Manuscript accepted on :February 16, 2017
Published online on: --
Plagiarism Check: Yes
C. R. Jayanthi, M. Renuka, and P. Panchaksharimath
Department, of pharmacology, Bangalore medical college and research institute, Bengaluru, India.
DOI : https://dx.doi.org/10.13005/bpj/1115
Abstract
Adverse drug reactions [ADRs] are more frequently encountered in the elderly (> 60 years) population. The etiology is multifactorial and often interconnected with interplay of many factors like polypharmacy, altered drug pharmacokinetic and pharmacodynamics responses, drug interactions that increase their risk for ADR, making them a vulnerable population. Hence, the present study was taken up.To evaluate clinical pattern, causality, severity and preventability of ADR’s in the elderly population. To evaluate Potentially inappropriate medicines [PIM] leading to ADRs using Beer’s criteria. An observational study was conducted from 2011 to 2015 to analyze ADRs in elderly reported from Victoria hospital attached to BMC&RI. Relevant data on patient’s demographics, details of ADR’s, causal drug details, outcome were collected as per CDSCO ADR reporting form. Causality was assessed using WHO causality assessment scale, severity using modified Hartwig and Siegel severity scale and preventability by modified Thornton and Schumock scale. Potentially inappropriate medicines (PIM) were determined according to Beer’s criteria. A total of 89 ADRs were reported during the study period, out of which 11% were reported in elderly. Majority (86%) were noted in the age group of 60-70 years. Dermatological (34%) followed by GIT (24%) system was predominantly affected due to ADR’s. Maculopapular rash (29.21%) was the most common ADR followed by gastritis (7.86%) and diarrhea (5.61%). Major contribution to the ADR’s was from J01cephalosporins (22.5%), N02 NSAID’s (20.22%) and J05 antiviral (6.7%) of the ATC groups. 87.6% of the ADR’s were probable and 12.4% were possible on WHO causality scale. Most ADRs were mild (51.68%) and moderate (44.94%). Majority were type A (98.87%) ADRs and probably preventable (92.1%). According to Beer’s criteria 30.33% of drugs causing ADR were PIM with NSAID’s (20.22%) being the most common inappropriately prescribed drugs. Most of the ADRs in elderly are predictable and preventable and are caused by commonly prescribed drugs like antibacterial and analgesics. Nearly one fourth of the ADRs were due to PIM which can be minimized by careful application of Beer’s criteria.
Keywords
adverse drug reactions; elderly; causality; Beer’s criteria; Thornton and Schumock; Hartwig scale
Download this article as:| Copy the following to cite this article: Jayanthi C. R, Renuka M, Panchaksharimath P. An observational Study to analyze the Adverse drug Reactions among the Elderly at A Tertiary Care Hospital. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Jayanthi C. R, Renuka M, Panchaksharimath P. An observational Study to analyze the Adverse drug Reactions among the Elderly at A Tertiary Care Hospital. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13648 |
Introduction
ADRs are one of the leading causes of morbidity and mortality in health care. They are the fourth largest cause of death ahead of pulmonary disease, diabetes, AIDS, pneumonia. The elderly people appear to be particularly at risk of experiencing an ADR due to the interplay of range of factors like polypharmacy, altered drug pharmacokinetic profiles and pharmacodynamics responses, drug interactions and cognitive problems that increase the risk in this group.(1)
According to the World Health Organization (WHO), world’s elderly population i.e., people 60 years of age and older is approximately 650 million at present and by 2050, it is forecast to reach 2 billion with 80% of them living in developing countries.(2)
Many epidemiological studies have reported high prevalence rate i.e 24% hospital admissions are due to ADRs in elderly as compared to 3 to 6% in other age groups. These ADRs increase the economic burden and contribute to excessive health care costs through increased patient morbidity and mortality. They contribute up to 5 to 10% of hospital costs. (3) The US FDA states that an estimate of the cost of drug-related morbidity and mortality is $136 billion annually, which is more than the total cost of cardiovascular or diabetic care in the United States. The Indian scenario as reported by a study show that total cost of hospital stay due to ADRs is estimated to be US $4350 that is US $80.5 per patient (108.7% of per capita per year expenditure on health).(4)
Many studies on the reporting of ADRs are available. However an emphasis on identifying ADRs and related problems in elderly in India is limited which continues to face an increase in elderly population and chronic conditions. Hence the present study aims to evaluate the clinical pattern, causality, severity & preventability of ADRs, potentially inappropriate medicines causing ADRs among elderly population in our teaching hospital.
Materials and Methods
It is a prospective observational study carried out in Bangalore Medical College and Research Centre, Bangalore. After obtaining approval from the ethics committee all the ADRs reported from patients ≥60 years from various departments of Victoria hospital attached to Bangalore medical college and research institute were collected from June 2011 to December 2015.
Relevant data on patient’s demographics, causal drug details, dosage, duration of therapy, route of drug administration, details of ADR’s like date of occurrence of events, brief description of the reaction, duration of reaction, treatment given with relevant investigation reports were collected as per CDSCO ADR reporting form.
Adverse drug reaction was defined as an effect that is noxious and unintended, and which occurs at doses used in man for prophylaxis, diagnosis and therapy as per WHO guidelines. We followed the United Nations agreed cutoff ≥60 years to refer to the older population/elderly. (5) International Classification of Disease (ICD-10)11 was used for coding the diagnosis and Anatomical Therapeutic Chemical (ATC) 12 classifications was used for medications.
Causality and severity assessment: The causality was assessed by using WHO causality assessment scale and severity was assessed by using the Hartwig severity assessment scale according to the recommendation by the WHO Uppsala Monitoring Center.
ADRs were assessed for preventability using modified Thornton and Schumock scale. Potentially inappropriate medicines (PIM) were determined according to Beer’s criteria.
Results
During the study period, a total of 809 ADRs were collected from the various department of Victoria hospital attached to Bangalore medical college and research institute. Out of the total, 89 ADRs were reported from the elderly age group which constitutes for 11% of the total ADRs. [Figure 1]
The gender distribution among the patients, who experienced ADRs were 56 (62.9%) males and 33 (37.1%) females [Figure 2]. Similarly, among total ADRs reported, 77 (86.51%) patients were 60-70 years, 11 (12.35%) were 71-80 years and 1(1.12%) patient was more than 80 years.
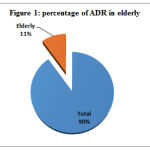 |
Figure 1: percentage of ADR in elderly
|
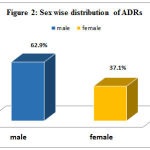 |
Figure 2: Sex wise distribution of ADRs
|
Distribution of ADRs across therapeutic classes was as follows: Antimicrobials (22.5%) J01cephalosporins, Analgesics (20.22%) N02 NSAID’s, Anti-hypertensives (7.86%), Antivirals J05 (6.7%) [Figure 3] Among the individual drugs, Ceftriaxone was associated with maximum cases of ADRs (15.73%) followed by Diclofenac (11.23%) and Aspirin (3.37%). In case of fixed dose drug combinations, isoniazid + rifampicin + ethambutol + pyrazinamide combination was responsible for 5.61% ADRs.
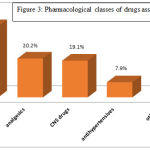 |
Figure 3: Pharmacological classes of drugs associated with ADRs
|
Table 1: Major drugs implicated with ADRs
| Drug class | Causal drugs | ADR(n) | |
| Antimicrobials (20) | Cephalosporins | Ceftriaxone | 14 |
| Fluroquinolones | Cefotaxime | 1 | |
| Ciprofloxacin | 5 | ||
| NSAIDs(18) | Diclofenac | 12 | |
| Aspirin | 3 | ||
| Paracetamol | 3 | ||
| Antiviral (6) | Zidovudine | 4 | |
| Stavudine | 1 | ||
| Atazanavir | 1 | ||
| Antitubercular (5) | Rifampicin | 5 | |
The frequency of ADRs associated with different routes of administration was as follows: Oral (n 48), parenteral (n 29), and topical (n 12).
The dermatological side effects (e.g. papular rash, itching etc.) were at the top with 34.83% followed by gastrointestinal disorders (29.21%) and neurological disorders (12.35%). The detailed description of organ systems affected by ADRs is shown in Table 2.
Table 2: ADRs and the organ system involved
| Organ system involved | No. of. ADRs (%) |
| Skin and mucous membrane | 31 (34.83%) |
| Gastrointestinal disorders | 26 (29.21%) |
| CNS and neurological disorders | 11 (12.35%) |
| Hematological disorders | 8 (8.98%) |
| Ophthalmological | 5 (5.61%) |
| Others | 8 (8.98%) |
Out of the 89 ADRs, 46 (51.68%) were found to be mild, 35 (39.32%) moderate and 8 (8.98%) severe. [Figure 4] Most of the severe ADRs were associated with anti-tubercular and antiretroviral drugs.
In 66.29% of cases the drug was withdrawn, dose unchanged (28%), dose reduced (5.6%). 74%
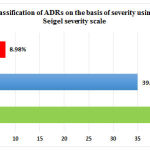 |
Figure 4 : Classification of ADRs on the basis of severity using Hartwig & Seigel severity scale
|
of the patients recovered symptomatically of the ADRs while 26% were in recovering stage.
Causality assessment revealed that 78 ADRs (87.6%) belonged to “probable” category, whereas 11 (12.40%) were of “possible” type according to the WHO-UMC scale. No case could be labeled “certain”, as re-challenge was not attempted by the health care professional, once a drug was withdrawn [Figure 5]. Majority were type A (98.87%) ADRs and probably preventable (92.1%).
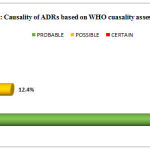 |
Figure 5: Causality of ADRs based on WHO cuasality assessment scale |
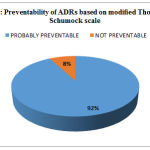 |
Figure 6: Preventability of ADRs based on modified Thornton and Schumock scale |
According to Beer’s criteria 30.33% of drugs causing ADR were PIM with NSAID’s (20.22%) being the most common inappropriately prescribed drugs.
Table 3 : Potentially inappropriate medicines causing ADRs according to Beers criteria
| Potentially inappropriate drugs | No of ADRs |
| Diclofenac | 10 |
| Aspirin | 3 |
| Nifedipine | 1 |
| Imipramine | 1 |
| Dicyclomine | 1 |
| Esomeprazole | 1 |
Discussion
The prevalence of disease increases with age and elderly are frequent medication users. Increased sensitivity to drug effects among the elderly results from changes in pharmacokinetics and pharmacodynamics. Age related losses of physiologic function also may predispose the older patient to adverse drug reactions.
Recognizing ADRs elderly becomes highly important since only around 3000 subjects receive a medicine prior to marketing, and often many (particularly type B) ADRs are often recognized only after marketing. These premarketing studies include only limited numbers of patients in the age group of 65 or older in trials and that even smaller numbers of the oldest old. In addition, long latency diseases like cancer are difficult to detect on account of the short duration of study.
In our study, we found that, there was a male preponderance 56 (62.92%). This is correlated with a study conducted by Veena et al. in Bengaluru which reported male patients were dominated with 55.66%. The same study demonstrated age wise distribution in between the age group of 65 and 70 years was 79.24%. These results are similar as shown by our study which shows frequency of ADRs in that age group around 86.51%. Another study by Lohani et al. in Nepal, shows similar results for age wise distribution of ADRs. (6, 7)
In the study majority of the ADRs were caused by antimicrobials cephalosporins followed by NSAIDs. This is in contrast with another study conducted by Mandavi et al who reported calcium channel blockers, ACE inhibitors as the major causative drugs for ADRs in elderly.
In our study among the antimicrobials cephalosporin’s and fluoroquinolones was the most common antibiotic class affecting the skin and GIT system used in the inpatient setting. These drugs caused generalized maculopapular rashes. A study conducted by Stavreva et al. also revealed the predominance of cephalosporins whereas fluoroquinolones were most accounted in a study conducted by Hussain et al. (8, 9,) A study of antibiotic dosage errors involving 1044 hospitalized patients >80 years of age revealed an overall rate of dosing error of 34% (10). Third-generation cephalosporins and gentamicin were most commonly associated with dosing errors among the antibiotics studied, with error rates of 50% and 65%, respectively.
Antimicrobials are frequently used to treat most common infections seen in elderly like pneumonia, urinary tract infections. An insufficient adjustment to the dosage for patients with renal dysfunction remains a significant contributor to antimicrobial-induced adverse events. These drugs should be initiated only when there is a clear potential clinical benefit and discourage irrational use that promote resistance. Empirical broad-spectrum antibiotics should be narrowed when a pathogen is identified.
Our study reported NSAIDs as the second most offending agents causing ADRs in elderly. Multiple studies support the finding of an increased risk of GI-related adverse drug events due to NSAIDs (11) which was also observed in our study. Most of these agents are extensively hepatically metabolized by Phase I cytochrome P450 iso enzymes. (12, 13, 14) Most NSAIDs are well-absorbed and highly plasma protein bound. Therefore, frail elders with hypoalbuminemia are likely to have higher free drug concentrations. Some agents have longer half-lives in older adults when compared to those determined in younger adults (i.e., celecoxib).
Antiretrovirals, like most chronically administered drugs, are reported to have adverse reaction. Zidovudine (ZDV) is the preferred nucleoside reverse transcriptase inhibitor in the first line antiretroviral regimen in India. It is known to be associated with life threatening toxicity like anaemia. The prevalence of zidovudine induced anaemia vary widely (5.42-9.62%), in studies from different parts of the world. In our study we found that antiviral agents caused 6 ADRs out of which 4 were zidovudine induced anaemia. These can be managed by careful monitoring of hemoglobin levels, switch over to alternative drugs like tenoforvir or treatment with eptoein alpha. These antiviral agents were the causative drugs for serious ADRs encountered in our study to be followed by anti- tubercular drugs.
In this study the causal relation for 87.6 per cent ADRs with drug was probable; corroborating with other results showing majority of reactions as probable. (15) Mandavi et al in 2009 evaluated ADRs and their risk factors encountered in ambulatory elderly patients reported majority 88.6% of ADRs on causality scale were probable. This can be explained since most of the ADRs occur with a reasonable time sequence to administration of the drug, unlikely to be attributed to concurrent disease, followed a clinically reasonable response on withdrawal (dechallenge)
Application of Beers list showed that 30.33% of drugs causing ADR were PIM. This revealed nearly one fourth of the ADRs were caused by PIM. NSAID’s (20.22%) being the most common inappropriately prescribed drugs. The PIM encountered in our study can be listed as follows like Aspirin, Diclofenac, Dicyclomine, Chlorzoxazone, Respiridone, Imipramine, and Nifedipine which is in concordance to the list of drugs observed in another study by Kamath et al in 2010. (16)
A study conducted by Zaveri et al. in Ahmedabad evaluated prescriptions of 407 geriatric patients in medicine department reported that 7.42% of the total drugs prescribed were PIMs. Similarly, another Indian study reported 4.1% of drugs prescribed were PIMs. (17, 18)
ADRs in elderly are largely contributed by prescribing error e.g., large doses of drugs without taking into account, the effect of age and frailty on drug disposition, especially renal and hepatic clearance. Increased pharmacodynamic sensitivity of the elderly to several commonly used drugs, e.g., central nervous system and cardiovascular drugs should also be considered while prescribing in elderly. Maintaining accurate record of all medications, monitoring to balance the need and avoiding polypharmacy, titrating from a small dose and individualizing dose to each patient, involving patient in decision of their therapy and educating them about the side effects of the drug are the strategies that can be employed by the physicians which will decrease the potential adverse drug reactions.
The present study is consistent in results with previous studies but the limitations were the lack of availability of information on polypharmacy and comorbidities due to incomplete documentation of data.
Conclusion
The present study attempted to study the pattern of ADRs and their distribution among different elderly age groups, organ systems affected, and therapeutic classes of medicines. Such studies conducted across multiple centers will serve as geriatric ADR database that can provide early warning signals of drug-reaction links if kept under active scrutiny.
The study reveals majority of the ADRs in elderly are predictable and preventable and are caused by commonly prescribed drugs like antimicrobials and analgesics. Nearly one fourth of the ADRs were due to PIM which can be minimized by careful application of Beer’s criteria. Multiple methods with the aim of reducing the occurrence of ADR, including trying to identify at risk patients in order to target additional attention and intervention are necessary.
In addition to aims to promote understanding, education and clinical training in pharmacovigilance to various target groups, it is imperative that the information collected is effectively communicated back to the public to ensure pharmacovigilance delivers its full benefit.
Reference
- Lavan AGallagher P. Predicting risk of adverse drug reactions in older adults, Ther Adv in Drug Saf. 2015; 7(1):11-22.
- World Health Organization. 10 facts on ageing and the life course. [Last accessed on 2016 Dec 3]. Available from: http://www.who.int/features/factfiles/ageing/en/index.html .
- Srinivasan R, Ramya G. Adverse drug reaction-causality assessment, IJRPC. 2011;1(3): 606-612.
- Harugeri A, Parthasarathi G, Ramesh M, Guido S, Basavanagowdappa H. Frequency and nature of adverse drug reactions in elderly in-patients of two Indian medical college hospitals, J Postgrad Med. 2011;57:189–95.
- World health organization programmes and projects: Health statistics and health information Systems-Definition of an older or elderly person [www.who.int/healthinfo/survey/ageingdefnolder/en/index.html]
- Veena DR, Padma L, Patil S. Drug prescribing pattern in elderly patients in a teaching hospital, J Dent Med Sci .2012; 1(5):39-42.
- Lohani SP, Thapa P, Aryal UR, Satyal KR. Polypharmacy and geriatric patients: Patterns of prescribing in the Tribhuvan University Teaching Hospital in Nepal, J Nepal Health Res Council. 2006;4(1).
- G. Stavreva, D. Pendicheva, A. Pandurska, R. Marev Detection of adverse drug reactions to antimicrobial drugs in hospitalized patients Trakia J. Sci., 6 (1) (2008), pp. 7–9
- Hussain Mohammed Misbah, Girhepunje Kundlik, Pal Ranju, Siddiqua Shahina Sugra Incidence of adverse drug reactions in a tertiary care hospital: a systematic review and meta-analysis of prospective studies Der Pharmacia Lettre, 2 (3) (2010), pp. 358–368.
- Hu K, Matayoshi A, Stevenson FT. Calculation of the estimated creatinine clearance in avoiding drug dosing errors in the older patient. Am J Med Sci2001; 322:133-136.
- Chang CH, Chen HC, Lin JW, Kuo CW, Shau WY, Lai MS. Risk of hospitalization for upper gastrointestinal adverse events associated with nonsteroidal anti-inflammatory drugs: a nationwide case-crossover study in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:763–771.
- Hanlon JT, Guay DRP, Ives T. Oral analgesics: efficacy, mechanism of action, pharmacokinetics, adverse effects, drug interactions, and practical recommendations for use in older adults. In: Gibson SJ, Weiner DK, editors. Pain in Older Persons, Progress in Pain Research and Management. Vol. 35. Seattle: IASP Press; 2005. pp. 205–222.
- Grosser T. Non-steroidal anti-inflammatory. In: Burton ME, et al., editors. Applied Pharmacokinetics and Pharmacodynamics: Principles of therapeutic drug monitoring. 4. Philadelphia: Lippincott; 2006. pp. 752–780.
- Woodhouse KW, Wynne H. The pharmacokinetics of non-steroidal anti-inflammatory drugs in the elderly. Clin Pharmacokinet. 1987;12:111–122.
- Mandavi, D’Cruz S, Sachdev A, Tiwari P. Adverse drug reactions and their risk factors among Indian ambulatory elderly patients. Indian J Med Res. 2012;136(3):404–410.
- Romana A, Kamath L, Sarda A, Muraraiah S, Jayanthi CR. Polypharmacy leading to adverse drug reactions in elderly in a tertiary care hospital, Int J Pharm Bio Sci .2012; 3(3): 218 – 224.
- Zaveri HG, Mansuri SM, Patel VJ. Use of potentially inappropriate medicines in elderly: A prospective study in medicine outpatient department of a tertiary care teaching hospital, Indian journal of Pharmacology. 2010; 42 (2): 95-98.
- Shenoy S. Evaluation of drug prescribing pattern in the elderly patients in tertiary care hospital, Indian journal of Pharmacology. 2006; 38:90.








