Blessing Chekwube Eluke1, Obianuju Francisca Ndubuisi2, Chidiebere Eluke3, Enerst Ukaejiofo4 and Silas Ufelle5
1Department of Medical Laboratory Science Faculty of Health Sciences and Technology College of Medicine University of Nigeria Enugu Campus. Enugu Nigeria.
2Department of Haematology Annunciation Specialist Hospital Emene Enugu.
3Department of Morbid Anatomy University of Nigeria.
4Department of Medical Laboratory Science Faculty of Health Sciences and Technology College of Medicine University of Nigeria.
5Faculty of Health Sciences College of Medicine University of Nigeria Enugu Campus. Enugu Nigeria.
Corresponding Author E-mail: blessingeluke@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1082
Abstract
Alteration in endothelial function may precede the development of morphological changes in disorders and may contribute to morbid development and clinical complications. Therefore, this work attempted to evaluate the levels of endocan (endothelial specific molecule-1) and other coagulation parameters and find their prognostic significance with respect to severity of human immuno-deficiency virus (HIV) infection. Sixty HIV infected patients on drugs and antiretroviral (ART) naïve were enrolled in a prospective, cross- sectional study while thirty HIV non reactive, apparently healthy individuals were recruited as control. Endocan was measured using high sensitive Enzyme linked immunosorbent assay. Plasma levels of prothrombin time and activated partial thromboplastin time were determined to check both intrinsic and extrinsic coagulation pathways. CD4+ count and platelet count were also analyzed by standard methods. HIV positive patients who are already on antiretroviral therapy (ART) had significantly increased endocan levels (471.134+92.84 pg/ml) compared to normal control (208.277+106.60 pg/ml) (p<0.05) while patients that are ART naïve had significantly increased endocan levels when compared to those already on drugs (611.60+608.77pg/ml) (p<0.05). HIV – 1 infected subjects not on drugs had significantly increased platelet count (145.1+580 cumm) when compared with normal subjects (90.100+40.00 cumm) (P< .0001) however, group on drugs had marginal decrease compared to normal group (85.000+192cumm). Markers of intrinsic and extrinsic coagulation- APTT and PT were significantly elevated in HIV positive patient when compared with apparently healthy controls. This is significantly associated with severity.
Keywords
Endocan; CD4+count; Antiretroviral therapy; Human Immunodeficiency virus; coagulation; prothrombin time
Download this article as:| Copy the following to cite this article: Eluke B. C, Ndubuisi O. F, Eluke C, Ukaejiofo E, Ufelle S. Changes in Endocan Levels and Blood Coagulation in HIV Infection. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Eluke B. C, Ndubuisi O. F, Eluke C, Ukaejiofo E, Ufelle S. Changes in Endocan Levels and Blood Coagulation in HIV Infection. Biomed Pharmacol J 2017;10(1).Biomed Pharmacol J 2017;10(1) Available from: http://biomedpharmajournal.org/?p=14150 |
Introduction
only health but also social, economic and quality of life1. One of the characteristics of the infection is its HIV infection continues to spread in recent ability to cause death due to opportunistic infection.
times despite advances in the study of virology and However, the use anti-retroviral drugs has limited it has come with devastating effects as it affects not the frequency of opportunistic infection and thus increased the life span of those infected 2. Although the use of antiretroviral drugs to a great extent, reduces viral replication and preserves the CD4+ lymphocyte count, other complications caused by HIV infection has begun to be noticed by researchers3. Haemostatic abnormalities have been recognized as a major contributor to survival 4. The emergence of other major causes of mortality like venous thrombotic event and cardiovascular disease has also been implicated5,6. HIV infected patients are identified as having increased risk for arterial and venous thromboembolic events 7. Preliminary evidence had suggested the culpable involvement of inflammation and chronic immune activation 8-10.
Currently, endothelial dysfunction has been suggested as one mechanism that may be contributory to cardiovascular risk involvement in these patients 11. There is increasing evidence that high levels of some biomarkers like circulating endothelial cells, D-dimer levels, micro particles tissue factor expression and platelet micro particles may predict cardiovascular disease in these patients 12-14. Endothelial dysfunction may be considered as a shift from the quiescent state of the endothelium into a more activated state. This state may lead to a pro inflammatory, procoagulant state and changes in availability of nitric oxide15. Damage to the endothelium will result in an imbalance between vasoconstriction and vasodilatation which may ultimately exacerbate atherosclerosis. Therefore, endothelial dysfunction maybe an early marker of atherosclerosis and a response to risk factors of cardiovascular disease16.
Experimental evidence has implicated elevated endocan levels in several diseases especially as it relates to endothelial activation, endothelial dysfunction and neovascularization17. Furthermore, endocan is a potential immune inflammatory marker that may be linked with cardiovascular disease18.
The continuous use of HAART has now been linked with cardiovascular disease in HIV infection. Some researchers are convinced that cardiovascular and thrombotic events in these individuals may be as a result of long term use of HARRT probably because HAART can modify lipid profile and may lead to atherosclerosis19. In contrast, other studies agree that cardiovascular events may be due to the HIV infection itself 20.
There is growing awareness on the abnormal coagulation in HIV infection21. Recent studies have demonstrated that changes in coagulation and inflammation may be responsible and predict risk for non-AIDS defining events 22. Of which cardiovascular disease is one. In this respect, studies on the levels of biomarkers of coagulation and inflammation have been associated with risk of mortality due to cardiovascular disease 23.
In summary, alteration in the endothelial biology and coagulation may explain non-AIDS defining clinical events 18. It is still debated on the mechanism behind the abnormal coagulation in both those HIV reactive on drugs and those not on drugs. Changes in the endothelium, coagulation and platelet activation in association with CD4+ count may help link severity of infection in HIV infections. The aim of this study is to estimate the level of endothelial specific molecule-1, biomarkers of intrinsic and extrinsic (APTT and PT) pathways especially in association with CD4+count and treatment.
Material and Method
Study Population
Study participants enrolled included HIV positive subjects who were on drugs, those that were not on highly anti- retroviral therapy (HAART) and drug naïve patients attending Enugu State University Teaching Hospital Parklane, Enugu, Nigeria. The normal control consisted of non-infected individuals that screened negative for HIV and other viral, bacterial infections.
Ethical Considerations
The study was approved by the ethics and review committee of Enugu State University Teaching Hospital. Informed consent was duly provided by each participant as a factor to recruitment.
Study Design
The study design was a cross sectional prospective study conducted on outpatient HIV positive subjects between December 2015 and August 2016. Patients attending Hematology Clinic of Enugu State University Teaching Hospital Parklane, Enugu, were enrolled for the study. Ninety subjects were included into the study consisting of sixty HIV positive patients and thirty apparently healthy HIV negative individuals. HIV positive patients were further grouped into those that were antiretroviral naïve and those on drugs. Peripheral blood samples were collected by venipuncture from all participants into sodium citrate; for the determination of prothrombin time and activated partial thromboplastin time, ethylene diamine tetra acetic acid(EDTA) tube; for the measurement of platelet and CD4+ count and plane tubes; serum was expressed for the measurement of endothelial specific molecule -1(endocan). The study was performed according to the guidelines as stipulated by the Helsinki declaration.
Measurements of coagulation parameters: prothrombin time, partial thromboplastin time and platelet count were analyzed based on manual method according to Baker and Sylverton 24 while CD4+ counts were assayed using Cyflow (Partec, serial number 090439122 2009)
Measurement of Endocan was performed using commercially available high sensitive Enzyme Linked Immunosorbent (ELISA) kits by Cloud Clone Corp Houston TX USA. The assay range of the ESM-1 ELISA kit was 15.6 to1000pg/ml. Intra-assay and the inter-assay variation coefficients were <10% and <12%, respectively.
Data Analysis
The data was analyzed using Statistical Package for Social Sciences version 20. The values were reported as mean ± standard deviation. One way analysis of variance was the statistical technique used for continuous variable while Pearson’s correlation was used to ascertain relationship.
Results
A total of sixty HIV infected patients; thirty treatment naïve, thirty on drugs and thirty HIV non infected subjects were enrolled into the study. Equal gender distribution was sought among participants. The mean ages of participants were 38.7 ± 14. 9years. The mean values of CD4+ counts of normal subjects, those on drugs and those not on drugs were 838±137, 800±19 and 359±56µml respectively (P=0.022).
Levels of endocan (endothelial specific molecule), APTT (activated partial thromboplastin time), PT (prothrombin time) and platelet count in HIV reactive patients on drugs and drug naïve patients compared with non-reactive apparently healthy subjects are shown in table 1.
Table 1: levels of endocan, APTT, PT and platelet count in HIV reactive patients on drug and drug naïve compared with non reactive apparently healthy subjects.
| Test | Reactive Not on
Drugs |
Reactive on Drugs |
Non-Reactive Control |
|
CD4+ µml |
800+19 | 359+156 | 838+137 |
| ESM1 pg/ml | 611.60+608.77 | 471.134+92.841 | 208.277+106.60 |
| Platelet cumm | 145.1+580 | 85.000+192 | 90.100+40.00 |
| Prothrombin Time (seconds) | 26.0±14.4 | 20+ 17.5 | 14+18 |
| Partial Thromboplastin Time (seconds) | 43.5+16.9
|
38.3+24.3 | 34.5+11.15 |
Patients on treatment with HAART showed significant decrease in the mean levels of endocan (471.134+92.84) when compared to those not on drugs (611.60+608.77pg/ml). However, there was a significant increase (p<0.05) when mean endocan values of HIV infected not on drugs were compared with healthy controls (208.277+106.60pg/ml).
Treatment with HAART decreased progressively the mean levels of platelet counts of HIV patients (p= 0.000) when compared with normal healthy uninfected subjects while those infected but without treatment remained elevated.
Markers of intrinsic and extrinsic coagulation- APTT and PT were measured in all subjects. Those patients on drugs and those not on drugs all had significantly elevated levels (in seconds) of platelet count, APTT and PT when compared with apparently healthy controls. Also, there was a statistically significantly decrease when levels of APTT and PT of HIV infected patients on drugs were compared to those that were not on treatment.
Figure 1 shows the levels of endocan levels in normal and HIV subjects as stratified by their CD4+ count. CD4+ counts of non-reactive were decreased when compared to others. No significant correlations were observed between endocan and prothrombin time p=0.41, r=0.32; endocan and activated partial thromboplastin time p=0.30, r=-0.63; and between endocan and platelet count p=0.20, r=-0.18
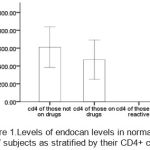 |
Figure 1: Levels of endocan levels in normal and HIV subjects as stratified by their CD4+ count |
Discussion
Alteration in endothelial function may precede the development of morphological changes in disorders and may contribute to morbid development and clinical complications 25,26. In this respect, endothelial activation with resultant coagulation anomalies has been implicated in defining non-AIDS clinical events27,28. Therefore, this work attempted to evaluate the levels of endocan (endothelial specific molecule-1) and other coagulation parameters (activated partial thromboplastin time, prothrombin time and platelet count) with a view in finding their prognostic significance with respect to severity of HIV infection and treatment.
It this study, we revealed that endocan levels were quite elevated in HIV positive patients that are not on treatment when compared to those on treatment and normal healthy controls. It has been previously indicated that HIV positive patients may be at a higher risk of cardiovascular disease because they are more prone to vascular changes brought about by inflammation 29. Already biomarkers of inflammation have been found in these patients 30. Immune activation probably due to co- factors such as co-infection with cytomegalovirus and bacteria or as a result of cytokines, elaboration of adhesion molecules and activation of innate/adaptive immunity has been suggested as reason for this situation 31.
The findings also revealed that though HAART may be effective in reducing viral multiplication and increasing CD4+ count, there may still be a low grade persistent inflammation and immune activation. This is because serum endocan levels were still significantly increased in patients already on treatment when compared with normal control found in this study. Viral replication has been suggested as being responsible for endothelial injury. Endocan is a soluble proteoglycan secreted by the vascular endothelium and has been associated with other bioactive molecules with properties such as cellular signaling, adhesion properties, regulation of proliferation and differentiation of cell types 32,33. Endocan has also shown promising result as a biomarker of inflammation 34. An increase in endocan levels may also suggest endothelial activation 35,36. In contrast, other authors argue that added clinical changes may be as a result of ART37. Though in this case those not already on drugs had increased endocan levels.
Furthermore, derangement in the endothelium may lead to blood clotting activation[]. Previous study had suggested decreased levels of markers of endothelial activation and coagulation markers in those receiving antiretroviral treatment 38. The result the current study showed a marked increase in platelet count, activated partial thromboplastin time and prothrombin time of HIV patients not on treatment when compared to non infected control. Increased procoagulant state has been linked to non AIDS defining characteristics. Increased expression of tissue factor with subsequent generation of fibrin cloth may have initiated coagulation in this group of subjects 39. Furthermore, this is in agreement with some authors who had formerly reported increased markers of platelet activation 13. They had argued that platelet activation markers may offer novel ways of looking at disease progression probably because platelet activation plays a role in inflammation and thrombosis. Also, selectin, an important molecule in regulation of haemostasis may stimulate other mediators such as platelet factor 4 that is important for inflammation40. Intrinsic and extrinsic coagulation cascade activation may lead to formation of thrombosis probably through the elaboration of tissue factor41.
Conclusion
The findings from this study revealed an increase in an serum endocan levels, platelet count, activated partial thromboplastin and prothrombin time both in ART naïve and ART treated individuals.
References
- Sepkowitz K.A. AIDS – the first 20 years. N Engl J Med2001;344(23):1764-72.
CrossRef - Palella F.J., Jr., Delaney K.M., Moorman A.C., Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD, the HIV Outpatient Study Investigators. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med1998;338(13):853-860.
CrossRef - Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506-2512.
CrossRef - Majluf-Cruz A. Changes in blood coagulation in HIV infection. Rev Invest Clin. 1997. 49(1):51-66.
- Passalaris J.D., Sepkowitz K.A., Glesby M.J. Coronary artery disease and human immunodeficiency virus infection. Clin Infect Dis 2000;31(3):787-797.
CrossRef - Fultz SL, McGinnis KA, Skanderson M, Ragni MV, Justice AC. Association of venous thromboembolism with human immunodeficiency virus and mortality in veterans.Am J Med.2004;116(6):420–423.
CrossRef - Lafon ME, Steffan AM, Royer C, Jaeck D, Beretz A, Kirn A,Gendrault J.HIV-1 infection induces functional alterations in human liver endothelial cells in primary culture. AIDS 1994; 8:747-752.
CrossRef - Baker JV. Chronic HIV Disease and Activation of the Coagulation System Thromb Res. 2013 132(5): 495–499.
- Aziz N, Nishanian P, Fahey JL Levels of cytokines and immune activation markers in plasma in human immunodeficiency virus infection: quality control procedures. Clin Diagn Lab Immunol.1988. 5: 755–761.
- Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton N I, Neaton JD, Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008; 5(10):e203.
CrossRef - Chi D, Henry J, Kelley J, Thorpe R, Smith JK, Krishnaswamy .The effects of HIV infection on endothelial function. Endothelium 2000;7:223-242.
CrossRef - López M, San Román J, Estrada V, Vispo E, Blanco F, Soriano V Endothelial dysfunction in HIV infection–the role of circulating endothelial cells, microparticles, endothelial progenitor cells and macrophages. AIDS Rev. 2012 14(4):223-30.
- Nkambule BB, Davison GM, Ipp H The evaluation of platelet indices and markers of inflammation, coagulation and disease progression in treatment-naïve, asymptomatic HIV-infected individuals. Int J Lab Hematol. 2015 37(4):450-458.
CrossRef - Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, Natarajan V, Rehm C, Hadigan C, Sereti I. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. Aids. 2010; 24(10):1509–1517.
CrossRef - Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 2006;113:1708-14.
CrossRef - Migliacci R, Becattini C, Pesavento R, Davi G, Vedovati MC, Guglielmini G,et al.Endothelial dysfunction in patients with spontaneous venous thromboembolism. Haematologica 2007; 92:812-818.
CrossRef - Pawlak K,Mysliwiec M,Pawlak D Endocan–the new endothelial activation marker independently associated with soluble endothelial adhesion molecules in uraemic patients with cardiovascular diseas Clin Biochem. 2015 48(6):425-30.
CrossRef - Andrade ACO; Bruno Cotter BR Endothelial function and cardiovascular diseases in HIV infected patient. Braz J Infect Dis. 2006. 10 (2) :139-145.
CrossRef - Stein JH, Klein MA, Bellehumer JL, McBride PE, Wiebe DA, Otvos JD, Sosman JM. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation 2001; 104:257-262.
CrossRef - De Larranaga GF, Petroni A, Deluchi G, Alonso BS, Benetucci JA. Viral load and disease progression as responsible for endothelial activation and/or injury in human immunodeficiency virus-1-infected patients. Blood Coagul Fibrinolysis 2003; 14:15-18.
CrossRef - Stricker RB Hemostatic abnormalities in HIV disease. Hematol Oncol Clin North Am.1991. 5(2):249-265.
- Balta S,Mikhailidis DP,Demirkol S, Ozturk C, Celik T, Iyisoy A Endocan: A novel inflammatory indicator in cardiovascular disease? Atherosclerosis. 2015 243(1):339-43.
CrossRef - Duprez DA1, Neuhaus J, Kuller LH, Tracy R, Belloso W, De WitS, Drummond F, Lane HC, Ledergerber B, Lundgren J, Nixon D, Paton NI, Prineas RJ, Neaton JD. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals.PLoS One. 2012;7(9):e44454.
- Baker, F.J., Silverton, R.E., Pallister, C.J. Introduction to Medical Laboratory Technology. 7th Edition 2001. Bounty press limited, 69-70.
- Vittecoq D., Escaut L., Merad M., et al. Coronary heart disease in HIV-infected individuals. Adv Cardiol2003;40:151-62.
CrossRef - Vallance P., Collier J., Bhagat K. Infection, inflammation, and infarction: does acute endothelial dysfunction provide a link? Lancet1997;349(9062):1391-1392.
CrossRef - Nordell AD,McKenna M,Borges ÁH, Duprez D, Neuhaus J, Neaton JD Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation J Am Heart Assoc. 2014; 3(3):e000844.
CrossRef - Hong Mu, Hong Chai, Peter H. Lin, Qizhi Yao, Changyi Chen Current Update on HIV-associated Vascular Disease and Endothelial Dysfunction 2007,31 (4): 632–643.
- Dombrowski JC, Kitahata MM, Van Rompaey SE, Crane HM, Mugavero MJ, Eron JJ, Boswell SL, Rodriguez B, Mathews WC, Martin JN, Moore RD, Golden MR. High Levels of Antiretroviral Use and Viral Suppression Among Persons in HIV Care in the United States, 2010. J Acquir Immune Defic Syndr. 2013; 63(3):299–306,
CrossRef - Funderburg NT. Markers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patients. Curr Opin HIV AIDS. 2014 Jan;9(1):80-86.
CrossRef - Nordoy I, Aukrust P, Muller F, Froland SS. Abnormal levels of circulating adhesion molecules in HIV-1 infection with characteristic alterations in opportunistic infections. Clin Immunol Immunopathol 1996; 81:16-21.
CrossRef - Sarrazin S,Adam E,Lyon M, Depontieu F, Motte V, Landolfi C, Lortat- Jacob H, Bechard D, Lassalle P, Delehedde M. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy Biochim Biophys Acta. 2006. 1765(1):25-37.
- Rubanyi GM: The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993, 22 (Suppl 4): S1-S14.
CrossRef - Sevket Balta, Dimitri P. Mikhailidis, Sait Demirkol, Cengiz Ozturk, Turgay Celik, Atila Iyisoy. Endocan: A novel inflammatory indicator in cardiovascular disease? Atherosclerosis. 2015 243(1):339-343.
CrossRef - Yilmaz MI, Siriopol D, Saglam M, Kurt YG, Unal HU, Eyileten T, Gok M, Cetinkaya H, Oguz Y, Sari S, Vural A, Mititiuc I, Covic A, Kanbay M. Plasma endocan levels associate with inflammation, vascular abnormalities, cardiovascular events, and survival in chronic kidney disease. Kidney Int. 2014 86(6):1213-1220.
CrossRef - Yilmaz MI,Siriopol D,Saglam M, Kurt YG, Unal HU, Eyileten T, Gok M, Cetinkaya H, Oguz Y, Sari S, Vural A, Mititiuc I, Covic A, Kanbay M.Plasma endocan levels associate with inflammation, vascular abnormalities, cardiovascular events, and survival in chronic kidney disease. Kidney Int. 2014 86(6):1213-20.
CrossRef - Francisci D, Giannine S, Baldelli F,Leone M, Belfiori B, Guglielmini G, Malincarne L, Gresele P HIV Type 1 Infection, and Not Short-term HAART, Induces Endothelial Dysfunction AIDS 2009 23(5):589-596.
- Wolf K, Tsakiris DA, Weber R, Erb P, Battegay M. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients with human immunodeficiency virus type 1. J Infect Dis 2002.185: 456–462.
- Mackman N. Role of tissue factor in hemostasis and thrombosis.Blood Cells Mol Dis. 2006;36(2):104–107.
CrossRef - Nkambule BB,Davison G,Ipp H. Platelet leukocyte aggregates and markers of platelet aggregation, immune activation and disease progression in HIV infected treatment naive asymptomatic individuals. J Thromb Thrombolysis. 2015 40(4):458-67.
CrossRef - Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development.Arterioscler Thromb Vasc Biol.2004;24(6):1015–22.
CrossRef








