Eman Turky Shamkhy, Vean Sabah Ali, Heba Fadhil Hassan, Suha Talal Abd, Ghada Ibraheem Al-Duboni and Rasha Abbas Azeez
Department of Basic Science, College of Dentistry, University of Baghdad, Baghdad-Iraq.
Correspondent Author E-mail: dr.alkarkhi@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1079
Abstract
The biological actions of ligand 2-{(E)-[2-hydroxyphenyl)imino] methyl}phenol [LF] and its metallic complexes of Cu (II), Co (II) and Rh (III) ions were examined against isolated bacteria include: Escherichia coli (EC),Staphylococcus aureus (SA), Candida albicans (CA), Acintobacterbaumani (AB), Enterobacter spp.(ES), Pseudo fluresence (PF). The organometallic complexes showed diverse properties reliant on the concentration and the cellular kind, cobalt complex demonstrated extra action against CA, ES. PF and SA, while rhodium metal complex display additional activity towards EC only.
Keywords
Ligand; Organometallic; Bioactivity
Download this article as:| Copy the following to cite this article: Shamkhy E. T, Ali V. S, Hassan H. F, Abd S. T, Al-Duboni G. I, Azeez R. A. Bioactivity of Formerly Synthesized Imino Phenol Ligand and its Organometallic Complexes. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Shamkhy E. T, Ali V. S, Hassan H. F, Abd S. T, Al-Duboni G. I, Azeez R. A. Bioactivity of Formerly Synthesized Imino Phenol Ligand and its Organometallic Complexes. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13983 |
Introduction
Cyclic compounds and theirderivatives possess remarkable capacity for coordination withtransition metals giving rise to coordination compounds with variable structural geometry, presently, there is a rising attention in the ligand chemistry of structurally modified bio-ligands. Organometallic complexes with possible biological activity are the spotlight of wide investigations[1-3].
A formerly synthesized 2-{(E)-[2-hydroxyphenyl)imino] methyl}phenol [LF] was prepared according to the below equation [4]:
![Scheme 1: Synthesis equation of the 2-{(E)-[2 hydroxyphenyl)imino] methyl}phenol](https://biomedpharmajournal.org/wp-content/uploads/2017/03/Vol10No1_Bioa_Eman_sch1-150x150.jpg) |
Scheme 1: Synthesis equation of the 2-{(E)-[2 hydroxyphenyl)imino] methyl}phenol
|
Cu(II), Co(II), and Rh(III) metal complexes were prepared and characterized with the ligand (LF) using IR, UV-Vis spectroscopy, metal investigation spectrophotometer, magnetic susceptibility and conductivity measurements[5-9]. Scheme 2 shows the predictable geometry of the organometallic complexes:
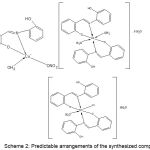 |
Scheme 2: Predictable arrangements of the synthesized complexes
|
Specifically, ligands composed of salicyaldehydes are very hopeful in the exploration of new efficient materials. They shows a diversity of biological activities [10] as well as show important photochromism where light absorption lead to interconversion between enol- imine and keto-amine tautomers via intramolecular hydrogen transfer [6].
As part of our efforts in this study is to inspect the antimicrobial action of ligand and its organometallic complexes towards Escherichia coli (EC),Staphylococcus aureus (SA), Candida albicans (CA), Acintobacterbaumani (AB), Enterobacter spp.(ES), Pseudo fluresence (PF). The complexes shows diverse action reliant to the concentration and the cellular type, cobalt complex demonstrate extra activity toward CA, ES. PF and SA, while rhodium metal complex exhibit more activity against EC only.
Materials and Methods
Dilution for the chemical compounds (ligands)
From Stock solution of Schiff organic ligand in concentration 0.1M, make the dilution of concentration 0.01M by using the dilution rule (M1V1 = M2V2), and use DMSO as a solvent or dilute for the stock solution
Test organisms
Tested bacterial and fungal were isolated from diverse scientific specimens, samples were isolated and identify according to typical laboratory methods [11]. Isolated bacteria include: Escherichiacoli, Staphylococcus aureus, Candida albicans, Acintobacterb-aumani, Enterobacter spp., Pseudo fluresence.
Bacterial and fungal Media (Agar Media)
Muller Hinton Agar set according to manufacturer’s regulations which involved dissolving 38 grams in one liter of de-ionized water with boiling, it was sterilized by autoclave at 15 lb pressure for 15 minutes. , then left to cool at 45- 50°C, poured and left to harden then put them in incubator at 37°C for 24 hours then kept in fridge till being used.
Antimicrobial Screening (in Vitro)
The antimicrobial activity of the ligand compounds LF, RhLF, CuLF, COLF and DMSO were measured by well diffusion technique [12, 13]. The prepared culture plates were immunized with different selected strains of bacteria and fungi using dispersal method. Wells were made on the agar surface with 6 mm cork borer. The location of the wells for each extract was marked at the external walls of plates before addition of chemical complexes and DMSO. The agents were discharged into the well. Each well was filled with 100µl with corresponding agents with the assistance of a micropipette. The plates were incubated at37±2°C for 24 hours for bacterial and 25±2°C for 48 hours for fungal action. The plates were observed for the zone clearances around the wells.The resulting zones of inhibition were uniformly circular. The widths of the zones of complete inhibition were measured, with the diameter of the disc. Regions are measured to the nearest whole millimeter, using sliding calipers or a ruler, which is held on the back of the reversed petri plate.
Results and Discussion
The main purpose of the production and preparation of any antimicrobial complex is to prevent the causal microbe without any side effects on the patients. Furthermore, it is well-intentioned to stress here on the basic idea of applying any chemotherapeutic agent which hinge on basically on the specific control of only one biological function and not multiple ones. The antibacterial activity of the parental ligand and its metal complexes against Escherichia coli (EC),Staphylococcus aureus (SA), Candida albicans (CA), Acintobacterbaumani (AB), Enterobacter spp.(ES), Pseudo fluresence (PF) [14].
Two concentrations were used to the bioactivity test 0.01 and 0.1. The data that was collected are recorded in Table 1 and Table 2 sequentially.
Table 1: Antimicrobial activity of Ligand; and its metal complexes in concentration 0.1M
| Inhibition zone diameter (mm) |
Agents |
|||||
|
Staphylococcus aurous Mean±SD |
Pseudo Fluresence Mean±SD |
Enterobacter sp. Mean±SD |
E.Coli Mean±SD |
Candida albicans Mean±SD |
Acintobacterbaumani Mean±SD |
|
| 0 | 0 | 0 | 0 | 0 | 0 | DMSO |
| 35.9 ± 0.99 | 32.8 ± 2.15 | 36.0 ± 1.41 | 39.0 ± 0.82 | 30.5 ± 0.71 | 25.4 ± 1.07 | LF (0.1) |
| 22.5 ± 0.70 | 33.0 ± 1.57 | 28.0 ± 1.25 | 37.7 ± 0.95 | 24.9 ± 0.74 | 16.3 ± 0.95 | RhLF(0.1) |
| 13.0 ± 0.66 | 13.2 ± 1.03 | 18.0 ± 1.15 | 20.6 ± 0.70 | 15.7 ± 0.95 | 7.2 ± 0.79 | CuLF(0.1) |
| 37.6 ± 1.17 | 35.5 ± 1.57 | 34.0 ± 1.42 | 30.0 ± 0.82 | 33.9 ± 0.74 | 24.9 ± 0.99 | COLF(0.1) |
Weak – < 10 mm in diameter, Moderately active – 10 – 15 mm in diameter,Strongly active – >15 mm in diameter
Table 2: Antimicrobial activity of Ligand; and its metal complexes in concentration 0.01M
| Inhibition zone diameter (mm) |
Agents |
|||||
|
Staphylococcus aurous Mean±SD |
Pseudo Fluresence Mean±SD |
Enterobacter sp. Mean±SD |
E.Coli Mean±SD |
Candida albicans Mean ±SD |
Acintobacterbaumani Mean±SD |
|
| 0 | 0 | 0 | 0 | 0 | 0 | DMSO |
| 28.0 ± 0.66 | 24.8 ± 1.32 | 22.7 ± 0.95 | 34.2 ± 0.79 | 27.6 ± 0.83 | 23.9 ± 0 74 | LF (0.01) |
| 15.4 ± 0.84 | 18.2 ± 1.03 | 18.5 ± 0.85 | 25.0 ± 0.82 | 20.8 ± 0.79 | 14.8 ± 0.79 | RhLF(0.01) |
| 11.3 ± 0.94 | 12.4 ± 1.07 | 14.7 ± 0.67 | 19.5 ± 1.08 | 12.3 ± 0.48 | 7.3 ± 0.48 | CuLF(0.01) |
| 17.6 ± 1.3 | 12.5 ± 1 08 | 20.4 ± 1.07 | 25.1± 0.74 | 22.6 ± 0.84 | 10.7 ± 0.82 | COLF(0.01) |
Weak – < 10 mm in diameter, Moderately active – 10 – 15 mm in diameter,Strongly active – >15 mm in diameter
As it appears clearly from the tables above using concentration of 0.1 M is more active against 0.01 M concentration. COLF is the strongest compound against AB, CA,EC, ES, PF and SA while RHLF shows more activity against EC only.
Figures 1, 2, 3, 4, 5 and 6 shows the inhibition zones of the DMSO and the complexes using the 0.1 M concentration on different microbs cell lines.
 |
Figure 1: Inhibition zone diameter of ligand; and its metal complexes in concentration 0.1 for Staphylococcus aurous bacteria
|
 |
Figure 2: Inhibition zone diameter of ligand; and its metal complexes in concentration 0.1 for Acintobacterbaumani bacteria
|
 |
Figure 3: Inhibition zone diameter of ligand; and its metal complexes in concentration 0.1 for Pseudo Fluresence bacteria
|
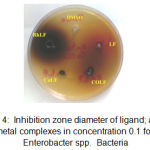 |
Figure 4: Inhibition zone diameter of ligand; and its metal complexes in concentration 0.1 for Enterobacter spp. Bacteria
|
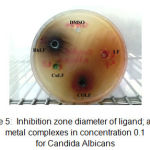 |
Figure 5: Inhibition zone diameter of ligand; and its metal complexes in concentration 0.1 for Candida Albicans
|
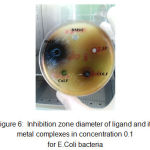 |
Figure 6: Inhibition zone diameter of ligand and its metal complexes in concentration 0.1 for E.Coli bacteria
|
Conclusion
Bioactivity was measured to the formerly prepared coordination compounds LF, CoLF, CuLF and RhLF, against Escherichia coli (EC),Staphylococcus aureus (SA), Candida albicans (CA), Acintobacterbaumani (AB), Enterobacter spp.(ES), Pseudo fluresence (PF), the research conluded to the following points:
The concentration of 0.1 M is shows extra activity in 0.01M concentration.
CoLF shows additional activity than other complexes.
References
- Etcheverry, S.B, Barrio, D.A, Cortiz, A.M and William, P.A.M. (2002) .Three new vanadyl(IV) complexes with non-steroidal anti-inflammatory drugs (Ibuprofen, Naproxen and Tolmetin). Bioactivity on osteoblast-like cells in culture. Journal of Inorganic Biochemistry 88: 94–100.
CorssRef - Singh, B.K, Jetley, U.K, Sharma, R.K and Garg, B.S. (2007). Synthesis, characterization and biological activity of complexes of 2-hydroxy-3,5-dimethylacetophenoneoxime (HDMAOX) with copper(II), cobalt(II), nickel(II) and palladium(II). SpectrochimicaActa Part A 68: 63–73.
CrossRef - Asegbeloyin, J.N., Agbo, I.C, Ukoha, P.O, Babhan, I. and Okafor, E.C. 2014. Synthesis, Characterization and in vitro Antibacterial Activity of Co(II), Cu(II) and Ni(II) Complexes with 4-Acylpyrazol-5-one Schiff Bases. Asian Journal of Chemistry; Vol. 26, 23: 8127-8133.
CrossRef - Eman, T. S. 2015.Synthesis, Characterizationand Spectroscopic Studies of 2-{(E)-Hydroxyphenyl)imino]methyl}Phenol Schiff Base with Some Metal Complexes. Journal of Al-Nahrain University Vol.18 (1): 39-45.
CrossRef - Yang-Hui L, Guo-Gan W, Shu-Lin M and Bai-Wang S. 2013. Complexation of different metals with a novel N-donor bridging receptor and Hirshfeld surfaces analysis. InorganicaChimicaActa. Vol. 397: 1– 9.
CrossRef - Suvra, A., Ambica, K., Achintesh N. B., Purak D., Debatra N. N. and Pinaki B.2013. Synthesis, characterization, X-ray structure and spectroscopic study of platinum (II) complexes with tridentate diazene ligands having O,N,S donor set. InorganicaChimicaActa. Vol. 394: 757–764.
CrossRef - Abeer A. Faheim, Safaa N. AbdouandZeinab H. Abd El-Wahab.2013. Synthesis and characterization of binary and ternary complexes of Co(II), Ni(II), Cu(II) and Zn(II) ions based on 4-aminotoluene-3-sulfonic acid. SpectrochimicaActa Part A: Molecular and Biomolecular Spectroscopy. Vol. 105: 109-124.
CrossRef - Manimaran, A. and Jayabalakrishnan, C.2010. Synthesis, characterization, redox, catalytic, and antibacterial activities of binuclear ruthenium(III) Schiff base complexes containing triphenyl phosphine as co-ligandSynthesis and Reactivity in Inorganic. Metal-Organic and Nano-Metal Chemistry. Vol. 40(2): 116-128.
- Hajria, A, Smirani, W. and Abderrahim, R. 2011. Synthesis, spectroscopic characterization and X-ray structure of [1,2a]benzimidazol-2-yl amidine. SpectrochimicaActa Part A.Vol.79: 1856-1859.
CrossRef - Felicia N. E., Tolulope M. F., Oluwole B. F. and Folashade T. Ogunsola. 2013. Substituent effect on spectral and antimicrobial activity of Schiff bases derived from aminobenzoic acids. Advances in Biological Chemistry, 3: 475-479.
CrossRef - Cheesbrough, M. 2000. District Laboratory Practice Manual in Tropical Countries Part Cambridge University Press, Cambridge. 136-137. 158,165, 180.
- Bauer AW, Kibry WM, Sherris JC, Turck M. 1966. Antibiotic susceptibility testing by a standardized single disc method. Am J ClinPathol. 45:493.
- Perez C. and Anesini Ethnopharmacol1994. In Vitro Antibacterial Activity Of Argentine Folk Medicinal Plants Against Salmonella Typ HI. Univ Buenos Aires CateraFarmaFacodontologia Buenos Aires Argentina. 44 (1): 41-46.
- Mostafa M. H. K., Eman H. I., Gehad G. M., Ehab M. Z. AND Ahmed B. 2012. Synthesis and characterization of a novel Schiff base metal complexes and their application in determination of iron in different types of natural water. Open Journal of Inorganic Chemistry. 2: 13-21.
CrossRef







