.9
(43,4-48,3) |
27.4
(23,3-31,2) |
0.0004 | 34.2
(31,3-36,2) |
0.0003 | 0.0005 |
| CI | 0,38 (0,1-0,7) | 3,7 (2,9-4,2) | 0.0001 | 2,1 (1,1-2,4) | 0.0005 | 0.0001 |
Note: The level of statistical significance of the differences of indications: p1-1st day thrombosis group in comparison with control, p2-7th day thrombosis group in comparison with control, p3-1st and 7th days thrombosis groups.
Table 2: Indicators of platelet aggregation and masses of the drawn blood clots of rats on 1st and 7th days of acute thrombosis of the IVC, Me (25-75)
| № | Indicator | Control | Thrombosis the 1st day | p1 | Thrombosis the 7th day | p2 | p3 |
| 1 | Platelets*109 /l | 724.4
(703,4-741,8) |
711.5
(701,2-722,3) |
0.2 | 715.6
(702,4-728,3) |
0.4 | 0.6 |
| 2 | Collagen, mm | 55.9
(53,6-58,2) |
68.6
(64,9-72,4) |
0.0001 | 62.4
(59,7-64,2) |
0.006 | 0.001 |
| 3 | lag-period, sec | 62.7
(61,4-64,3) |
47.6
(41,3-52,4) |
0.004 | 59.4
(58,4-63,1) |
0.3 | 0.001 |
| 4 | MPA count., r.u. | 6.5
(5,3-7,2) |
8.9
(7,2-9,6) |
0.0002 | 7.1
(6,4-7,2) |
0.002 | 0.001 |
| 5 | tg α collagen, sec | 38.5
(36,9-40,2) |
58.3
(55,4-63,1) |
0.0003 | 44.3
(41,2-46,3) |
0.0004 | 0.001 |
| 6 | ADP, mm | 52.4
(47,9-54,3) |
66.2
(57,5-71,2) |
0.0001 | 58.4
(56,3-60,1) |
0.003 | 0.001 |
| 7 | MPA ADP, r.u. | 6.7
(5,2-7,4) |
9.2
(8,9-10,6) |
0.0004 | 7.6
(7,2-8,3) |
0.007 | 0.0003 |
| 8 | tg α ADP, sec | 44.7
(41,2-45,4) |
53.4
(51,2-55,3) |
0.0003 | 48.4
(44,3-52,1) |
0.001 | 0.0007 |
| 9 | Adrenaline, mm | 49.8
(47,6-51,3) |
54.2
(51,2-56,3) |
0.001 | 51.4
(48,6-52,4) |
0.005 | 0.001 |
| 10 | MPA adren., r.u. | 4.8
(4,1-5,3) |
7.3
(6,8-9,2) |
0.0003 | 6.3
(5,9-7,1) |
0.0002 | 0.0001 |
| 11 | tg α adren., sec | 34.1
(32,7-36,5) |
49.4
(44,3-54,7) |
0.0004 | 41.4
(39,6-42,4) |
0.001 | 0.0003 |
| 12 | Ristocetin, mm | 57.3
(56,4-59,3) |
68.7
(64,2-73,1) |
0.001 | 62.1
(58,7-64,3) |
0.0002 | 0.001 |
| 13 | MPA rist., r.u. | 6.2
(5,7-6,6) |
9.4
(8,2-10,3) |
0.0003 | 8.4
(7,9-8,7) |
0.0001 | 0.002 |
| 14 | tg α rist., sec | 47.5
(45,2-49,3) |
55.3
(52,4-58,2) |
0.001 | 50.4
(49,6-52,1) |
0.0001 | 0.0002 |
| 15 | Wu-Hoak, % | 6,8 (4,6-9,1) | 17.5
(14,2-19,4) |
0.0002 | 15.9
(14,7-16,3) |
0.001 | 0.2 |
| 16 | M “wet” blood clot, g | – | 11,3 (8,9-10,6) | – | 9,4 (8,2-10,3) | – | 0.0001 |
| 17 | M “dry” blood clot, g | – | 4,6 (4,4-5,2) | – | 3,7 (3,2-4,1) | – | 0.0002 |
| 18 | Δ m, g | – | 4,4 (4,3-4,9) | – | 5,4 (5,2-5,9) | – | 0.0004 |
Note: M-mass; The level of statistical significance of the differences of indications: p1-1st day thrombosis group in comparison with control, p2-7th day thrombosis group in comparison with control, p3-1st and 7th days thrombosis groups
Table 3: The indicators of coagulogram of rats on the 1st and 7th day of acute thrombosis of the IVC, Me (25-75)
| № | Indicator | Control | Thrombosis the 1st day | p1 | Thrombosis the 7th day | p2 | p3 |
| 1. | APTT, sec | 23.1
(21,6-24,7) |
26.5
(24,7-28,3) |
0.7 | 24.3
(21,2-26,9) |
0.1 | 0.8 |
| 2. | TT, sec | 27.2
(26,4-28,9) |
29.3
(27,5-30,6) |
0.2 | 28.3
(26,1-29,4) |
0.4 | 0.3 |
| 3. | PT, sec | 12.4
(11,5-13,9) |
14.3
(12,5-16,1) |
0.3 | 13.3
(12,1-15,4) |
0.7 | 0.2 |
| 4. | Fibrinogen, sec | 24.3
(22,5-26,7) |
21.2
(19,4-24,3) |
0.2 | 22.4
(21,1-23,0) |
0.4 | 0.6 |
| 5. | SFMC, g/l × 10-2 | 3.1
(2,7-3,6) |
4.9
(3,1-5,2) |
0.001 | 4.7
(4,3-6,5) |
0.003 | 0.7 |
| 6. | AT III,% | 95.4
(94,7-97,5) |
67.2
(64,3-78,2) |
0.004 | 83.5
(81,1-87,4) |
0.0001 | 0.002 |
| 7. | D-dimer, µg/ml | 2.2
(1,7-2,6) |
6.3
(5,7-6,5) |
0.001 | 6.1
(5,9-6,4) |
0.0001 | 0.2 |
Note: The level of statistical significance of the differences of indications: p1-1st day thrombosis group in comparison with control, p2-7th day thrombosis group in comparison with control, p3-1st and 7th days thrombosis groups
Table 4: Indicators of antithrombotic activity of pentoxifylline in modeling of thrombosis of the IVC, Ме (25-75)
| № | Group | ADP,
mm |
Collagen, mm | Wu-Hoak,
% |
M wet,
g |
M dry,
g |
Δ M,
g |
| 1 | Sham operated rats | 46.5
(44,6-53,1) |
43.7
(41,2-47,5) |
6.8
(4,6-9,1) |
– | – | – |
| 2 | Group of thrombosis without treatment | 66,2**
(57,5-71,2) |
68,6**
(58,9-72,4) |
17,5*
(14,2-19,4) |
11.3
(8,9-10,6) |
4.6
(4,4-5,2) |
4.4
(4,3-4,9) |
| 3 | Group of thrombosis + pentoxifylline | 38.3
(37,1-43,2) |
33.2
(24,9-36,3) |
15,8**
(13,6-16,9) |
8.2
(7,1-8,6) |
3.7
(2,2-4,1) |
4.5
(3,6-5,7) |
Note: The level of statistical significance of the differences between the control and experimental groups: * p ≤ 0.05, * p ≤ 0.001.
Analysis of the functional activity of platelets (table 2) shows that the rate of platelet aggregation induced by ADP and collagen, is higher in comparison with intact rats by 35.1% (p = 0.0001) and 27.8% (p = 0.0001), respectively. The reaction rate of the release, the lag-period during collagen-induced platelet aggregation decreased by 1.3 times (p = 0.004) compared to intact rats, which suggests early activation of the platelets. Indicators of platelet aggregation induced by adrenaline and ristocetin, similar to ADP and collagen, showed expressed hyperaggregation, average amplitude increased for adrenaline and ristocetin by 9.0%, (p = 0.001) and 16.5% (p = 0.001), respectively. Platelet aggregation rate and the average size of platelet aggregates significantly increased regardless of the selected inductor. Rate of circulating platelet aggregates as per the Wu-Hoak method was recorded at an average of 18.3%. Clottage markers, D-dimers and SFMC, naturally have risen to the level of 6.3 µg/ml (p = 0.001) and 4.9×10-2 g/l (p = 0.001), respectively. According to coagulation diagram (table 3), there was a slight increase in time of APTT, PT, TT and decrease in fibrinogen concentration. However, these changes were not statistically significant compared to the intact rats indicators. AT III activity rate decreased by 29.5% (p = 0.004) relative to intact rats which is logical due to their enhanced consumption in the zone of massive thrombosis.
Table 5: Evaluation of the effectiveness of pentoxifylline as a means to prevent thrombosis of inferior vena cava according to TЕG, Ме (25-75)
| Indicator | Control
(intact) |
Group of thrombosis without treatment | Group of thrombosis + pentoxifylline | p |
| R, min | 11,6 (9,7-13,2) | 5,4 (4,4-7,2) | 12,8 (10,1-14,4) | 0.002 |
| K, min | 4,3 (4,1-5,7) | 5,6 (3,4-6,3) | 5,7 (5,1-6,3) | 0.1 |
| Angle, deg | 43,7 (42,4-44,3) | 73,2 (71,4-75,3) | 42,3 (41,4-45,3) | 0.0001 |
| MA, mm | 55,9 (51,2-57,8) | 71,4 (69,3-73,1) | 51,3 (49,7-54,3) | 0.004 |
| TMA, min | 35,4 (32,4-38,5) | 29,4 (24,2-31,3) | 34,7 (32,1-35,7) | 0.002 |
| G, dyne/cm2 | 6,3 (5,7-6,4) | 6,1 (5,4-7,3) | 5,2 (5,1-7,3) | 0.03 |
| E, dyne/cm2 | 127,3 (115,4-142,3) | 135,2 (124,2-139,7) | 120,4 (117,3-123,5) | 0.001 |
| TPI/sec | 15,1 (13,2-17,1) | 19,3 (17,9-22,4) | 14,7 (13,2-17,5) | 0.001 |
| CL30, % | 91,6 (87,3-94,8) | 100,0 (100,0-100,0) | 94,6 (91,3-98,3) | 0.01 |
| LY30, % | 29,6 (27,2-31,9) | 85,9 (82,1-94,7) | 29,4 (27,4-32,4) | 0.0004 |
| CLT, min | 47,9 (43,4-48,3) | 27,4 (23,3-31,2) | 47,1 (47,2-49,3) | 0.003 |
| CI | 0,38 (0,1-0,7) | 3,7 (2,9-4,2) | 0,42 (0,37-0,51) | 0.0005 |
Note: p – the level of statistical significance for comparison between groups of thrombosis without treatment and treatment with pentoxifylline.
The 7th day of thrombosis the thromboelastography data show normalization of indicators characterizing functional activity of platelets and coagulative link of the hemostatic system. Indicators of R and K were at the level of values of intact and sham operated animals. However, hyperaggregation of platelets and increased fibrinolytic activity of blood preserved. The 7th day of thrombosis the MA and Angle indicators averaged 63.7 mm (p = 0.0001) and 54.7 degrees, which exceeds values of intact animals, at 13.1% and 25.1%, respectively. This led to the displacement of the common coagulation potential in the direction of hypercoagulation, index of common coagulation potential, CI, averaged 2.1 (p = 0.0005), however, the settings and the TPI, TMA by the 7th days of thrombosis match the values of the intact rats. CL30 and LY30 parameters describing a system of fibrinolysis, accounted for 98.7% (p = 0.01) and 47.4% (p = 0.007), which remained higher than in intact rats. Complete clot lysis time (CLT) averaged 34.2 minutes (p = 0.0003), which shows efficacy in reference values in intact rats at 28.6% (p = 0.0003). The aggregometry data showed normalization of platelet functional activity indicators, platelet aggregation induces by ADP and collagen, decreased relative to 1 day by 12.0% and 14.5%, respectively, and statistically is not distinguished from groups of intact and sham operated rats. Coagulation diagram showed values of APTT, PT, TT and fibrinogen concentration corresponding to the values of intact rats. D-dimer level equal to 6.1, and CTA as per Wu-Hoak > 15% still, naturally, demonstrated the presence of inferior vena cava thrombus. This is also proven by the results of autopsy. Weight of fresh blood clots, drawn during autopsy, averaged 9.4 g, dry ones – 3.6 g. Histologically (figure 2) the vein lumen is expanded, the lumen showed an obstructive thrombus of mixed nature, composed of fibrin, erythrocytes, accumulations of platelets, there are foci of lymph hystocytic infiltration with an additive of neutrophils. Large surface has plots with absent endothelial cells.
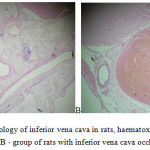 |
Figure 2: Histology of inferior vena cava in rats, haematoxylin-eosin x 100: A -control group, B – group of rats with inferior vena cava occlusion for the 7th day. |
The study was completed by evaluating the inferior vena cava thrombosis as a model for preclinical studies on the example of the use of pentoxifylline. Out of all the indicators the efficacy criterion was defined as fresh and dry mass of blood clots, results of thromboelastometry and indicators of platelet aggregation. It is discovered that in the group of sham operated rats the clot formation did not occur. The average weight of a wet clot in the group of rats with thrombosis was 11.3 g., that of dry clots was 4.4 g. Pentoxifylline statistically significantly reduced the massiveness of thrombosis by 34.5% (p < 0.001) and 27.4% (p < 0.001), respectively, in comparison with the control. Despite the occurence of circulating platelet aggregates ≥ 15% (p < 0.0001) by the Wu-Hoak method, the platelet aggregation rates remained on the level of the stated values. Thromboelastography data (table 5) demonstrate effective correction of hypercoagulation state and platelets hyperaggregation caused by thrombosis. TEG indicators correspond to the values of the intact animals.
Thus, it is established that hemostasis system during modeling of the inferior vena cava thrombosis in rats is characterised by natural changes that are platelets hyperaggregation, the emergence of circulating platelet aggregates and hypercoagulation on coagulative component of hemostasis system. These indicators are recorded quite efficiently by method of classical aggregatometry, thromboelastometry and histological studies. The study of preventive effect of pentoxifylline demonstrates that inferior vena cava thrombosis model can be used in preclinical studies.
References
- European Society of Cardiology. Guidelines on the diagnosis and management of acute pulmonary embolism. European Heart Journal, 2014; 35: 3033-80.
- Langevelde, K., Šrámek, A., Vincken, P.W.J., Rooden, J.K., Rosendaal, F.R., Cannegieter, S.C. Finding the origin of pulmonary emboli with a total-body magnetic resonance direct thrombus imaging technique. Haematologica, 2013; 98: 309-15.
- Zhou, J., May, L., Liao, P., Gross, P.L., Weitz, J.I. Inferior vena cava ligation rapidly induces tissue factor expression and venous thrombosis in rats. Arterioscler Thromb Vasc Biol, 2009; 29 :863-9.
- Воrn, G.V. Aggregation of blood platelets by adenosinediphosphate and its reversal. Nature, 1962; 194: 927-9.
- Kohanna, F.H., Smith, M.H., Salzman, E.W. Do patients with thromboembolic disease have circulating platelet aggregates? Blood, 1984; 64(1): 205-9.







