Manuscript accepted on :January 16, 2017
Published online on: --
Plagiarism Check: Yes
Mohammad Mustufa Khan1, Gyanendra Kumar Sonkar2, Roshan Alam1, Sangeeta Singh2, Sudhir Mehrotra3 and Satyendra Kumar Sonkar4
1Department of Biochemistry, Integral Institute of Medical Sciences and Research, Integral University, Lucknow, India.
2Department of Biochemistry, King George’s Medical University, Lucknow, India.
3Department of Medicine, Integral Institute of Medical Sciences and Research, Integral University, Lucknow, India.
4Incharge of Hemodialysis Unit, Department of Medicine, King George’s Medical University, Lucknow, India.
Corresponding Author E-mail: gyanendrakrsonkar@kgmcindia.edu
DOI : https://dx.doi.org/10.13005/bpj/1123
Abstract
Adiponectin gene (ADIPOQ) has association with circulatory adiponectin levels, metabolic disorders and type 2 diabetes mellitus (T2DM) in different population. We aimed to evaluate the relationship between SNP rs266729 in promoter region of ADIPOQ gene with T2DM patients, circulatory adiponectin levels and other clinical and anthropometric parameters in North Indian adult population. This case-control study was conducted on 300 subjects (150 T2DM and 150 healthy controls), aged between 25-75 years. Biochemical investigations performed included fasting and post prandial blood sugar level, lipid profile and serum creatinine. Glycated haemoglobin (HbA1c) and circulatory adiponectin levels were assayed using commercially available kits. PCR-RFLP method was used for genotyping. Mean levels of various anthropometric and biochemical parameters were significantly higher in T2DM than healthy controls (p<0.001). The levels of circulatory adiponectin was found significantly lower in T2DM as compared to healthy controls (p=0.014). There was no significant association of CC and CG genotypes with T2DM patients (p=0.81). C and G allele frequencies of the rs266729 were also not significantly associated with T2DM cases as compared to healthy controls (p=0.23). It was observed that significant impact on circulatory adiponectin levels for rs266729 polymorphism with GG genotype having very low circulatory adiponectin level (p<0.001). There was no significant association of CC, CG genotype and C, G allele frequencies of rs266729 in with T2DM cases as compared to healthy controls. However, it was observed that GG genotype of rs266729 has significant impact on circulatory adiponectin levels in T2DM cases. The GG carrier females have two fold increased risk for diabetes than men.
Keywords
ADIPOQ gene; SNP rs266729; Type 2 Diabetes Mellitus; Adiponectin
Download this article as:| Copy the following to cite this article: Khan M. M, Sonkar G. K, Alam R, Singh S, Mehrotra S, Sonkar S. K. Association of ADIPOQ Gene Variant Rs266729 with Circulatory Adiponectin levels in Patients with type 2 Diabetes in North Indian Population: A Case-Control Study. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Khan M. M, Sonkar G. K, Alam R, Singh S, Mehrotra S, Sonkar S. K. Association of ADIPOQ Gene Variant Rs266729 with Circulatory Adiponectin levels in Patients with type 2 Diabetes in North Indian Population: A Case-Control Study. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13433 |
Introduction
Diabetes is one of the most challenging non-communicable disease in the current century worldwide. T2DM, represents 90–95% of the total diabetes cases, ranging from predominantly insulin resistance, insulin deficiency to insulin secretory defect1. 422 million people are affected worldwide2 and its prevalence is increasing rapidly because of population and surge of obesity in many countries including India3. Prevalence of diabetes in total is 7.8%, while in males 7.9% and females 7.5% in Indian population2.
The exact pathogenesis of T2DM is unclear, it is generally accepted that T2DM is a multifactorial disorder resulting from genetic polymorphisms and several environmental factors.(4) Genome Wide Association Studies (GWAS) showed that T2DM has strong genetic link and many genes are responsible to develop T2DM and overt its complications also5. GWAS among European and Asian populations indentified ADIPOQ locus as the major gene for variation in the serum adiponectin levels6. The most widely studied adiponectin gene (ADIPOQ) has direct and indirect association with obesity, insulin resistance and metabolic traits that contributes in development of T2DM7, 8.
Adiponectin is an adipokine protein and secreted from adipocyte cells which plays a major role in regulating blood glucose levels, insulin sensitivity, and lipid metabolism9. Adiponectin levels differ according to age, sex, and body mass index with lower levels in obese individuals10. Its level is low in T2DM11. Consequently, low levels of plasma adiponectin might play a role in the etiology of insulin resistance and T2DM. There is growing evidence demonstrating the association of single nucleotide polymorphisms (SNPs) of the ADIPOQ gene with varying levels of circulating adiponectin. SNP rs266729 (−11377 C > G) and rs1501299 (+276 G > T) in the proximal promoter of ADIPOQ gene has been widely studied. This allelic variant is associated with lower adiponectin levels, has also been shown to be related with obesity12.
A functional single nucleotide polymorphism (SNP), rs266729, in the promoter region of the ADIPOQ gene causes an amino acid change leading to replacement of cytosine with guanine at nucleotide position -11377 (11377C > G) and is associated with T2DM13. Although the G allele of the ADIPOQ rs266729 SNP may appear important in associations with T2DM risk in various populations14, 15 yet genetic evidence of its effect on T2DM has been ambiguous.
Thus, these uncertain results motivated us to carry on a replication study and evaluate the relationship between SNP rs266729 in promoter region of ADIPOQ gene with T2DM in North Indian adult population. We also estimated the potential effects of its association with circulatory adiponectin levels, other clinical and anthropometric parameters. Clinical variables of T2DM was also assessed according to adiponectin gene rs266729 genotypes distribution.
Materials and Methods
Subject Selection
This is a case-control study and all subjects (T2DM and healthy controls) were enrolled from outpatients Diabetes Clinic of IIMS&R, Integral University, Lucknow (India) and King George’s Medical University, Lucknow (India). Study was approved by ethical committee of the institution. Written informed consent was taken from each subject and all procedures performed in this study involving human participants were in accordance with the ethical standards of this university and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards16.
This study was conducted on 300 subjects (150 T2DM and 150 healthy controls), aged between 25-75 years. T2DM was defined according to the criteria provided by World Health Organisation (2006): fasting blood sugar ≥126mg/dl, or 2 hour post 75g blood glucose load ≥200mg/dl. Subjects with ischemic heart disease, angina, myocardial infarction (MI), and electrocardiogram abnormalities, those with other concurrent sickness like chronic liver disease, hypothyroidism or those on drugs like diuretics, pregnant women and women using oral contraceptives were excluded from the study.
Laboratory Investigations
5ml venous blood was taken from each subject in EDTA, fluoride and plain vials after overnight fasting for laboratory investigation. Investigations performed included fasting and post prandial blood sugar, lipid profile and serum creatinine using of Vitros 250, Johnson & Johnson fully automated analyser and HbA1c by high performance liquid chromatography of Bio-Rad D10 analyser.
Estimation of Serum Adiponectin
Serum levels of adiponectin were evaluated using standard commercially available ELISA kit (USCN, Life Science Inc. Wuhan). The test was conducted in duplicate and as per manufacturer protocol. The inter-assay variation of adiponectin was CV<12% and intra-assay variation was CV<10%. The detection limit of the assay was 0.156-10ng/ml, and its sensitivity; the minimum detectable dose of human ADP is typically less than 0.065ng/ml).
Anthropometric Parameters
Age, gender, systolic and diastolic blood pressure (SBP and DBP respectively), body mass index (BMI), waist circumference (WC), waist hip ratio (WHR) were recorded as per standard protocol17.
DNA Extraction and Genotyping
Genomic DNA was extracted from peripheral blood mononuclear cells (PBMCs) using salting out method18. Isolated genomic DNA was quantified by spectrophotometer (Systronics-2205). Agarose gel electrophoresis was done to confirm the purity of isolated DNA containing 10μg/ml ethidium bromide and was observed under Gel Dock system (Bio-Rad, Gel Doc XR+, Universal Hood II).
PCR-RFLP method was used for genotyping. Primers were designed using Primer3 software. The ADIPOQ rs266729 (-11377C>G) polymorphic locus was amplified using the forward primer, 5’-ACTTGCCCTGCCTCTGTCTG-3’and the reverse primer, 5’-CCTGGAGAACTGGAAGCTG-3’19. Amplifications were performed using a thermal cycler (Applied Biosystems, US). Each PCR reaction consisted of a total of 15μl containing 100 ng template DNA, buffer (100mM Tris, pH 9.0; 500mM KCl; 0.1% gelatin), 200μM dNTP, 10 pmol of each primers and 1.0 unit Taq DNA polymerase. PCR reaction conditions, after an initial step of 5 min at 94°C, followed by 35 cycles of 30s at 94°C, 30s at 58°C, 30s at 72 °C, and a final extension step at 72°C for 7 min. PCR products were verified on 2% agarose gel containing 10μg/ml ethidium bromide and visualized by UV light. Following PCR, the products were digested with 10U of HhaI enzyme (New England Biolabs, UK), at 37°C for overnight. The restriction fragments of PCR products were separated on a 2.5% agarose gel. 50bp DNA ladder was included in each run.
Statistical Analysis
All the statistical analysis was done using SPSS software version 20.0 (Chicago, US). All the phenotypic data were compared by using ANOVA or unpaired t-test. Values were given as mean ± SD (Standard Deviation). Allelic and genotypic frequencies were presented with 95% confidence interval (CI) and were analyzed using χ2 test. Genotypes of ADIPOQ rs266729 (-11377C>G) were tested for Hardy–Weinberg equilibrium. A p value <0.05 was considered as statistically significant for all the data analyzed.
Results
Anthropometric and Clinical Characteristics
A total of 300 subjects were enrolled for this case control study (150 T2DM and 150 healthy controls). Age and gender were matched between the cases and control groups (p>0.05). Of these, 61.3% were males and 38.7% were females. The anthropometric and biochemical profile of both the groups are given in Table 1. Mean levels of anthropometric parameters i.e. WC, WHR, SBP and DBP were significantly higher in T2DM cases whereas BMI remained indifferent between the two groups. We found gender specific differences for WC and WHR, male had significantly more WC and WHR. Similarly, biochemical parameters such as blood sugar, HbA1c, SCr were also significantly raised in T2DM cases as compared to healthy controls (p<0.001). There was significant elevation in triglyceride and VLDL levels in T2DM cases as compared to healthy controls (p=0.002, p=0.003, respectively). The levels of circulatory adiponectin was found significantly lower in T2DM as compared to healthy controls (p=0.014), (Table 1).
Table 1: Clinical and Anthropometric parameters of Case (T2DM) and Control Groups
|
Parameters |
Case
(n=150) |
Control
(n=150) |
P-value |
|
| AGE (years) | 48.31±10.88 | 48.03±11.83 | 0.83 | |
| Gender (M/F) | 97/53 | 87/63 | 0.28 | |
| BMI (kg/m2) | 24.96±4.68 | 24.73±4.74 | 0.67 | |
| Waist circumference
(cm) |
Male | 99.46±5.49 | 95.18±6.16 | <0.001* |
| Female | 95.13±7.32 | 96.57±8.58 | 0.34 | |
| Waist-Hip Ratio
(WHR) |
Male | 0.99±0.06 | 0.95±0.06 | <0.001* |
| Female | 0.95±0.07 | 0.97±0.09 | 0.19 | |
| SBP (mmHg) | 140.75±26.65 | 114.45±7.52 | <0.001* | |
| DBP (mmHg) | 82.39±15.67 | 70.21±8.69 | <0.001* | |
| FBS (mg/dl) | 167.01±73.41 | 93.83±11.47 | <0.001* | |
| PPBS (mg/dl) | 260.59±112.52 | 127.29±24.41 | <0.001* | |
| HbA1c (%) | 8.01±2.09 | 5.30±0.72 | <0.001* | |
| Total Cholesterol (mg/dl) | 167.81±53.94 | 157.73±46.27 | 0.083 | |
| Triglyceride (mg/dl) | 182.27±112.76 | 148.35±68.36 | 0.002* | |
| HDL (mg/dl) | Male | 37.39±10.48 | 38.91±11.34 | 0.34 |
| Female | 39.39±12.61 | 39.58±11.11 | 0.93 | |
| LDL (mg/dl) | 92.59±47.59 | 91.15±47.11 | 0.79 | |
| VLDL (mg/dl) | 36.14±22.57 | 29.67±13.72 | 0.003* | |
| Serum Creatinine (mg/dl) | 2.44±2.11 | 0.91±0.25 | <0.001* | |
| Adiponectin (μg/ml) | 1.82±1.05 | 2.22±1.68 | 0.014* | |
Values are expressed as Mean ± Standard Deviation
*Significant considered as P<0.05. M: Male, F: Female, FBS: Fasting Blood Sugar, PPBS: Post-Prandial Blood Sugar, HbA1c: Glycated Haemoglobin, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Sugar, BMI: Body Mass Index, WC: Waist Circumference, TC: Total Cholesterol, TG: Triglyceride, HDL: High-Density Lipoprotein, LDL: Low-Density Lipoprotein, VLDL: Very Low-Density Lipoprotein,
Genotypes and Alleles Distribution
Fragment of 250bp was detected for the CC homozygote wild type (absence of HhaI restriction site). Fragments of 138bp and 112bp were detected for the GG mutant homozygote (presence of HhaI restriction site). The CG heterozygous contained the three fragments of 250bp, 138bp and 112bp (Fig. 1).
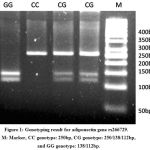 |
Figure 1: Genotyping result for adiponectin gene rs266729. M: Marker, CC genotype: 250bp, CG genotype: 250/138/112bp, and GG genotype: 138/112bp.
|
The genotype and allele frequencies of the rs266729 promoter region ADIPOQ gene polymorphism in T2DM patients and healthy controls are shown in Table 2. The frequencies of the CC, CG and GG genotypes of rs266729 were 52%, 36.7%, 11.3% in T2DM cases and 56.7%, 36.7%, 6.6% in healthy controls respectively. The allele frequencies of the C and G were 70.3%, 29.7% in T2DM and 75%, 25% in healthy controls respectively. There were no significant association of homozygous CC and heterozygous CG genotype with T2DM patients (OR: 0.92; CI: 0.57-1.49; p=0.81) and C, G allele frequencies of the rs266729 had no significant association with T2DM cases as compared to healthy controls (OR: 0.79; CI: 0.55-1.13; p=0.23). We also analysed the dominant genotype (CC vs. CG+GG) and found no significant difference between T2DM cases and healthy controls (OR: 0.83; CI: 0.53-1.31; p=0.49). Similarly, the recessive genotype (CG+CC vs. GG) did not show significant difference between T2DM cases and healthy controls (OR: 0.56; CI: 0.25-1.26; p=0.23).
Table 2: Genotypes and Allele Distribution of Adiponectin rs266729 Gene Polymorphism in Case (T2DM) and Control Groups
| rs266729 Polymorphism | Case
N (%) |
Control
N (%) |
OR (95% CI) | P-value |
| Co dominant
CC CG GG |
78 (52%) 55 (36.7%) 17 (11.3%) |
85 (56.7%) 55 (36.7%) 10 (6.6%) |
1.00 0.92 (0.57-1.49) 0.54 (0.23-1.25) |
– 0.81 0.21 |
| Dominant
CC CG+GG |
78 (52%) 72 (48%) |
85 (56.7%) 65 (43.3%) |
1.00 0.83 (0.53-1.31) |
– 0.49 |
| Recessive
CG+CC GG |
133 (88.7%) 17 (11.3%) |
140 (93.4%) 10 (6.6%) |
1.00 0.56 (0.25-1.26) |
– 0.23 |
| Alleles
C G |
211 (70.3%) 89 (29.7%) |
225 (75%) 75 (25%) |
1.00 0.79 (0.55-1.13) |
– 0.23 |
Values are expressed as Number (N) and Percentage (%)
OR: Odd Ratio, CI: Confidence Interval
Significant considered as P<0.05.
Impact of Genotypes Distribution on Clinical Characteristics
Clinical characteristics of the T2DM cases according to adiponectin rs266729 genotypes are shown in Table 3. There was significant impact of rs266729 polymorphism on circulatory adiponectin level (p<0.001). Serum adiponectin level was significantly lower in GG genotype as compared to CC & CG genotypes. Serum creatinine levels were also gradually raised from 14% to 16% for rs266729 genotypes CG and GG, respectively as compared to CC, but it was not significantly raised.
Table 3: Clinical variables of Cases (T2DM) according to adiponectin gene rs266729 genotypes.
| Genotypes→
Variables↓ |
CC(n=78) |
CG (n=55) |
GG (n=17) |
P-value |
| FBS (mg/dl) | 167.99±78.09 | 167.36±73.71 | 161.37±44.29 | 0.94 |
| PPBS (mg/dl) | 264.26±123.70 | 261.64±106.17 | 240.31±67.52 | 0.73 |
| HbA1c (%) | 8.14±2.23 | 7.85±1.97 | 7.95±1.72 | 0.73 |
| TC (mg/dl) | 172.23±56.84 | 163.61±52.33 | 161.11±42.35 | 0.57 |
| TG (mg/dl) | 177.27±105.20 | 192.93±130.13 | 170.72±77.64 | 0.66 |
| HDL (mg/dl) | 39.75±11.20 | 38.55±13.09 | 37.75±9.77 | 0.75 |
| LDL (mg/dl) | 97.32±50.90 | 86.91±44.40 | 89.24±38.71 | 0.44 |
| VLDL (mg/dl) | 34.89±21.05 | 38.54±26.02 | 34.09±15.51 | 0.61 |
| Serum Creatinine (mg/dl) | 2.22±1.55 | 2.53±1.63 | 2.57±2.18 | 0.49 |
| Adiponectin (μg/ml) | 1.97±1.05 | 1.97±0.99 | 0.71±0.36 | <0.001* |
Values are expressed as Mean ± Standard Deviation
*Significant considered as P<0.05.
FBS: Fasting Blood Sugar, PPBS: Post-Prandial Blood Sugar, HbA1c: Glycated Haemoglobin, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Sugar, BMI: Body Mass Index, WC: Waist Circumference, TC: Total Cholesterol, TG: Triglyceride, HDL: High-Density Lipoprotein, LDL: Low-Density Lipoprotein, VLDL: Very Low-Density Lipoprotein
Study of SNP rs266729 of ADIPOQ gene with gender distribution showed that GG genotype was almost two folds higher in females (12.9%) than males (6.5%), but it was not significantly associated (Table 4).
Table 4: Genotype and Allele frequency distribution of SNP rs266729 of ADIPOQ gene in Genders (M/F) of T2DM and controls
| rs266729 Polymorphism | Male (M)
N (%) |
Female (F)
N (%) |
OR (95% CI) | P-value |
| Co dominant
CC CG GG |
101 (54.9%) 71 (38.6%) 12 (6.5%) |
62 (53.4%) 39 (33.6%) 15 (12.9%) |
1.00 0.89 (0.54-1.48) 2.04 (0.89-4.64) |
– 0.76 0.13 |
| Dominant
CC CG+GG |
101 (54.9%) 83 (45.1%) |
62 (53.4%) 54 (46.6%) |
1.00 1.06 (0.66-1.69) |
– 0.90 |
| Recessive
CG+CC GG |
172 (93.5%) 12 (6.5%) |
101 (87.1%) 15 (12.9%) |
1.00 2.13 (0.96-4.73) |
– 0.09 |
| Alleles
C G |
273 (74.2%) 95 (25.8%) |
163 (70.3%) 69 (29.7%) |
1.00 1.22 (0.84-1.75) |
– 0.34 |
Values are expressed as Number (N) and Percentage (%)
OR: Odd Ratio, CI: Confidence Interval
Significant considered as P<0.05.
A multiple linear regression was performed so as to find the predictor of SNP rs266729. Model showed adiponectin as the only strong predictor (ß= -0.233, p=0.000, CI: -0.15 to -0.05), (Table 5).
Table 5: Multiple linear regression analysis to show the dependence of SNP rs266729 of ADIPOQ gene on study parameters in T2DM cases (N=150)
| Unstandardized Coefficients | Standardized Coefficients | t | p-value | 95% Confidence Interval for B | ||||
| B | Std. Error | Beta | Range (CI) | |||||
| (Constant) | -1.674 | 0.604 | 2.770 | 0.006 | 0.484 | 2.863 | ||
| HbA1c | -0.010 | 0.028 | -0.031 | -0.351 | 0.726 | -0.064 | 0.045 | |
| FBS | 0.002 | 0.001 | 0.164 | 1.227 | 0.221 | -0.01 | 0.004 | |
| PPBS | 0.000 | 0.001 | -0.147 | -1.083 | 0.280 | -0.003 | 0.001 | |
| TC | 0.000 | 0.002 | -0.033 | -0.212 | 0.832 | -0.004 | 0.004 | |
| TG | -0.001 | 0.004 | -0.161 | -0.267 | 0.790 | -0.009 | 0.007 | |
| HDL | 0.000 | 0.004 | -0.010 | -0.153 | 0.879 | -0.008 | 0.007 | |
| LDL | 0.000 | 0.002 | -0.031 | -0.228 | 0.820 | -0.004 | 0.003 | |
| VLDL | 0.010 | 0.022 | 0.276 | 0.442 | 0.659 | -0.033 | 0.052 | |
| BMI | -0.005 | 0.011 | -0.039 | -0.449 | 0.618 | -0.027 | 0.016 | |
| WHR
SCr |
0.354
0.038 |
0.727 | 0.038 | 0.486 | 0.627 | -1.078 | 1.786 | |
| 0.025 | 0.099 | 1.505 | 0.133 | -0.012 | 0.088 | |||
| ADP | -0.106 | 0.027 | -0.233 | -4.014 | 0.000* | -0.159 | -0.054 | |
| SBP | 7.007E-5 | 0.003 | 0.003 | 0.028 | 0.978 | -0.005 | 0.005 | |
| DBP | -0.002 | 0.004 | -0.050 | -0.565 | 0.573 | -0.010 | 0.006 | |
Dependent variable: SNP rs266729 of ADIPOQ gene
* p<0.05 is considered significant at 95% confidence interval
SBP – systolic blood pressure, DBP – diastolic blood pressure, FBS – Fasting Blood Sugar; Post – Prandial Blood Sugar; HbA1c – glycated haemoglobin; TC – total cholesterol, HDL – High-Density Lipoprotein; LDL – Low-Density Lipoprotein; VLDL – Very Low-Density Lipoprotein, SCr – serum creatinine, WHR – waist hip ratio, ADP- Adiponectin
Receiver operating characteristics (ROC) curve of adiponectin illustrated that area under curve was 0.446. When we take the cut-off value of adiponectin as 1.7μg/ml (sensitivity 48% and specificity 50%), then 52% T2DM patients have adiponectin levels below 1.7μg/ml.
Discussion
In present study, we investigated the possible association of ADIPOQ gene (rs266729 SNP) with levels of adiponectin and other clinical and anthropometric parameters in patients with T2DM in North India population. The baseline characteristics of clinical parameters i.e. blood sugar, HbA1c, SCr, triglyceride and VLDL were significantly raised while serum adiponectin level was significantly lower in T2DM cases as compared to healthy controls. Similarly, anthropometric parameters i.e. WC, WHR, SBP and DBP were significantly higher in T2DM cases than healthy controls which are consistent with other reports from various ethnic groups20, 21. Studies have also reported that serum adiponectin level was lower in T2DM patients than healthy controls and hypoadiponectinemia was strongly associated with T2DM, insulin resistance, obesity and other metabolic diseases22, 23.
The frequencies of the CC, CG and GG genotypes of SNP rs266729 of ADIPOQ gene, had no significant differences in the homozygous CC and heterozygous CG genotypes in T2DM patients and healthy controls. We did not find any association between SNP rs266729 with C and G allele. Other research groups also found similar genotypic and allelic frequencies15, 24, 25. Whereas, a study on Iraqi population reported that the frequency of the G allele of rs266729 (C/G) polymorphism was significantly higher in diabetic subjects (28%) compared to that in normal subjects (14%). The homozygous genotype (GG) significantly increased the risk of T2DM by three folds and the heterozygous CG genotype significantly raised the risk of T2DM by two folds with respect to those of wild type26.
SNP rs266729 of ADIPOQ gene demonstrated that it has a significant association with lower circulatory adiponectin levels in T2DM and we also found that GG genotype of SNP rs266729 was significantly associated with lower adiponectin levels in T2DM than healthy control. About half of the T2DM patients had adiponectin level below 1.7μg/ml in our diabetic group. Contrary results were reported by other previously published reports in different population14, 27 as well as Indian population25. A study from US on multi-ethnic population reported that G allele was significantly associated with hypoadiponectinemia but there was no significant association of this SNP with adiponectin27. A very recent study on Chinese population also observed no significant association of this polymorphism with circulatory adiponectin level15 but various other Asian studies support our results25, 26, 28, 29. Serum creatinine levels were also gradually raised for rs266729 genotypes CG and GG, respectively as compared to CC. The higher serum creatinine levels were reported in diabetic and diabetic nephropathy patients who carry GG genotype and have higher frequency of G allele in various ethnic populations30. HbA1c was significantly elevated in T2DM as compared to healthy controls, but HbA1c was not associated by SNP rs266729 of ADIPOQ gene. A recent study observed that serum adiponectin was decreased in T2DM and negatively correlated with HbA1c22. Adiponectin was significantly associated with altered glucose metabolism and independently contributed to the variance of HbA1c in a population31.
In our study, T2DM patients have higher WC, WHR, SBP, DBP, TG, FBS and PPBS than healthy controls. A study from UK reported that the C11377G SNP in the adiponectin gene promoter is associated with blood pressure and waist–hip ratio. Replacing C by G caused an increase in DBP by 1.63%, SBP by 1.83% and WHR by 1.61%, per gene copy of which DBP and WHR had significant association32. However, a meta-analysis did not find any association of the ADIPOQ polymorphism (rs266729) with blood lipids and blood pressure in East Asian population33.Adiponectin has shown a negative correlation to SBP and DBP levels in healthy control34. Adiponectin level was positively associated with HDL cholesterol and negatively associated with BMI, WHR, TG, FBS, fasting insulin, SBP and DBP. The rs266729 minor G allele was associated with lower adiponectin27. Contrary, Adiponectin levels were inversely correlated with WC, WHR, weight, BMI and physical activity, but were less influenced by the polymorphisms of ADIPOQ studied35. Lipid profile was correlated with circulatory adiponectin level36. A significant association was observed for gender with diabetes. Significantly higher WC and WHR were found in diabetic males of our study group. Results showed that SNP rs266729 was significantly associated with body weight, WC, BMI and percentage of total body fat37.
Genotypic analysis showed that GG genotype was more common in females (12.9%) as compare to male (6.5%). Though it was not significant yet it may double the risk for diabetes in females as compare to males. Zhong et al. studied rs266729 SNP and found no significant difference in allelic frequency between the two genders but they observed that females with G allele at rs266729 had higher risk for coronary artery disease compared to females with C allele38.
Conclusion
There was no significant association of CC, CG genotype and C, G allele frequencies of the rs266729 with T2DM cases as compared to healthy controls. However, it was observed that GG genotype of rs266729 has significant impact on circulatory adiponectin levels in T2DM cases. GG genotype was more prevalent in females than males in T2DM cases in our study group. Therefore we can say that GG carrier females have two fold increased risk for diabetes than men and subjects with adiponectin below1.7μg/ml doubles the risk for developing the T2DM.
Recommendations
We have studied one SNP of promoter region of ADIPO gene. Haplotypes and block study with another SNP of promoter region of ADIPOQ gene are warranted in this population. Linkage disequilibrium of this SNP with various variants of ADIPOQ gene is also needed. Our sample size might be smaller to validate this SNP. Study should be replicated in larger sample size of this population.
Key messages
Lower circulatory adiponectin and higher serum creatinine were observed. GG genotype and G allele frequency are strongly associated with hypoadiponectinemia in T2DM patients. Most of the T2DM patients have hypoglycaemia, hypertension and hypoadiponectinemia which are leading to cause CKD, CVD and other comorbidities of metabolic diseases.
Acknowledgments
We are grateful to the residents of Medicine department and PhD scholars of Biochemistry department including the technical staffs for their help and support in carrying out my thesis work. We also acknowledge Honorable Vice-Chancellor, Professor S. W. Akhtar, Integral University, Lucknow, India for the invaluable help and financial support to carry out research work without any hindrance.
Conflict of Interest
All Authors declare that they have no conflict of interest.
References
- IDF Diabetes Atlas, 5th edition annual update, International Diabetes Federation 2012.
- NCD Risk Factor Collaboration (NCD-RisC) members. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. April 6, 2016. http://dx.doi.org/10.1016/S0140-6736(16)00618-8.
- Shaw JE,Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4-14.
- Ben-Salem A, Ajina M, Suissi M, Daher HS, Almawi WY, Mahjoub T. Polymorphisms of transcription factor-7-like 2 (TCF7L2) gene in Tunisian women with polycystic ovary syndrome (PCOS). 2014;533(2): 554-7.
- Phani NM, Adhikari P, Nagri SK, et al. Replication and Relevance of Multiple Susceptibility Loci Discovered From Genome Wide Association Studies for Type 2 Diabetes in an Indian Population. PLoS ONE. 2016;11(6):e0157364.
- Heid IM, Henneman P, Hicks A, Coassin S, Winkler T, et al. Clear detection of ADIPOQ locus as the major gene for plasma adiponectin: results of genomewide association analyses including 4659 European individuals. 2010;208(2):412-420.
- Tsai MK, Wang HMD, Shiang JC, et al. Sequence Variants of ADIPOQ and Association with Type 2 Diabetes Mellitus in Taiwan Chinese Han Population. Scientific World Journal. Volume 2014, Article ID 650393,7 pages. http://dx.doi.org/10.1155/2014/650393.
- Davis SK, Xu R, Samson Y, et al. Association of ADIPOQ gene with type 2 diabetes and related phenotypes in African American men and women: the Jackson Heart Study. BMC Genetics. 2015;16:147.
- Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. J. Clin. Nutr. 2010;91(1):258S–261S.
- Lang HF, Chou CY, Sheu WH, Lin JY. Weight loss increased serum adiponectin but decreased lipid levels in obese subjects whose body mass index was lower than 30 kg/m2. Res. 2011;31(5):378–386.
- Medina-Bravo P, et al. Decrease in serum adiponectin levels associated with visceral fat accumulation independent of pubertal stage in children and adolescents. Med. Res. 2011;42(2):115–121.
- Wang X, Zhang S, Chen Y, Liu H, Lan C, Chen X, et al. APM1 gene variants -11377C/G and 4545G/C are associated respectively with obesity and with non-obesity in Chinese type 2 diabetes. Diabetes Res Clin Pract. 2009;84(3):205-10.
- Hsiao TJ, Lin E. A Validation Study of Adiponectin rs266729 Gene Variant with Type 2 Diabetes, Obesity, and Metabolic Phenotypes in a Taiwanese Population. Biochem Genet. DOI 10.1007/s10528-016-9760-y.
- Prior SL, Gable DR, Cooper JA, Bain SC, Hurel SJ, Humphries SE, et al. Association between the adiponectin promoter rs266729 gene variant and oxidative stress in patients with diabetes mellitus. Eur Heart J. 2009;30(10):1263-9.
- Li Y, Wu QH, Jiao ML, et al. Gene-environment interaction between adiponectin gene polymorphisms and environmental factors on the risk of diabetic retinopathy. J Diabetes Invest. 2015;6(1):56-66.
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA.2013;310(20):2191-4.
- Deepa M, Farooq S, Deepa R, Manjula D, Mohan V. Prevalence and significance of generalized and central body obesity in an urban Asian Indian population in Chennai, India (CURES: 47). European Journal of Clinical Nutrition. 2009;63(2):259–267.
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988;16(3):1215.
- Suriyaprom K, Phonrat B, Tungtrongchitr R. Association of adiponectin gene -11377C>G polymorphism with adiponectin levels and the metabolic syndrome in Thais. Asia Pac J Clin Nutr. 2014;23(1):167-173.
- Radcliffe NJ, Seah J, Clarke M et al. Clinical predictive factors in diabetic kidney disease progression. J Diabetes Investig. 2016 doi: 10.1111/jdi.12533.
- Sung KC, Park HY, Kim MJ and Reaven G. Metabolic markers associated with insulin resistance predict type 2 diabetes in Koreans with normal blood pressure or prehypertension. Cardiovasc Diabetol. 2016;15:47.
- Andersson DP, Laurencikiene J, Acosta JR, Rydén M, Arner P. Circulating and adipose levels of adipokines associated with insulin sensitivity in non-obese subjects with type 2 diabetes. J Clin Endocrinol Metab. doi: 10.1210/jc.2016-1883.
- Barbarash O, Gruzdeva O, Uchasova E, et al. The role of adipose tissue and adipokines in the manifestation of type 2 diabetes in the long-term period following myocardial infarction. Diabetol Metab Syndr. 2016;8:24.
- Alkhateeb A, Al-Azzam S, Zyadine R, Abuarqoub D. Genetic association of adiponectin with type 2 diabetes in Jordanian Arab population. 2013;512(1):61–63.
- Ramya K, et al. Genetic Association of ADIPOQ gene variants with type 2 diabetes, obesity and serum adiponectin levels in south Indian population, 2013, http://dx.doi.org/10.1016/j.gene.2013.09.012.
- Kaftan AN, Hussain MK. Association of adiponectin gene polymorphismrs266729 with type two diabetes mellitus in Iraqi population. A pilot study. 2015, http://dx.doi.org/10.1016/j.gene.2015.06.004.
- Mente A, Meyre D, Lanktree MB, Heydarpour M, Davis AD, et al. Causal Relationship between Adiponectin and Metabolic Traits: A Mendelian Randomization Study in a Multiethnic Population. PLoS ONE. 2013;8(6): e66808.
- de Faria APC, Modolo R, Sabbatin AR, et al. Adiponectin -11377C/G and+276G/T Polymorphisms affect Adiponectin Levels but do not Modify Responsiveness to Therapy in Resistant Hypertension. Basic & Clinical Pharmacology & Toxicology. 2015;117:65–72.
- Khabour OF, Abu-Rumeh L, Al-Jarrah M, et al. Association of adiponectin protein and ADIPOQ gene variants with lumbar disc degeneration. Experimental and Therapeutic Medicine. 2014;8(4):1340-1344.
- Nazir N, Siddiqui K, Al-Qasim S, Al-Naqeb D. Meta-analysis of diabetic nephropathy associated genetic variants in inflammation and angiogenesis involved in different biochemical pathways. BMC Medical Genetics. 2014;15:103.
- Fernandez ´ -Real JM, Castro A, V´Azquez G, et al. Adiponectin Is Associated With Vascular Function Independent of Insulin Sensitivity. Diabetes Care.2004;27(3): 739-745.
- Avery PJ, Patel SK, Ibrahim IM, Walker M, Keavney BD. Common variation in the adiponectin gene has an effect on systolic blood pressure. Journal of Human Hypertension. 2011;25:719–724.
- Zhao T, Zhao J. Genetic effects of adiponectin on blood lipids and blood pressure. Clin Endocrinol (Oxf). 2011;74(2):214-22.
- Baden MY, Yamada Y, Takahi et. al. Association of adiponectin with blood pressure in healthy people. Clin Endocrinol (Oxf). 2013;78(2):226-31.
- Lanas F, Seron P, Saavedra N, Ruedlinger J, Salazar L. Genetic and Non-Genetic Determinants of Circulating Levels of Adiponectin in a Cohort of Chilean Subjects. Mol Diagn Ther. 2015;19(4):197-204.
- Eslamian M, Mohammadinejad P, Aryan Z, Nakhjavani M, Esteghamati A. Positive Correlation of Serum Adiponectin with Lipid Profile in Patients with Type 2 Diabetes Mellitus is Affected by Metabolic Syndrome Status. Arch Iran Med. 2016;19(4):269 – 274.
- Zadjali F, Al-Yahyaee S, Hassan MO, et al. Association of adiponectin promoter variants with traits and clusters of metabolic syndrome in Arabs: family-based study. 2013;527(2):663-9.
- Zhong C, Zhen D, Qi Q, Genshan M. A lack of association between adiponectin polymorphisms and coronary artery disease in a Chinese population. Genetics and Molecular Biology. 2010;33(3):428-433.







