Mohammed Alsbou1, Gadeer Abdeen2, Adel Batarseh3, Nidda Bawaresh4, Jaber Jaber4, Gadeer Qawasmi4, Taqwa Maqatef4, Hayat Banat4 and Abdelrahman Batayneh4
1Department of Pharmacology, Faculty of Medicine, Mutah University, Jordan.
2King Hussein Medical Center (KHCC), Jordan.
3Royal Medical Services, Jordan.
4Rational Drug Use and Pharmacovigilance Department, Jordan Food & Drug Administration, Jordan.
Corresponding Author E-mail: mohsb74@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1112
Abstract
The aims of this study were to analyze the adverse drug reactions reports (ADRs) submitted to the Jordan Pharmacovigilance (PV) department at Jordan Food and Drug Administration (JFDA) in the period from 2010 to 2014, determine the rate of reporting of ADRs per year, identify the most common drugs involved in ADRs, and finally the most commonly body systems implicated in ADRs. The total number of ADRs reports was 428. There was a 5-fold increase in the rate of reporting over the study period. The most commonly classes of drugs implicated in ADRs were antineoplastics (37.6%), followed by immunomodulators (14.1%), antibiotics (10.3%) and analgesics (6.6%). The most commonly reported system organ classes involved in these ADRs were skin and subcutaneous (19.2%), followed by gastrointestinal (16.5%) and nervous system (11.5%). This is the first study to analyze the Jordan national pharmacovigilance database and the results of this study are considered the cornerstone of post-marketing surveillance and it could be used an essential tool for signal generation in Jordan. More educational programs and awareness campaigns are needed to promote the concept of PV and to increase the role of healthcare professionals in the reporting of ADRs in Jordan.
Keywords
Pharmacovigilance; adverse drug reactions; Jordan
Download this article as:| Copy the following to cite this article: Alsbou M, Abdeen G, Batarseh A, Bawaresh N, Jaber J, Qawasmi G, Maqatef T, Banat H, Batayneh A. Analysis of the National Pharmacovigilance Database in Jordan (2010-2014). Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Alsbou M, Abdeen G, Batarseh A, Bawaresh N, Jaber J, Qawasmi G, Maqatef T, Banat H, Batayneh A. Analysis of the National Pharmacovigilance Database in Jordan (2010-2014). Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13844 |
Introduction
Pharmacovigilance (PV) also known as drug safety surveillance is the science of enhancing patient safety through collecting, monitoring, assessing and preventing of adverse drug reactions (ADRs) 1. The objectives of PV are to improve public health and safety in relation to the use of medicines, to contribute to the assessment of benefit, harm, and risks associated with the use of medicines and to encourage the safe, rational and more effective use of drugs 2. PV is an important and integral part of clinical research. Both clinical trials safety and post-marketing PV are critical throughout the product lifecycle. Once released into the market, a medicine leaves the secure and protected scientific environment of clinical trials and is legally set free for consumption by the general population. At this point, most medicines will only have been tested for short-term safety and efficacy on a limited number of carefully selected individuals 3.Therefore, it is essential that new and medically still evolving treatments are monitored for their effectiveness and safety under real-life conditions post release 4.
Good pharmacovigilance practice will identify the risks in the shortest possible time after the medicine has been marketed and will help to establish and/or identify risk factors. When communicated effectively, this information allows for intelligent, evidence-based prescribing with potential for preventing many adverse reactions and will ultimately help each patient to receive optimum therapy at a lower cost 5.The post-marketing assessment of the benefits and risks of medical products can be achieved through collaborative efforts from regulatory bodies, healthcare providers, industry and the patients. Therefore, effective pharmacovigilance systems should communicate with the patients and healthcare professionals to ensure sharing of information related to drug safety 6. In order to prevent unnecessary suffering by patients and to decrease the financial loss sustained by the patient due to the inappropriate or unsafe use of medicines, it is essential that a monitoring system for the safety of medicines is supported by doctors, pharmacists, nurses and other healthcare professionals in the country 7.
In Jordan, the PV system was established in 2001 and Jordan joined the WHO programme for international drug monitoring in 2002. In 2006, the first PV guidelines were approved based on the International Council for Harmonization (ICH)-Guidelines, which clarify the relation among stakeholders (Health authorities, healthcare providers, industry and patients) 8. In order to increase the awareness about PV and promote reporting of ADRs, five PV regional centers have been established recently in the north, middle and south part of Jordan 9. In this study, we aimed to analyze the national ADRs reports submitted to the PV department at Jordan Food and Drug Administration (JFDA).
Methods
ADRs reports submitted to the rational drug use and pharmacovigilance department at JFDA from 2010 to 2014 were analyzed. The aims of analysis of ADRs reports were to create national PV database for the JFDA, to determine the rate of reporting per year, classes of drugs involved in causing ADRs, the most common reported drugs, the most frequently ADRs and system organ classes involved in these ADRs.System organ classes and body systems involved in ADRs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) terminology 10.
Results
The total number of ADRs reports received was 428 over the 5-year period. Eighty reports were excluded from the analysis as they were related to quality issue; therefore 348 reports were included in the study.The annual rate of reporting increased gradually over the study period. There was about a 5-fold increase in the number of received ADR reports (Figure 1).
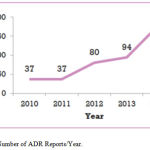 |
Figure 1: Number of ADR Reports/Year.
|
Classes of Drugs Involved in Adrs
Seventeen classes of drugs were involved in causing ADRs. The most common classes were antineoplastics (37.6%), immunomodulators (14.1%), antibiotics (10.3%) and analgesics (6.6%) (Table 1).
Table 1: Classes of drugs implicated in causing ADRs
|
Classes of Drugs
|
Total No. of reports (348) |
Percentage (%) |
| Antineoplastics | 131 | 37.6% |
| Immunomodulators | 49 | 14.1% |
| Antibiotics | 36 | 10.3% |
| Analgesics | 23 | 6.6% |
| Antihypertensives | 19 | 5.5% |
| Antivirals | 15 | 4.3% |
| Antiepileptics | 13 | 3.7% |
| Anticoagulants | 9 | 2.6% |
| Antidiabetics | 9 | 2.6% |
| Corticosteroids | 7 | 2% |
| Antihyperlipidemics | 4 | 1.2% |
| Hormones | 4 | 1.2% |
| Antipsychotics | 2 | 0.6% |
| Vitamins & iron | 2 | 0.6% |
| Anti-acne | 2 | 0.6% |
| Peptic ulcer-healing | 2 | 0.6% |
| Antidepressants | 1 | 0.3% |
| Others | 20 | 5.7% |
A total of 125 drugs were involved in causing ADRs. Antineoplastics were the first most common class of drugs, 131 reports. The most frequent antineoplastic drugs were docetaxel (28) reports, followed by oxaliplatin (15) reports. Immunomodulators were the second most common class of drugs involved in ADRs, 49 reports. The most commonly drugs were lenalidomide (12), and thalidomide (10) reports. Antibiotics were the third most commonly class of drugs involved in ADRs, 36 reports. The most common drugs were ceftriaxone (8) reports, and vancomycin (6) reports (Table 2).
Table 2: Drugs involved in causing ADRs
| No of reports | Drugs | Total no of reports | No of drugs | Classes of drugs |
| N= 348 | ||||
| 28 | Docetaxel | 131 | 25 | Antineoplastics |
| 15 | Oxaliplatin | |||
| 11 | Nilotinib | |||
| 11 | Capcitabine | |||
| 10 | Rituximab | |||
| 10 | Filgrastim G-CSF | |||
| 8 | Bevacizumab | |||
| 5 | Erlotinib | |||
| 5 | Cabazitaxel | |||
| 4 | Imatinib | |||
| 3 | Everolimus | |||
| 3 | Paclitaxel | |||
| 2 | Carboplatin | |||
| 2 | Fluorouracil | |||
| 2 | Trastuzumab | |||
| 2 | Pegfilgrastim | |||
| 2 | Hydroxyurea | |||
| 1 | Cisplatin | |||
| 1 | Cyclophosphamide | |||
| 1 | Cytarabine | |||
| 1 | Dacarbazine | |||
| 1 | Ruxolitinib | |||
| 1 | Vincristin | |||
| 1 | Bortezomib | |||
| 1 | Vemurafenib | |||
| 12 | Lenalidomide | 49 | 10 | Immunomodulators |
| 10 | Thalidomide | |||
| 6 | Adalimumab | |||
| 4 | Cyclosporine | |||
| 4 | Infliximab | |||
| 4 | fingolimod | |||
| 4 | Tacrolimus | |||
| 3 | Tocilizumab | |||
| 1 | Mycophenolate | |||
| 1 | Basiliximab | |||
| 8 | Ceftriaxone | 36 | 15 | Antibiotics |
| 6 | Vancomycin | |||
| 4 | Doxycylcine | |||
| 3 | Teicoplanin | |||
| 2 | Ciprofloxacin | |||
| 2 | Gemifloxacin | |||
| 2 | Imipenem + cilastatin | |||
| 2 | Amoxicillin | |||
| 1 | Azithromycin | |||
| 1 | Amikacin | |||
| 1 | Cefdinir | |||
| 1 | Cefuroxime | |||
| 1 | Erythromycin | |||
| 1 | Metronidazole | |||
| 1 | Tigecycline | |||
| 8 | Diclofenac | 23 | 10 | Analgescis |
| 4 | Aspirin | |||
| 3 | Paracetamol | |||
| 2 | Pethidine | |||
| 1 | Codeine | |||
| 1 | morphine | |||
| 1 | Piroxicam | |||
| 1 | Lornoxicam | |||
| 1 | Etoricoxib | |||
| 1 | Ibuprofen | |||
| 4 | Amlodipine | 19 | 10 | Antihypertensives |
| 4 | Furosemide | |||
| 3 | Irbesrtan | |||
| 2 | Candesartan | |||
| 1 | Enalapril | |||
| 1 | Valsartan | |||
| 1 | Hydrochlorothiazide | |||
| 1 | Atenolol | |||
| 1 | Metoprolol | |||
| 1 | Amiloride | |||
| 8 | Peg interferon alfa 2a | 15 | 7 | Antivirals |
| 2 | Valganciclovir | |||
| 1 | Ganciclovir | |||
| 1 | Micafungin | |||
| 1 | Interferon alpha | |||
| 1 | Acyclovir | |||
| 1 | Ribavirin | |||
| 5 | Lamotrigine | 13 | 6 | Antiepileptics |
| 3 | Carbamazepine | |||
| 2 | Topiramate | |||
| 1 | Phenobarbital | |||
| 1 | Oxcarbazepine | |||
| 1 | levetiracetam | |||
| 3 | Enoxaparin | 9 | 4 | Anticoagulants &Fibrinolytics |
| 2 | Heparin | |||
| 2 | Bemiparin Sodium | |||
| 2 | Streptokinase | |||
| 4 | Metformin | 9 | 4 | Antidiabetics |
| 3 | Insulin | |||
| 1 | Vildagliptin | |||
| 1 | Glibenclamide | |||
| 3 | Prednisolone | 7 | 5 | Corticosteroids |
| 1 | Dexamethasone | |||
| 1 | Fluticasone | |||
| 1 | Hydrocortisone | |||
| 1 | Betamethasone | |||
| 2 | Atorvastatin | 4 | 3 | Antihyperlipidemics |
| 1 | Gemfibrozil | |||
| 1 | Simvastatin | |||
| 2 | Oxytocin | 4 | 3 | Hormones |
| 1 | levothyroxin | |||
| 1 | Progesterone | |||
| 2 | Palipeidone | 2 | 1 | Antipsychotics |
| 1 | Alfacalcidol | 2 | 2 | Vitamins & iron |
| 1 | Iron | |||
| 2 | Isotretinoin | 2 | 1 | Anti-acne |
| 1 | Famotidine | 2 | 2 | Peptic ulcer-healing |
| 1 | Omeprazole | |||
| 1 | Venlafaxine | 1 | 1 | Antidepressants |
| 2 | Immunoglobulin | 20 | 17 | Others |
| 2 | Atracurium | |||
| 2 | Zoledronic acid | |||
| 1 | Cyclopentolate | |||
| 1 | Omalizumab | |||
| 1 | Brimonidine | |||
| 1 | Misicrom | |||
| 1 | Sulbutamol | |||
| 1 | Hydroxychloroquine | |||
| 1 | Deferasirox | |||
| 1 | Rifampicin | |||
| 1 | Epoetin beta | |||
| 1 | Ibandronic acid | |||
| 1 | Salbutamol | |||
| 1 | Midazolam | |||
| 1 | Hydroxychloroquine | |||
| 1 | Pseudoephedrine |
System Organ Classes Involved in Adrs
The total number of ADRs was (417). The most frequently reported systems were skin and subcutaneous 80 ADRs (19.2%), gastrointestinal (GI) 69 ADRs (16.5%) and nervous system 48 ADRs (11.5%) (Tables 3 & 4).
Table 3: System organ classes involved in ADRs
|
System Organ Class
|
Total No. of ADRs (417) |
Percentage (%) |
| Skin & Subcutaneous | 80 | 19.2% |
| Gastrointestinal | 69 | 16.5% |
| Nervous | 48 | 11.5% |
| Blood | 39 | 9.4% |
| Respiratory | 31 | 7.4% |
| General Disorder | 31 | 7.4% |
| Musculoskeletal | 30 | 7.2% |
| Vascular | 21 | 5% |
| Endocrine | 16 | 3.8% |
| Cardiac | 15 | 3.6% |
| Renal & Urinary | 10 | 2.4% |
| Hepatobiliary | 9 | 2.2% |
| Immune | 8 | 1.9% |
| Psychiatric | 4 | 1% |
| Infections | 3 | 0.7% |
| Eye | 2 | 0.5% |
| Ear | 1 | 0.2% |
Table 4: Systems involved in ADRs according to MedDRA terminology
| Systems | No of ADRs | ADRs | No of ADRs |
| Skin & subcutaneous | 80 | Redness | 31 |
| Itching | 18 | ||
| Acral erythema | 13 | ||
| Hand & foot syndrome GIII | 4 | ||
| Angioedema | 4 | ||
| Urticaria | 4 | ||
| Sweating | 3 | ||
| Photosensitivity | 2 | ||
| Vomiting | 1 | ||
| Diarrhea | 22 | ||
| GI bleeding | 11 | ||
| Duodenal ulcer | 6 | ||
| Abdominal pain | 6 | ||
| Nausea | 6 | ||
| Constipation | 5 | ||
| 3 | |||
| Gastrointestinal | 69 | Erosions antralgastropathy | 3 |
| Heartburn | 1 | ||
| Gingival hyperplasia | 1 | ||
| Dysphagia | 1 | ||
| Loss of taste | 1 | ||
| Localized small bowel angioedema | 1 | ||
| Poor appetite | 1 | ||
| Abdominal Distension | 1 | ||
| Headache | 7 | ||
| Convulsions | 7 | ||
| Generalized weakness | 6 | ||
| Numbness | 5 | ||
| Drowsiness | 4 | ||
| Neuropathy | 3 | ||
| Extrapyramidal symptoms | 2 | ||
| Coma | 2 | ||
| Nervous | 48 | Speaking disturbances | 2 |
| Hyperthermia | 2 | ||
| Tremor | 2 | ||
| Neuralgia | 1 | ||
| Increased intracranial pressure | 1 | ||
| Disorientation to time, place | 1 | ||
| Sleep disturbance | 1 | ||
| Vertigo | 1 | ||
| Vocal cord paralysis | 1 | ||
| Anemia | 9 | ||
| Neutropenia | 9 | ||
| Thrombocytopenia | 7 | ||
| Pancytopenia | 5 | ||
| Blood | 39 | Septicemia, Septic cholangitis | 3 |
| Bleeding | 2 | ||
| Leukopenia | 2 | ||
| Leukocytosis | 1 | ||
| Febrile neutropenia | 1 | ||
| Difficulty in breathing | 20 | ||
| Cough | 5 | ||
| Respiratory | 31 | Respiratory depression | 3 |
| Chest infection | 2 | ||
| Candida infection in lungs | 1 | ||
| General disorders | 31 | Fever | 27 |
| Chills | 4 | ||
| Musculoskeletal | 30 | Back pain | 19 |
| Myalgia | 3 | ||
| Muscle weakness | 3 | ||
| Arthralgia | 2 | ||
| Muscle cramps | 2 | ||
| Sitting imbalance | 1 | ||
| Hypotension | 10 | ||
| Hypertension | 5 | ||
| Vascular | 21 | Septic shock | 2 |
| Pulmonary embolism | 2 | ||
| Leg edema | 1 | ||
| Arterial thromboembolism | 1 | ||
| Hyperglycemia | 4 | ||
| Hypoglycemia | 3 | ||
| Hypocalcemia | 2 | ||
| Hyponatremia | 2 | ||
| Endocrine | 16 | Hypercalcemia | 1 |
| Hypertrichosis | 1 | ||
| Elevated TSH | 1 | ||
| Thyroid disorders | 1 | ||
| Hypokalemia | 1 | ||
| Palpitation | 10 | ||
| Cardiac arrest | 2 | ||
| Cardiac | 15 | Ischemia | 1 |
| Myocardial infarction | 1 | ||
| Bradycardia | 1 | ||
| Renal impairment | 4 | ||
| Hematuria | 2 | ||
| Renal & urinary | 10 | Renal colic | 1 |
| Urinary tract infection | 1 | ||
| Acute urinary retention | 1 | ||
| Micro albuminuria | 1 | ||
| Elevation of liver enzymes | 3 | ||
| Biliary colic | 2 | ||
| Hepatobiliary | 9 | Crigler-najjar syndrome | 2 |
| Jaundice | 1 | ||
| Hyperbilirubinemia | 1 | ||
| Anaphylaxis | 5 | ||
| Immune | 8 | Anaphylactic shock | 1 |
| Reactivation of chicken box | 1 | ||
| Arthritis | 1 | ||
| Psychiatric | 4 | Hallucination | 4 |
| Infections | 3 | Herpes Zoster | 2 |
| Mucositis | 1 | ||
| Retinopathy | 1 | ||
| Eye | 2 | Eyelid edema | 1 |
| Ear | 1 | Tinnitus | 1 |
Discussion
The rationale drug use and pharmacovigilance department at JFDA with the cooperation of Health Hazard Evaluation Committee (HHEC) has analyzed the domestic adverse drug reactions (ADRs) reports submitted to JFDA. The results of this study summarized the last 5 years experience of PV in Jordan. This study shows that there was a 5-fold increase in the number of received ADRs reports. Although these results indicated that reporting rate increased over the study period, however, the rate of reporting is still low in Jordan. Under-reporting of ADRs is a challenge for PV system worldwide, this is because most countries including Jordan follow the spontaneous or voluntary reporting system of ADRs 11-14. A study was conducted in the UK by Venulet et al. showed that about 85-98 % of doctors never submitted an ADR report to the national authority 15. A recent study was conducted by Suyagh et al. to evaluate the pharmacist’s knowledge, practice and attitude toward ADRs reporting in Jordan. This study suggested that the majority of pharmacists have insufficient knowledge about PV and ADRs reporting and the authors recommended that more educational programs are needed to increase the pharmacists role in the process of reporting 16. A cross-sectional study by Abu Farah et al. was conducted to evaluate knowledge and perceptions of PV among pharmacy students in Jordan. This study found that the majority of students had lack of knowledge of PV and reporting, and PhamD students had better knowledge about PV and ADRs reporting system than Bachelor of pharmacy students. The authors suggested incorporation of PV into pharmacy curriculum in order to increase the awareness among pharmacy students 17.
According to the results of this study, seventeen classes of drugs were involved in causing ADRs. The most common classes were antineoplastics (37.6%), immunomodulators (14.1%), antibiotics (10.3%) and analgesics (6.6%). These results are similar to previous studies. A study by Ozcan et al. demonstrated that antineoplastics, immunomodulators, and anti-infective agents were the most frequently reported drug groups involved in ADRs, they accounted for about 50% of all reported drugs 18. A study by Khan et al. showed that antibiotics and anticancer drugs were the most frequent classes of drugs implicated in ADRs 19. A study by Gharaibeh et al. was conducted to assess the prevalence rate of drug-induced admissions to the medical ward at Jordan University Hospital. They found that 3.6% of admissions were drug-induced, and chemotherapeutic drugs were the most common involved drugs, they were implicated in 36% of cases 20. A recent study by Alsbou et al. showed that the prevalence rate of ADRs was 3.2%, and antibiotics and analgesics were the most common classes of drugs involved in ADRs, they were involved in 33% and 25% of ADRs, respectively 21. Another pilot study by Alsbou et al. showed that 8% of patients admitted to the internal medical department experienced an ADR, and antibiotics and analgesics were the most commonly drugs involved in causing ADRs 22.
According to our results, the most common system organ classes involved in ADRs were skin and subcutaneous 80 ADRs (19.2%), gastrointestinal 69 ADRs (16.5%) and nervous system 48 ADRs (11.5%). These results are consistent with previous studies. Analysis of ADRs reports submitted to the WHO-ADR-VigiBase showed that skin and subcutaneous tissue disorders, nervous system and GI disorders were the most commonly reported ADRs 23. A recent study was conducted to analyze the ADR reports submitted to the Turkish PV center showed that skin and subcutaneous tissue, general disorders and administration site conditions, GI and nervous disorders were the most frequently reported ADRs, they were implicated in 15.3%, 13.5%, 10.7%, 9.6% of ADRs, respectively 18. Another study by Khan et al. found that the most frequent body systems implicated in ADRs were GI, skin and nervous systems and the GI symptoms were vomiting, nausea and diarrhea, and the symptoms related to the skin were rash and urticaria 19. A study by Alsbou et al. showed that GI symptoms (vomiting, diarrhea, bleeding, peptic ulcer and nausea) and allergic reactions (skin rash) were the most commonly identified ADRs 21. A pilot study showed that skin rash and GI bleeding were the most common reactions involved in ADRs 22. Another study by Garaibeh et al found that bone marrow was the most affected body organ implicated in drug-induced admissions (32%), followed by the nervous system (24%), and then the GI system (23%) 20.
Conclusion
In conclusion, this is the first detailed study to analyze the national PV database in Jordan. The results of this study is considered as a useful tool for JFDA to look for new safety concerns that might be related to the marketed drugs in Jordan and it will enable the health authority to take an appropriate action toward drugs at the proper time to ensure patient safety and improve public health.The success of PV system in Jordan depends upon government support and public awareness on need to report suspected ADRs.
References
- Meyboom RHB, Egberts ACG, Gribnau FWJ, Hekster YA.. Pharmacovigilance in perspective. Drug Saf. 1999;21(6): 429-447.
- Sumit K, Ashish B. Pharmacovigilance in India: perspectives and prospects. J Drug Deliv and Ther 2013; 3 (4):237-246.
- Hannan EL. Randomized clinical trials and observational studies: Guidelines for assessing perspective strengths and limitations. JACC Cardiovasc Interv 2008;1:211-217.
- Sultana J, Cutrroneo P, Trifiro G. Clinical and economic burden of adverse drug reactions. J Pharmacol and Pharmacother 2013;4 (sup l1):s73-77.
- (2012). Safety of medicines: a guide to detecting and reporting adverse drug reactions. Geneva.
- (2006). The safety of medicines in public health programs: pharmacovigilance an essential tool. Geneva.
- Suke SG, Kosta P, Negi H. Role of Pharmacovigilance in India. Online J Public Health Inform.2015;1;7(2):e223.
- Yadav S. Status of adverse drug reaction monitoring and pharmacovigilance in selected countries. Indian J Pharmacol 2008, 40 (suppl 1): s4-9.
- Alsbou M, Alshagahin H, Abosamhadaneh N. Establishment of a new regional pharmacovigilance center for south Jordan: ten months experience. Biomed & Pharm J 2016,9 (2): 507-511.
- http://www.meddra.org. Accessed 15 July 2016.
- Tandon VR, Mahajan V, Khajuria V, Gillani Z. Under-reporting of adverse drug reactions: A challenge for pharmacovigilance in India. Indian J Pharmacol. 2015;47(1):65-71.
- Pushkin R, Frassetto L, Tsourounis C, Segal ES, Kim S. Improving the reporting of adverse drug reactions in the hospital setting. Postgrad Med. 2010; 122:154–64.
- Irujo M, Beitia G, Bes-Rastrollo M, Figueiras A, Hernández-Díaz S, Lasheras B. Factors that influence under-reporting of suspected adverse drug reactions among community pharmacists in a Spanish region. Drug Saf 2007; 30:1073–82.
- Hazell L, Shakir SA. Under-reporting of adverse drug reactions: A systematic review. Drug Saf 2006;29:385–96.
- Venulet J, Ten Ham M. Methods for monitoring and documenting adverse drug reactions. Int J Clin Phar Ther 1996;34:112-129.
- Suyagh M, Farah D, Abu Farha R. Pharmacist’s knowledge, practice and attitudes toward pharmacovigilance and adverse drug reactions reporting process. Saudi Pharm J. 2015;23(2):147-53.
- Abu Farha R, Alsous M, Elayeh E and Hattab D. A Cross-Sectional Study on Knowledge and Perceptions of Pharmacovigilance among Pharmacy Students of Selected Tertiary Institutions in Jordan. Trop J Pharm Res. 2015; 14(10): 1899-1905.
- Ozcan G, Aykac E, Kasap Y, Nemutlu NT, Sen E, Aydinkarahaliloglu ND. Adverse Drug Reaction Reporting Pattern in Turkey: Analysis of the National Database in the Context of the First Pharmacovigilance Legislation. Drugs Real World Outcomes. 2016;3:33-43.
- Khan LM, Al-Harthi SE, Saadah OI. Adverse drug reactions in hospitalized pediatric patients of Saudi Arabian University Hospital and impact of pharmacovigilance in reporting ADR. Saudi Pharm J. 2013;21(3):261-266.
- Garaibeh M, Zmeili S, Abu-rajab A, Daoud Z. Drug-induced admissions to medical wards at Jordan university hospital. Int J Clin Pharmacol Ther. 1998;36(9):478-82.
- Alsbou M, Alzubiedi S, Alzobi H, Abu Samhadanah N, Alsaraireh Y, Alrawashdeh O, Aqel A, Al-Salem K. Adverse drug reactions experience in a teaching hospital in Jordan. Int J Clinc Pharm 2015;37:1188-1193.
- Alsbou M. Incidence of adverse drug reactions in Alkarak governmental hospital: a pilot study. J MED J 2010;44 (4):444-446.
- Aagaard L, Strandell J, Melskens L, Petersen PS, Holme Hansen E. Global patterns of adverse drug reactions over a decade: analyses of spontaneous reports to VigiBase™.Drug Saf. 2012;1;35(12):1171-82.








