Manuscript accepted on :March 02, 2017
Published online on: --
Plagiarism Check: Yes
Kailas D. Datkhile, Pratik P. Durgawale, and Madhavi N. Patil
Molecular and Genetic Laboratory, Krishna Institute of Medical Sciences University, Karad.
Corresponding Author E-mail: kddatkhile@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1114
Abstract
Biogenic nanoparticles have received massive attention due to its increased application for production of nanomedicine which had proven to be of intense value in the field of biomedicine. The present study was aimed to compare cytotoxicity of biosynthesized silver nanoparticles with chemically synthesized silver nanoparticles.The cytotoxic properties of both AgNPs were demonstratedin vitro by assessing the cell morphology, cell proliferation and DNA fragmentation usinghuman leukemia K562 cell line. The results from performed assays showed that biosynthesized AgNPs has significant concentration dependent cytotoxic effect with a varied degree of alteration in morphology and cell proliferation on K562 cell line similar to chemically synthesized silver nanoparticles.Also, induction of apoptosis was checked by cellular DNA fragmentation which showed that the biogenic as well as chemically synthesized AgNPs treated cells exhibited extensive double strand breakage with concentration dependent manner.In summary the present findings clearly indicated that biogenic AgNPs showed decreased cell viability and inhibits cell proliferation with effective cytotoxicity and antiproliferative activity.
Keywords
Biogenic AgNPs; Silver Nanoparticles; Cytotoxicity; DNA fragmentation
Download this article as:| Copy the following to cite this article: Datkhile K. D, Durgawale P. P, Patil M. N. Biogenic silver nanoparticles are equally Cytotoxic as Chemically Synthesized silver nanoparticles. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Datkhile K. D, Durgawale P. P, Patil M. N. Biogenic silver nanoparticles are equally Cytotoxic as Chemically Synthesized silver nanoparticles. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13678 |
Introduction
An increasing application of nanomaterials is for production of nanomedicine had confirmed to be of deep importance in the field of biomedicine. Silver has long been recognized as one of the nanoparticles having importance in medical and industrial processes. Silver nanoparticles (AgNPs) are among the emerging nanoproducts reported to have many uses in variant fields of catalysis, photonics, biosensing and diagnostics(1). AgNPs have also gained interest in the field of nanonedicine due to their unique properties such as antibacterial (2), antifungal (3), anti-parasitic(4); antioxidant(5)and apparent therapeutic potential in treating a variety of diseasessuch as diabetes, AIDS, cancer etc. in the future(6-8). So far, several methods have been developed for the synthesis of AgNPs ranging from chemical (9), electrochemical(10) and photochemical methods (11). Very recently, biological synthesis of nanoparticles is in application for its ecofriendly mode of synthesis with promising biomedical applications.The biological or green synthesis has received complete attention in the area of nanotechnology research because nanoparticle synthesis by using living organism, cellular extracts and plant based products, bacteria and fungi could be as simple, viable and non-toxic forms compared to other methods of synthesis(12). Biological techniques are less toxic and cost effective in the synthesis of nanoparticles where plant extracts are used as reducing and capping agent. Earlier synthesis of silver nanoparticles using different medicinal plants for pharmaceutical and biological applications have been reported (13-14).
The use of nanoparticles for biomedical applications, such as drug delivery, biosensors, and diagnostic tools, has been extensively studied throughout the past decade. However, little is known about the health risks and toxicity of these nanomaterials. Due to the unintended adverse effect of chemically AgNPs synthesized biogenic nanoparticles is an emerging and growing concern both academically and socially. With this evidence, here we investigated the comparison of cytotoxicity effects of both biosynthesized and chemically synthesizedf AgNPs. We studied effect of both the AgNPs on cytotoxicity of human leukemia cell line, K562 in vitro. The plant extract derived nanoparticles exhibited strong cytotoxic effects against selected leukemia cells along with chemically synthesized nanoparticles, which suggest that biologically synthesized silver nanoparticles could be the most promising candidates as biomedicines for treatment of diseases.
Materials and Methods
Preparation of aqueous extract for Synthesis of Silver Nanoparticles
To prepare aqueous extract, 10 gms powder of medicinal plant Nathophodytes foetida leaves was mixed with 100 mL of double distilled water in a 250 mL capacity Erlenmeyer flask. The mixture was boiled at 80°C for 30 minutes. After cooling, the aqueous extract was filtered through a series of Whatman filters and finally passed through a 0.22μm filter and stored at 4°C for further experiments.
Biosynthesis and Purification of Silver Nanoparticles
Aqueous solutions of 1 milli molar (mM) silver nitrate (AgNO3) prepared and used for the synthesis of silver nanoparticles. The preparation of silver nanoparticles was carried out by adding 10 ml of plant extract to 90 ml of 1mM AgNO3 solution for reduction of Ag ions. The preparation was incubated at room temperature in dark. The reaction for synthesis of plant Ag-NPs was observed to 24 hours (h). Preliminary characterization of biosynthesized AgNPs was carried out using UV-Visible spectroscopy. The reduction of pure Ag ions was monitored by measuring UV visible spectrum of the reaction mixture at a wavelength of 300–700 nm for AgNPs by sampling the aliquots withdrawn from reaction mixture at different time intervals. The AgNPs were further purified by frequent centrifugation at 10,000 rpm for 30 minutes, washed with double-distilled water followed by redispersion of the pellet in deionized water for further experiments. Chemically synthesized silver nanoparticles (<100 nm particle size)were purchased from Sigma Aldrich.
Cell Line and Cell Culture
To investigate the in vitro inhibitory effects of the Ag-NPs & NFAgNPs, human leukemia cell line i.e., K562 cell lines were obtained from National Centre for Cell Sciences, Pune, India. The cells were maintained in T-25 flasks containing RPMI1640 liquid medium supplemented with 10% heat inactivated Fetal Bovine Serum (FBS), Penicillin-Streptomycin at 100 U/mL / 100μg/mL respectively and maineained under an atmosphere of 5 % CO2, 95% humidity and 37°C.
Effect on Cell Morphology
In a six-well culture plate, K562 cells were seeded at 1×105 cells/ well and incubated for 24hrs at 37°C in 5 % CO2. Later, the cells were treated with different concentrations (1, 2.5, 5, 7.5, 10, 15, 20, 25, 30 µg/mL) of AgNPs,NFAgNPs in RPMI1640 medium without FBS and further incubated for 48 h. The cytomorphology of the cells then examined in an inverted phase-contrast microscope (Primovert Carl Zeiss microscope).
In Vitro assay for Cytotoxicity Activity (MTT assay).
In vitro inhibitory effects of the biosynthesized and chemically synthesized AgNPs on human leukemia cell line was determined by the MTT colorimetric assay and vital dye exclusion/Trypan blue assay. The cells were maintained in RPMI16 40 medium supplemented with 10% FBS, Penicillin-Streptomycin at 100 U/mL / 100μg/mL in a humidified atmosphere of 5% CO2 at 37°C. Ten thousand cells in 200 μl of RPMI 1640 medium per well were seeded in a 96 well plate and incubated at 37°C, 5% CO2. After 24 hrs incubation the confluent cells were exposed to respective treatment of nanoprticles at different concentrations ((1, 2.5, 5, 7.5, 10, 15, 20, 25, 30 µg/mL) in RPMI 1640 medium without FBS and incubated for further 48h at 37°C& 5% CO2. The solvent DMSO treated cells served as treatment control. After completion of treatment of 48h, medium was removed and cells were washed with Hanks Balanced Salt Solution. There after, 20μl/well of 5mg/ml concentration of MTT(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), was added to the cells and further incubated for another 4 h at 37°C, and 5% CO2 atmosphere. After 4h incubation, 200 μl of dimethyl sulphoxide (DMSO) was added to each well to dissolve the formazan crystals. The Absorbane of purpule colour developed was measured at 560nm wavelength using UV-Vis 1800 spectrophotometer (Shimadzu) to determine percentage inhibition of growth of treated as well as untreated cells. All experiments were performed in triplicates. The effect of the AgNPs & NFAgNPs on the cell proliferation was expressed as the % growth inhibition, using the following formula: Percentage (%)inhibition = 100- (A560nm of treated cells / A560nm of control cells) × 100%.
Cell Viability Assay
K562 cells at a seeding density of 1 X 105 cells/ml were cultured in six well plates containing RPMI1640 medium with 10% FBS and Penicillin-Streptomycin at 100 U/mL / 100μg/mL. Cells were allowed to grow for 24 h for recovery. Such cells were then treated with AgNPs and NF AgNPs at different concentrations as mentioned earlier. Thereafter, viable cells in control and treated groups were counted by trypan blue staining using hemocytometer after 48 h of treatment. Percentage cell viability was determined by counting the cells using the following formula: Percentage cell viability = (Total number of viable cells/Total number of cells) × 100.
DNA fragmentation Assay
In order to check apoptosis, DNA fragmentation assay was performed according to the procedure described by (Herrmann et al., 1994). In brief, 1x 106 cells in 6 well plates were treated with different concentrations of NF AgNPs and AgNP along with untreated controls and incubated at 37°C in 5% CO2, for 48 hours. After 48 h of treatment, cells were harvested by centrifugation and washed once with HBSS. Such cells were lysed in 0.3 ml of cell lysis buffer containing (10mM Tris-HCl, pH 7.5, 1 mM EDTA, 0.2% triton-X 100, 0.5% sodium dodecyl sulphate (SDS). The cell lysate was incubated with 0.5 miligrams (mg)/ml RNase Aat 37°C for 2 h,thereafter, 0.2mg/mlat 55°C for 1h.DNA in Aqueous phase was proteinase K by adding 1/10th volume of 5M sodium chloride and equal volume of isopropanol at -20°C. After overnight precipitation, the suspension was centrifuged at 12000 x g for 30 minutes at 4°C followed by DNA pellet wash by 70% ice cold ethanol and air-dried DNA pellet for 10 minutes at room temperature to remove traces of ethanol. The DNA was resuspended in appropriate volume of T10E1 buffer (pH8.0). DNA dissolved in loading buffer were loaded on a 1.5% (w/v) low EEO agarose (GeNei) gel containing 1 μg/mL ethidium bromide and subjected to electrophoresis at 80 V for 1-2 h in Tris-Acetate-EDTA (TAE) buffer along with DNA molecular weight marker. The DNA fragments were visualized by exposing the gels to UV transilluminator followed by photography in gel documentation system (BioRad Laboratories).
Statistical Analysis
The Percentage inhibition of cell growth and percent cell viability are reported as Mean± SEM of three independent experiments. SPSS for windows version 11.0 software was used for statistical analysis.
Results
Biogenic Agnps Causes Decreased Cell Viability and Inhibition of Cell Proliferation
In order to determine the effect of biogenic and chemically synthesized AgNPs on cell viability and cell proliferation, trypan blue vital dye exclusion and MTT assay experiments were carried out. The cytotoxic activity of the biologically and chemically synthesized silver nanoparticles were evaluated in vitro against K562 cell lines after 48 h exposure and the cell viability measured over a range of concentrations between 1 and 30μg/mL . The results obtained from the revealed that biogenic silver nanoparticle exhibited a range of significant cytotoxic activities in a dose dependent manner varying from 1 μg/mL to 30 μg/. Their activity data is presented in the graphical representation also (Fig. 1). MTT assay done after AgNPs treatment revealed that both biogenic as well as chemically synthesized silver nanoparticles were inhibiting K562 cell proliferation in a concentration dependent manner (Table 1).It also has been reported that the cytotoxicity on K562 cell lines were increased with an increase in concentration of AgNPs. Complete mortality was observed in 30 µg/ml and higher concentrations of AgNPs. These results thus suggested that the stress caused by both AgNPs is affecting cell viability and inhibiting cell proliferation.
Table 1: Cytotoxic activity of (A) biogenic (NFAgNPs) and (B) chemically synthesized AgNPs against K562 cell line proliferation, determined by MTT assay. All the data were expressed in Mean ± SEM of three independent experiments
| (A) Concentration of NFAgNPs (µg/ml) | Absorbance @ 560nm Mean ± SEM | Percentage (%) Inhibition Mean ± SEM | (B) Concentration of AgNPs (µg/ml) | Absorbance @ 560nm Mean ± SEM | Percentage (%) Inhibition Mean ± SEM |
| Control | 1.94 ± 0.31 | 0.00 ± 0.00 | Control | 1.160 ± 0.038 | 0.00 ± 0.00 |
| 1.0 | 1.676 ± 0.25 | 13.57 ± 0.71 | 1.0 | 1.05 ± 0.0382 | 9.87 ± 0.31 |
| 2.5 | 1.58 ± 0.25 | 18.55 ± 0.70 | 2.5 | 0.90 ± 0.017 | 22.74 ± 1.00 |
| 5.0 | 1.353 ± 0.22 | 30.24 ± 0.87 | 5.0 | 0.77 ± 0.035 | 33.90 ± 0.84 |
| 7.5 | 1.11 ± 0.18 | 42.78 ± 1.45 | 7.5 | 0.67 ± 0.014 | 42.48 ± 0.61 |
| 10.0 | 0.845 ± 0.14 | 56.44 ± 1.33 | 10.0 | 0.53 ± 0.026 | 54.07 ± 0.75 |
| 15.0 | 0.656 ± 0.06 | 66.15 ± 1.10 | 15.0 | 0.39 ± 0.001 | 66.52 ± 1.08 |
| 20.0 | 0.345 ± 0.04 | 82.18 ± 0.80 | 20.0 | 0.245 ± 0.002 | 78.96 ± 0.92 |
| 25.0 | 0.053 ± 0.007 | 97.25 ± 1.03 | 25.0 | 0.139 ± 0.003 | 88.02 ± 0.44 |
| 30.0 | 0.0007 ± 0.00 | 99.96 ± 0.013 | 30.0 | 0.029 ±0.009 | 97.51 ± 0.96 |
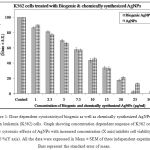 |
Figure 1: Dose dependent cytotoxicity of biogenic as well as chemically synthesized AgNPs on human leukemia (K562) cells. Graph showing concentration dependent response of K562 cells to show cytotoxic effects of AgNPs with increased concentration (X axis) inhibits cell viability up to 100 %(Y axis). All the data were expressed in Mean ± SEM of three independent experiments. Bars represent the standard error of mean. |
AgNPs causes cytomorphological alterations.
Microscopic observations of both biologically and chemically synthesized AgNPs treated K562 cells were monitored using Carl Zeissphase contrastinverted microscope where in treated cells showed distinct cell ular mor phological alteration indicating unhealthy cells, whereasthe control cells appeared normal confluent aggregates with rounded cells (Figure 2A). BiosynthesizedAgNPs treated cells showed the morphological variations such as loss of membrane integrity therefore appeared shrunken, inhibition of cell growth and cytoplasmic condensation when comparedto control. Similar results were observed by other groups in cells treated with chemically synthesized AgNPs (Figure 2B). These results indicate that the AgNPs treated cells at higher concentrations undergone cell death where as untreated cells were live.
 |
Figure 2: In vitro cytotoxicity of AgNPs.
|
Cytomorphological changes and growth inhibition of AgNPs treated K562 cells: Cells were treated with or without biogenic as well as chemically synthesized AgNPs for 48 h at different concentrations and microscopic photographswere taken using inverted phase contrast microscope microscope at 20×magnification. A.(a) Non treated K562 cells; (b) K562 cells treated with 2.5µg/ml biogenicAgNPs; (c) K562 cells treated with 5µg/ml biogenic AgNPs (d) K562 cells treated with 10µg/ml biogenicAgNPs; (e) K562 cells treated with 15µg/ml biogenicAgNPs. (f) K562 cells treated with 20µg/ml biogenic AgNPs. B. (a) Non treated K562 cells; (b) K562 cells treated with 2.5µg/ml chemically synthesized AgNPs; (c) K562 cells treated with 5µg/ml chemically synthesized AgNPs (d) K562 cells treated with 10µg/ml chemically synthesized AgNPs; (e) K562 cells treated with 15µg/ml chemically synthesized AgNPs. (f) K562 cells treated with 20µg/ml chemically synthesized AgNPs.
Agnps induced Stress Causes Apoptosis in K562 Cells
Evaluation of induction of apoptosis following treatment with increasing concentrations of biologically and chemically synthesized AgNPs was determined by DNA fragmentation pattern. The AgNPs treated cells exhibited extensive double strand breaks thereby yielding a ladder appearance shown in (Lane 4-8, Figure 3 A and B), while the DNA of control as well as minimum concentration of both the AgNPs treated cells exhibited minimum DNA breakage (Lane 2 and 3, ) (Fig. 3 A and B). There was dose dependent increase in DNA fragmentation in both biogenic as well as chemically synthesized treated K562 cells.
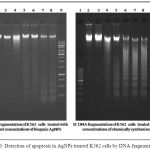 |
Figure 3: Detection of apoptosis in AgNPs treated K562 cells by DNA fragmentation.
|
Representative agarose gel images showing DNA fragmentation of K562 cells treated with biogenic silver nanoparticles (AgNPs), and chemically synthesized AgNPs at different concentrations. Lane1;100 base pair DNA marker, lane 2; DNA from untreated control K562 cells, lane 3; DNA from cells treated with 2.5 microgram AgNPs, lane 4; lanes 4-8; DNA from cells treated with 5, 10, 15, 20, 25 µg/ml of AgNPs respectively, lane 9 ;1Kb DNA marker.
Discussion
The toxicity of AgNPs to prokaryotic, eukaryotic cells and multicellular organisms has been investigated in a number of studies. But many studies failed to describe the behavior of nanoparticles in the particular biological media. Therefore the purpose of our study was to investigate the toxicity AgNPs with a specific focus on nature of synthesis and concentration dependent effects and to explore the possible differences in toxicity.In the present study we used exposure of concentrations in the range of 1–30 μg/ml, primarily based on previous studies of Ag nanoparticles and eukaryotic cells. We found biologically synthesized silver nanoparticles were more toxic to ward K562 cellssimilar tochemically synthesizedAgNPs in a concentration dependent manner. When cytoxicity is considered it is known that the cytotoxic effects of silver are the result of active physicochemical interaction of silver atoms with the functional groups of intracellular proteins, as well as with the nitrogen bases and phosphate groups in DNA but the actual mechanism linked to silver nanoparticle cytotoxicity is not completely understood. The cell viability assay is important method for toxicology analysis which explain the cellular response to a toxic materials, and it can provide information on cell death, survival, and metabolic activities (6). Earlier it has been reported that the cytotoxicity on HeLa cell lines increased with an increase in concentration of AgNPs(15). Also, enhanced cytotoxic activity of AgNPs on MCF7 cells observed due to the increased cytotoxicity, decreased viability and proliferation which result in apoptosis through induced programmed cell death (16). Piao et al.2011(17) reported that AgNPs and AgNO3 showed cytotoxicity in a dose-dependent manner in human liver cells; among these materials AgNPs showed higher cytotoxicity compared toAgNO3.Molina et al. 2010(18)reported that colloidal silver induced dose-dependent cytotoxic effect on breast cancer cells.To support these studies, wehereby confirmed that AgNPs induced morphological changes in K562 cells where AgNPs treated K562 cells showed apparent morphological variations such as alteration in cell shape compared to control cells. Biosynthesized AgNPs treated cells appeared to shrink when compared to control. It is clearly evidenced that synthesized AgNPs induces cell damage through the loss of cell membrane integrity and apoptosis. Several factors influence toxicity of AgNPs such as dose, time and size of the particles. DNA fragmentation is broadly considered as a characteristic feature of apoptosis. Nanoparticles easily cross the nuclear membrane and they can therefore interact with DNA directly or indirectly although the exact mechanism for this interaction is not yet known(18). In order to explore the cytotoxicity of AgNPs in human leukemia cells we used the agarose gel electrophoresis for DNA fragmentation analysis. In contrast to the concentration dependent effect on cell viability, we found that all tested AgNPs induced DNA damage after 48 h reported by the DNA fragmentation. The relation of effect on cell viability and the DNA damage may potentially be explained by reactive oxygen species (ROS) generation(19). However, we could not provide any evidence of intracellular ROS production preceding toxicity, thus contradicting many other published in vitro studies(20).Several studies have demonstrated that AgNPs significantly induce either apoptosis or necrosis in different celltypes(15, 21-23). Our results supported previous findings where AgNPs treatment significantly increased DNA fragmentation in K562 cells. Therefore these results suggest that the stress induced by AgNPs is responsible for inhibition of cell proliferation followed by induction of apoptosis in K562 cells.
Conflict of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
Acknowledgement
We greatly acknowledge Dr. Neelima Malik, Vice Chancellor, Krishna Institute of Medical Sciences for guidance & support to carry out this research. The experimental assistance of Mr. Santosh Jadhav is duly acknowledged. Krishna Institute of Medical Sciences University is duly acknowledged for for supporting the experimental work.
References
- Songping W, Shuyuan M. Preparation of ultra fine silver powder using ascorbic acid. Mater. Chem. Phy. 2005; 89: 423-427.
- Ravindra S, Mohan YM, Reddy NN and Raju KM . Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via Green Approach. Colloids and Surfaces A: Physicochemical and Engineering Aspects., 2010; 367:(1-3) 31-40.
- Duran N, Marcato PD, De Souza GI, Alves OL and Esposito E. Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. Journal of Biomedical Nanotechnology., 2007; 3(2): 203-208.
- Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, Van de Meent D, Dekkers S, de Jong WH, Van Zijverden M, Sips AJAM and Geertsma RE., Nano-silver: a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology., 2009; 3(2) :109-138
- Conde J, Doria G, Baptista P. Noble metal nanoparticles applications in cancer. J Drug Deliv., 2012;751075
- Rani APV, Hande M P. Valiyaveettil S Antiproliferative activity of silver nanoparticles. BMC Ceel Biol., 2009; 10:65.
- Lara HH, Ayala-Nu˜nez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol. 2010;8:1.
- Sukirtha R, Priyanka KM, Antony JJ, Kamalakkannan S, Thangam R, Gunasekaran P, Krishnan M and Achiraman S.Cytotoxic effect of green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochemistry., 2012; 47(2): 273–279.
- Lu, X., et al., Chemical synthesis of novel plasmonic nanoparticles. Annual review of physical chemistry, 2009; 60: 167-192.
- Yin B, Ma H, Wang S, Chen S. Electrochemical synthesis of silver nanoparticles under protection of poly(N-vinylpyrrolidone). J Phys Chem B., 2003;107:8898–8904.
- Callegari A, Tonti D, Chergui M. Photochemically grown silver nanoparticles with wavelength-controlled size and shape. Nano Lett., 2003; 3:1565–8.
- Iravani, S., Green synthesis of metal nanoparticles using plants. Green Chemistry, 2011; 13(10): 2638- 2650.
- Leela, A., Vivekanandan, M.Tapping the unexploited plant resources for the synthesis of silver nanoparticles. African Journal of Biotechnology., 2008; 7: 3162-3165.
- Song, J.Y., Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess and biosystems engineering., 2009; 32: 79-84.
- Jeyaraj M, Rajesha M, Arunb R, MubarakAlic D, Sathishkumara G, Sivanandhana G, et al. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf B: Biointerfaces., 2013;102:708–717.
- MfouoTynga I, ElHussein A, AbdelHarith M, Abrahamse H. Photodynamic ability of silver nanoparticles in inducing cytotoxic effects in breast and lung cancer cell lines. Int J Nanomed., 2014; 9(1):3771–3780.
- Piao MJ, Kang KA, Lee IK et al., Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondriainvolved apoptosis, Toxicology Letters, 2011; 201(1): 92–100.
- Molina MAF, E.Mendoza-Gamboa,C.A. Sierra-Rivera et al., “Antitumor activity of colloidal silver on MCF-7 human breast cancer cells,” Journal of Experimental and Clinical Cancer Research,2010;29 (1) 148.
- Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med.2008;44(9):1689–1699.
- Kim S, Ryu DY. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J Appl Toxicol. 2013;33(2):78–89
- Wright J B, Lam K, Buret A G, Olson M E and Burrell RE. Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regeneration., 2002; 10 (3):141–151
- Kang B, Mackey M A and El-Sayed M A. Nuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosis. J. Am. Chem. Soc. 2010; 132 (5): 1517–1519
- Foldbjerg R, Dang D A and Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011; 85: 743–750.







