Manuscript accepted on :29-07-2024
Published online on: 11-09-2024
Plagiarism Check: Yes
Reviewed by: Dr. Faheema Jabbar Aboalhor
Second Review by: Dr. Ketaki Kulkarni
Final Approval by: Dr. em. Hans-Joachim Freisleben
Nargiza Nusratovna Parpieva1 , Askar Anvarovich Adilkhodzhaev2
, Askar Anvarovich Adilkhodzhaev2 and Zarifa Abdiraubovna Muminova3
and Zarifa Abdiraubovna Muminova3
1Doctor of Medical Sciences, director of Ministry of Health of the Republic of Uzbekistan, Republican Specialized Scientific and Practical Medical Center of Phthisiology and Pulmonology named after Academician Sh. Alimov. Republic of Uzbekistan, Tashkent city, Yakkasarai district, Akilova street.
2Doctor of Medical Sciences, Scientific director of the Department of Extrapulmonary Organ Surgery of Ministry of Health of the Republic of Uzbekistan, Republican Specialized Scientific and Practical Medical Center of Phthisiology and Pulmonology named after Academician Sh. Alimov. Republic of Uzbekistan, Tashkent city, Mirabad district, A.S. Banokaty.
3Basic Doctoral student of Ministry of Health of the Republic of Uzbekistan, Republican Specialized Scientific and Practical Medical Center of Phthisiology and Pulmonology named after Academician Sh. Alimov. Republic of Uzbekistan, Tashkent city, Yashnabad district, Tuzel.
Corresponding Author E-mail: tolmas4th@mail.ru
Abstract
The incidence of tuberculosis caused by Mycobacterium bovis, not only the pulmonary form, but also the form developing in the extrapulmonary organs, is also increasing from year to year. Despite the large number of EPTB, TBPLN occupies a leading place among diseases of this type and the study of its pathogenic strains is an urgent task in ensuring the effectiveness of treatment. In this regard, the main purpose of the presented manuscript is to determine the frequency of M. bovis in TBPLN, its effect on the development and course of the disease, as well as the effectiveness of treatment. For this purpose, for the first time, the features of education that occur in patients in peripheral lymph nodes using instrumental methods of ultrasound, computed tomography, and magnetic resonance imaging have been identified. In subsequent studies, 110 patients with peripheral lymph node pathology were diagnosed with TBPLN by detecting mycobacteria in pathological material using general hematological, microbiological and gene-molecular (Gene Xpert) methods. In order to ensure the high effectiveness of drugs used for medicinal purposes, strains of the pathogen were detected using histological, cytological studies, BCG test and specific analyzes such as Diaskintest, Quantiferon test, immunological tests. The study showed that about 80% of patients had M bovis in the overall assessment, 76.4% of patients were sensitive to rifampicin, 9.1% of patients had rifampicin-resistant bacteria, and 14.5% of patients did not have mycobacteria. Therapeutic measures were carried out in 2 different modes, such as standard and individual or with replacement, when all patients were divided into 2 groups. During the period from the 56-day intensive phase of standard treatment to the 84-day intensive phase, a total of 40 patients had a sharp decrease in lymph nodes, elimination of purulent inflammation, and after a while 22 patients in this group had a relapse. In the individual treatment regimen, Levofloxacin and linezolid were used instead of pyrazinamide. While the effectiveness of treatment was achieved in 48 patients of group II after 56 and 84 days of the intensive phase, relapses after a certain time were observed in only 6 patients. When choosing an individual treatment regimen in patients diagnosed with M. bovis, a decrease in relapses to 11.5% is achieved. When M. bovis is detected, an individual scheme of antibacterial treatment of tuberculosis is selected, in which, instead of pyrazinamide, it is recommended to choose one of the reserve lines, depending on the sensitivity of the pathogen to drugs.
Keywords
Extra Pulmonary Tuberculosis; Lymphodissection; Pyrazinamide; Recurrence; Tuberculous Lymphadenopathy
| Copy the following to cite this article: Parpieva N. N, Adilkhodzhaev A. A, Muminova Z. A. Study the Occurrence of Mycobacterium Bovis in Tuberculosis of Peripheral Lymph Nodes and its Effect on the Course of the Disease. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Parpieva N. N, Adilkhodzhaev A. A, Muminova Z. A. Study the Occurrence of Mycobacterium Bovis in Tuberculosis of Peripheral Lymph Nodes and its Effect on the Course of the Disease. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/4ekTxJs |
Introduction
Tuberculosis, which causes Mycobacterium bovis infection, affects a wide range of mammals and the possibility of human transmission is often the main factor in the surveillance of livestock and wild animals. Compared to other serious diseases, this disease is considered the biggest killer in the last 200 years and therefore has become a heavy burden on the health care system in the last decade [1, 2, 3]. It is worth noting that Mycobacterium tuberculosis is a rod-shaped aerobic bacillus and causes pulmonary tuberculosis and extra pulmonary tuberculosis (EPTB). EPTB affects many organs including the meninges, lymph nodes, gastrointestinal tract, pleura, genitourinary tract and bones which are different from pulmonary tuberculosis. EPTB accounts for a growing proportion of cases worldwide, although there is no information on epidemiological, clinical or microbiological factors [4, 5, 6, 7, 8]. The incidence of EPTB and its dominant forms vary from country to country. According to WHO, from 8% to 46% of new cases of tuberculosis in different regions correspond to this. Of the 6.1 million cases of tuberculosis reported in 2012, 0.8 million (13.1%) were in the EPTB, while in 2020, the EPTB accounted for 18% of the 5.8 million cases of tuberculosis reported worldwide [9, 10, 11, 12, 13, 14]. Tuberculosis of the lymph nodes is a sedentary process initiated by the human body in relation to mycobacteria and pathogens of the disease cause pathological changes in the human body. In this case, lymphocytes eliminate only single attacks of bacilli, and mass attacks of triggers lead to damage to the lymph nodes. Scrofula is an older term for tuberculous lymphadenopathy in the neck [9, 15, 16, 17]. The share of tuberculosis of the peripheral lymph nodes (TBPLN) in the total incidence of tuberculosis ranges from 5% to 19%. The share of TBPLN in the structure of EPTB is 43.0% compared to 3.1%, and in some literature even 58% [10, 18, 19, 20, 21, 22, 23]. Almost every third patient (31.2%) was diagnosed with an epidemiologically dangerous variant of TBPLN, an leaky wound or a fistula, and this should be a warning to specialists in the primary care department that even EPTB has a risk of spreading tuberculosis infection [24, 25, 26, 27].
It is worth noting that in the diagnosis of TBPLN, the identification of mycobacterium strains in many cases is not given importance, the differentiation of M. bovis from M. tuberculosis is of great importance in the treatment of patients. Unlike other representatives of the M. tuberculosis complex, M. bovis is inherently resistant to the drug pyrazinamide.
The question of which strain of mycobacteria causes TBPLN is answered in various sources, and some medical literature provides sufficient data on the cause of mycobacterium tuberculosis in many cases (90%) of tuberculosis of the lymph nodes of the neck area. These mycobacteria infect the mucous membrane of the mouth or nose and travel through the lymph vessels to the lymph nodes, while the proportion of M. bovis is claimed to be 2.1% in pulmonary tuberculosis and 9.4% in EPTB in humans, while other researchers claim that, on the contrary, tuberculosis of the lymph nodes is called M. bovis. In 30-50% of patients diagnosed with tuberculous lymphadenitis during the studies, it was confirmed that the disease was caused by M. Bovis. Infection with mycobacteria in cattle (M. bovis) usually occurs through the mucous membrane of the mouth, eyes, skin, contact or gastrointestinal tract. With the practical detection of M. bovis, patients are treated in accordance with the standard course of anti-tuberculosis therapy, but pyrazinamide is excluded from the treatment regimen. A pyrazinamide sensitivity test can be used to screen M. Bovis [28, 29, 30, 31, 32].
Tuberculosis caused by M. bovis is clinically, radiologically and macroscopically indistinguishable from tuberculosis caused by M. tuberculosis. The most alternative method for detecting M. bovis is PZR with a sensitivity of 95%. The study of pathogenic strains in tuberculosis of peripheral lymph nodes plays an important role in ensuring the effectiveness of treatment of this pathology. In this presented manuscript, the main goal was to study the incidence of mycobacterium bovis in tuberculosis of peripheral lymph nodes and its effect on the course of the disease.
Materials and Methods
It is worth noting that, in 2019-2023, in the Department of Surgery of extrapulmonary organs of the Republican Specialized Scientific and Practical Center of Phthisiology and Pulmonology named after Academician Sh. Alimov was analyzed and treated for several types of tuberculosis. Of these patients, 110 were treated with tuberculosis of the peripheral lymph nodes. In this regard, most of the analyses conducted at the center consist precisely of a set of measures aimed at detecting extrapulmonary tuberculosis. To determine the early stage of tuberculosis, an ultrasound examination (Mindray DC-N6, China) of all patients was performed, which is one of the main methods for diagnosing extrapulmonary forms of tuberculosis [33, 34]. In order to clarify and differentiate from other lymphadenopathies of extrapulmonary forms of tuberculosis, computer and magnetic resonance tomography (Echelon Smart 1.5 T, Healthcare Americas Corporation, USA) were additionally performed, especially in the early stage of the disease. The results of the analyses showed that the ultrasound examination was compared with the data of computer and magnetic resonance tomography and this, in turn, requires general blood tests and microbiological analyses. Initially, a Mantoux test was conducted, which provides information about predominantly latent forms of tuberculosis in patients, but there was one antigenic effect of this test, as well as a high probability of a false positive result. In this regard, 2 additional Diaskintest antigen samples were conducted (developed in Russia and currently being implemented mainly in the Commonwealth of Independent States), this sample is considered to have high sensitivity and specificity compared to the Mantoux test (BCG vaccine, Russia). In addition, based on the venous blood of patients used all over the world, a QuantiFERON sample (Diaskintest®, Russia), QuantiFERON fracture analysis (QuantiFERON®-TV gold, Russia), was conducted, highly sensitive, highly specific and with a very low probability of a false positive result. To confirm the result obtained in the above-mentioned instrumental and specific samples, a general blood test (using the BC-760 hematological analyzer, MINDRAY, China), microbiological (bacteriological analyzer BD BACTEC FX40, Russia), genetic-molecular, morphological in particular histological, cytological (microtom Sanny MS-1, Avion TEX), immunological methods studies (GeneXpert gene-molecular analyzer, USAID, USA), and CD 4+, CD 8+, T lymphocytes (ELISA, BD Simultest CD4/CD8 reagent, MindrayBC320 (Mindray Biomedical electronic Corporation, China) in pathological materials were performed.
Histological examination revealed changes characteristic of tuberculous lymphadenitis in all 110 patients (100%).The patients were divided into two groups. In addition to histological examination to confirm the diagnosis in 58 patients in group I, the Xpert method examined the punctate gene from the lymph node and performed standard antibacterial therapy against tuberculosis; In order to confirm the diagnosis in 52 patients of group II, along with histological examination and genetic expert examination, the presence of M. bovis at the point from the lymph node was analyzed using polymerase chain reaction, among which an individual scheme of antibacterial therapy against tuberculosis was used in the treatment of one of the patients identified M. bovis.
Results
During the study, a comparison was carried out on all indicators in patients classified in group II, according to the above indications, until the end of the study. Initially, the incidence of TBPLN was determined by the age of patients, with the average age of patients with this pathology being 32.4 years, as shown in the figure below (figure 1). It is noteworthy that TBPLN is mainly observed between the ages of 18 and 44 with high labor and intellectual abilities and causes not only medical, but also socio-economic problems, creating serious health risks in this age range.
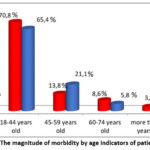 |
Figure 1: The magnitude of morbidity by age indicators of patients.
|
The stages of the disease in patients were classified according to E.N. Bellendir and the changes characteristic of each stage and the degree of their occurrence were recorded, as shown in Figure 2. The results of the study showed that complications or serious changes began to appear mainly from the second stage of the disease. In stage II, caseous changes in the lymph nodes developed, and these changes were observed in 11.5 – 15.5% of patients. At stage III, lymph node abscesses were observed in 43.1% – 55.8% of patients, and at stage IV -fistulous ulcers, and these changes were detected in 32.7-41.4% of patients. From the results obtained, it can be concluded that, in accordance with the stages of the disease, the development of serious complications also increases.
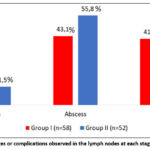 |
Figure 2: Changes or complications observed in the lymph nodes at each stage of the disease.
|
In patients in both groups, it was also found that along with TBPLN, there are many other concomitant diseases such as diabetes, HIV or AIDS, cardiovascular diseases, chronic bronchitis, diseases of the gastrointestinal tract and liver, Diseases of the gallbladder, anemia. However, the decompensated or severe degree of concomitant diseases has not been established. It is noteworthy that among the identified concomitant diseases, the incidence of anemia is high and amounts to 25-32.1% of the total incidence (Fig.3).
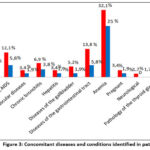 |
Figure 3: Сoncomitant diseases and conditions identified in patients.
|
Damage to all peripheral lymph nodes was found in patients with TBPLN. At the same time, it was noticed that with the defeat of the lymph nodes, the number of lesions of the lymph nodes located in the head, neck and jaw is clearly greater than in other areas. In particular, lesions were found in 62.1% and 67.2% of patients in the back and lower neck, respectively, while the largest number of lesions, including up to 86.2%, were found in the lymph nodes located in the front of the neck (Fig.4). At the same time, it is also noted in the scientific literature that damage to the lymph nodes located in the area of the burning neck is observed in up to 90% of cases [16, 18, 19].
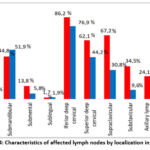 |
Figure 4: Characteristics of affected lymph nodes by localization in TBPLN.
|
For the purpose of surgical treatment of patients, the practice of opening and draining an abscess, scraping an leaky wound, fistulonecrectomy and regional lymph dissection was carried out (Fig. 5).
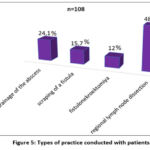 |
Figure 5: Types of practice conducted with patients.
|
The following are clinical examples from practice conducted with patients:
As noted above, TBPLN-related diseases are most common in the population aged 18-44 years and this disease is accompanied by various other concomitant diseases or complications. Below we will look at several cases where burning TBPLN has been diagnosed with concomitant diseases, as well as complications. Thus, a 34-year-old patient was clinically diagnosed with tuberculosis of peripheral (neck, armpit area) lymph nodes, an active period complicated by an abscess, HIV, in which stage 4 antiretroviral therapy is used as a concomitant disease. In this case, the patient is prescribed a one-stage “Bilateral regional lymph node dissection of the neck area.” Lymphodissection of the left armpit area” (Fig.6 – a, b,c,d,e). As a result of the operations, the affected lymph nodes with abscesses, caseous changes and necrotic tissues were removed, and the operation was completed by applying continuous atraumatic sutures to the skin (Fig.6-f,g,h, i).
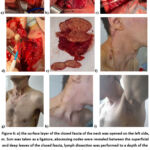 |
Figure 6: a) the surface layer of the closed fascia of the neck was opened on the left side, m. Scm was taken as a ligature, abscessing nodes were revealed between the superficial and deep leaves of the closed fascia, lymph dissection was performed to a depth of the sarotis of the vagina;
|
Patient Sh., 39 years old, clinical diagnosis “tuberculosis of peripheral lymph nodes, active period. Complication: abscess. Leaky wound.” The patient has an “abscess autopsy”. Removal of a leaky wound passage. The procedure of “regional lymph node dissection” was performed (Fig. 7).
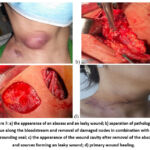 |
Figure 7: a) the appearance of an abscess and an leaky wound; b) separation of pathological tissue along the bloodstream and removal of damaged nodes in combination with the surrounding seal;
|
The pathological material obtained during practice from all patients in the study was systematically studied, and all patients were given a histological conclusion “tuberculous lymphadenitis”. The punctate from the lymph nodes of group I patients was examined using Gene Xpert, in group II patients, along with the Gene Xpert study, the punctate from the lymph node was examined. The presence of bovis was analyzed using polymerase chain reaction.
The Gene Xpert examination of 46 patients from group I (79.3%) revealed mycobacteria sensitive to rifampicin in 7 (12.1%) mycobacteria resistant to rifampicin, and the analysis of 5 patients from this group (8.6%) did not reveal mycobacteria. In 20 patients (38.5%) from group II, treatment was performed using PZR. It was discovered that bovis exists, and this indicator is variously cited in the scientific literature around the world, with different authors naming the figure from 2% to 50%. In addition, Gene Xpert detected mycobacteria sensitive to rifampicin in 38 patients (73.1%), mycobacteria resistant to rifampicin in 3 (5.8%), and the analysis in 11 patients (21.1%) did not reveal mycobacteria (Fig. 8).
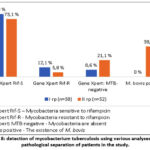 |
Figure 8: detection of mycobacterium tuberculosis using various analyses in the pathological separation of patients in the study.
|
In our observation, the treatment results were as follows: in 14 patients of group I (24.2%), at the 56-day intensive stage of treatment according to the standard regimen, there was a sharp decrease in lymph nodes, elimination of purulent inflammation, in 26 patients (44.8%), after the 84-day intensive phase of treatment according to the standard scheme, lymph nodes, and Also, inefficiency and relapses in the treatment of patients in this group were observed in 22 patients (37.9%). In turn, in 27 patients of group II (51.9%) at the 56-day intensive stage of treatment according to the standard scheme, there was a sharp decrease in lymph nodes, elimination of purulent inflammation, in 21 patients (40.4%) at the 84-day intensive stage of treatment according to the standard scheme. during treatment, lymph nodes were reduced to levels I and II, in 4 (7.7%) patients, nodes and foci of inflammation remained unchanged. Inefficiency and relapses of treatment of patients in this group amounted to 11.5% (6 patients) (Fig. 9).
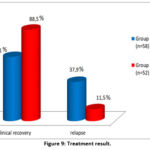 |
Figure 9: Treatment result.
|
Thus, the effectiveness of treatment was observed in 62.1% of patients receiving the standard treatment regimen, as shown in Figure 9, however, after a certain time, relapses were observed in 37.9% of patients in this group. While in patients receiving an individual regimen, the effectiveness of treatment was observed in 88.5%, relapses were observed only in 11.5% after a certain period of time. In this regard, it can be concluded that when treating TBPLN in an individual mode, not only higher treatment effectiveness is achieved, but also a sharp decrease in relapses.
Discussion
Tuberculosis is one of the most widespread diseases worldwide, according to the World Health Organization estimates of about 11 million cases, of which extrapulmonary tuberculosis accounts for about 30% of all cases of active tuberculosis and affects mainly children and adults with weakened immune systems. It is during the early diagnosis of the extrapulmonary form of tuberculosis that delays often occur due to circumstances such as sampling, which must be submitted, and low bacterial load in the samples taken. Also, despite the presence of various diagnostic tests, the lack of an appropriate diagnostic algorithm due to the different vagueness of the localization of the pathological process that has arisen reduces the ability of clinicians to accurately diagnose tuberculosis. In this regard, research has begun around the world to develop a protocol that allows early diagnosis of this specific form of tuberculosis. As a result of these studies, significant progress has been made in the timely detection and treatment of otolaryngological tuberculosis, as well as a decrease in the proportion of other similar extrapulmonary forms of tuberculosis in frequency from 0.4% to 1%. As already noted, cases of extrapulmonary tuberculosis of various localization, despite significant advances in prevention, diagnosis and treatment, tuberculosis of the lymph nodes due to its serious complications remains the leading cause of death worldwide. This, in turn, requires a projected increase in extrapulmonary tuberculosis and new approaches to improving detection and diagnosis methods at an early stage [35, 36, 37, 38, 39].
The presented manuscript describes cases of extrapulmonary tuberculosis, which occurs mainly in the peripheral lymph nodes and develops under the influence of Mycobacterium bovis, its effect on the course of the disease, as well as a change in treatment tactics in order to prevent serious side effects. Therapeutic measures were carried out in 2 different modes, such as standard and individual or with replacement, when all patients were divided into 2 groups. In total, during the 56-day intensive phase of standard treatment, 14 patients had a sharp decrease in lymph nodes, elimination of purulent inflammation, and 26 patients in the 84-day intensive phase had a decrease in lymph nodes to I and II degrees. In the individual treatment regimen, Levofloxacin and lineloside are used instead of pyrazinamide. While the effectiveness of treatment was achieved after 56 days in 27 patients of group II and in another 21 patients after 84 days of the intensive phase, no changes were observed in 4 patients, and relapses were observed in 6 patients. When choosing an individual treatment regimen in patients diagnosed with m bovis, a decrease in relapses to 11.5% is achieved. However, with standard TBPLN treatment, the recurrence rate was 37.9%.
Conclusions
In tuberculosis of peripheral lymph nodes, it is important to identify pathogenic strains, in particular M. bovis, since M. bovis pyrazinamide relatively stable in nature to the drug, and insufficient completeness of antibacterial therapy leads to an increase in the frequency of relapses of the disease (37.9%).;
When choosing an individual regimen of antibacterial therapy against tuberculosis in patients, pyrazinamide was replaced by the choice of one of the reserve drugs in accordance with the sensitivity of the pathogen to drugs, which allowed not only to reduce recidivism to a significant level, but also to alleviate the course of the disease.
It is also possible to prevent side effects such as hyperuricemia, hepatotoxic conditions, dysuria, arthralgia and sideroblastic anemia that may occur due to this by limiting the use of pyrazinamide in patients who have been diagnosed with M. bovis with pathological abnormalities.
Acknowledgement
We express our deep gratitude to the director and the Department of Science and Innovation, which regulates research activities of Ministry of Health of the Republic of Uzbekistan, Republican Specialized Scientific and Practical Medical Center of Phthisiology and Pulmonology named after Academician Sh. Alimov for their support and encouragement in conducting scientific research presented in this manuscript
Conflict of Interest
There are no conflict of interest.
Funding Source
This article was funded by the Foundation for the support of scientific research, established by the director of of Ministry of Health of the Republic of Uzbekistan, Republican Specialized Scientific and Practical Medical Center of Phthisiology and Pulmonology named after Academician Sh. Alimov. Grant number: GF (Global funding) 2501.
References
- Borham M, Oreiby A, El-Gedawy A, Hegazy Y, Khalifa HO, Al-Gaabary M, Matsumoto T. Review on Bovine Tuberculosis: An Emerging Disease Associated with Multidrug-Resistant Mycobacterium Species. Pathogens. 2022 Jun 21;11(7):715. doi: 10.3390/pathogens11070715.
CrossRef - Elsayed M.S.A.E., Amer A. The rapid detection and differentiation of Mycobacterium tuberculosis complex members from cattle and water buffaloes in the delta area of Egypt, using a combination of real-time and conventional PCR. Mol. Biol. Rep. 2019;46:3909–3919. doi: 10.1007/s11033-019-04834-3.
CrossRef - Gormley E, Corner LAL. Pathogenesis of Mycobacterium bovis Infection: the Badger Model As a Paradigm for Understanding Tuberculosis in Animals. Front Vet Sci. 2018 Jan 15;4:247. doi: 10.3389/fvets.2017.00247.
CrossRef - Jawed A, Tharwani ZH, Siddiqui A, Masood W, Qamar K, Islam Z, Jawed A, Shah M, Adnan A, Essar MY, Rackimuthu S, Head MG. Better understanding extrapulmonary tuberculosis: A scoping review of public health impact in Pakistan, Afghanistan, India, and Bangladesh. Health Sci Rep. 2023 Jun 22;6(6):e1357. doi: 10.1002/hsr2.1357.
CrossRef - Rolo M, González-Blanco B, Reyes CA, Rosillo N, López-Roa P. Epidemiology and factors associated with Extra-pulmonary tuberculosis in a Low-prevalence area. J Clin Tuberc Other Mycobact Dis. 2023 May 12;32:100377. doi: 10.1016/j.jctube.2023.100377.
CrossRef - Lee JY. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc Respir Dis. 2015;78(2):47‐55. 10.4046/TRD.2015.78.2.47
CrossRef - Patterson B, Wood R. Is cough really necessary for TB transmission? Tuberculosis. 2019;117:31‐35. 10.1016/J.TUBE.2019.05.003
CrossRef - Wurie FB, Lawn SD, Booth H, Sonnenberg P, Hayward AC. Bioaerosol production by patients with tuberculosis during normal tidal breathing: implications for transmission risk. Thorax. 2016;71:549‐554. 10.1136/thoraxjnl-2015-207295
CrossRef - Bellendir E.N. Patogeneticheskie predposыlki k razrabotke evolyusionnoy klassifikatsii gematogennыx («metastaticheskix») form vnelegochnogo tuberkuleza // Problemы tuberkuleza, 1986. № 8. S. 64-68.
- Vasileva I.A., S.A. Andronov, G.S. Balasanyans [i dr.]. – Tuberkulez u vzroslыx: Klinicheskie rekomendatsii / Moskva: Rossiyskoe obщestvo ftiziatrov, 2022. – 151 s.
- Krutko V.S., Poteyko P.I., Xodosh E.M. Tuberkulez perifericheskix limfaticheskix uzlov. Meditsina neotlojnыx sostoyaniy. 2013. №1. S. 151-153.
CrossRef - Parpieva N.N. «Zabolevaemost tuberkulezom snizilas v dva raza, a smertnost ot etogo zabolevaniya – v chetыre». Meditsinskiy jurnal «Sog‘lom xayot» №2. 2017:1-5.
- Fayzullaeva D.B., Parpieva N.N., Xakimov M.A. Diagnostika i lechenie tuberkuleznoy limfadenopatii u VICh infitsirovannыx. Uchebnoe posobie / Tashkent, 2021. – 157 s.
- Baykan AH, Sayiner HS, Aydin E, Koc M, Inan I, Erturk SM. Extrapulmonary tuberculosıs: an old but resurgent problem. Insights Imaging. 2022 Mar 7;13(1):39. doi: 10.1186/s13244-022-01172-0.
CrossRef - Perelman M.I., Bogadelnikova I.V. Ftiziatriya / M.I. Perelman, M.: GEOTAR – Media, 2010. 448 s.
- Poteyko P.I., Krutko V.S., Shevchenko O.S., Xodosh E.M. Tuberkulez perifericheskix limfaticheskix uzlov // Jurnal «Meditsina neotlojnыx sostoyaniy» 1 (48) 2013:12-18.
CrossRef - Assefa W, Eshete T, Solomon Y, Kassaye B. Clinicoepidemiologic considerations in the diagnosis of tuberculous lymphadenitis: evidence from a high burden country. Int J Infect Dis. 2022 Nov;124:152-156. doi: 10.1016/j.ijid.2022.09.030.
CrossRef - Kulchavenya Ye.V. Tuberkulez perifericheskix limfaticheskix uzlov: epidemiologicheskaya xarakteristika / Ye.V. Kulchavenya [i dr.] // Tuberkulyoz i bolezni lyogkix. – 2018. – T. 96, № 10. – S. 30–34.
- Chovdurbaev N.J. Kliniko-morfologicheskaya xarakteristika tuberkuleza perifericheskix limfaticheskix uzlov // Nauka, Novыe Texnologii i Innovatsii Kыrgыzstana №10, 2016, s.104-107
- Kimura Y, Shimada M, Kawashima M, Yamane A, Nagai H, Matsui H. Relapse of cervical tuberculous lymphadenitis immediately after completion of effective anti-tuberculosis treatments. Respirol Case Rep. 2020 Apr 7;8(4):e00555. doi: 10.1002/rcr2.555.
CrossRef - Amanjolova L. K., R. G. Dostarbaev, Z. S. Bekbenbetova, Ye. P. Telegina / Sluchay generalizovannoy formы rezistentnogo tuberkuleza v sochetanii s legochnoy i vnelegochnoy lokalizatsiey u rebenka (klinicheskiy sluchay) / / Ftiziopulmonologiya. – 2022. – № 2. – S. 4-8.
- Tutkyshbaev S.O., Omirzak Ye.J., Imangaliev N.K. Tuberkuleznыy limfadenit perifericheskix limfaticheskix uzlov i yego diagnostika. Ftiziopulmonologiya. 2015. № 1-2 (26). S. 27-28.
- Antel K, Oosthuizen J, Malherbe F, Louw VJ, Nicol MP, Maartens G, Verburgh E. Diagnostic accuracy of the Xpert MTB/Rif Ultra for tuberculosis adenitis. BMC Infect Dis. 2020 Jan 13;20(1):33. doi: 10.1186/s12879-019-4749-x. Erratum in: BMC Infect Dis. 2020 Mar 2;20(1):187.
CrossRef - Bhembe NL, Green E. Characterization of mutations in the rpoB gene conferring rifampicin resistance in Mycobacterium tuberculosis complex isolated from lymph nodes of slaughtered cattle from South Africa. Braz J Microbiol. 2020 Dec;51(4):1919-1927. doi: 10.1007/s42770-020-00356-4.
CrossRef - Ganchua SKC, Cadena AM, Maiello P, Gideon HP, Myers AJ, Junecko BF, Klein EC, Lin PL, Mattila JT, Flynn JL. Lymph nodes are sites of prolonged bacterial persistence during Mycobacterium tuberculosis infection in macaques. PLoS Pathog. 2018 Nov 1;14(11):e1007337. doi: 10.1371/journal.ppat.1007337.
CrossRef - Manicheva O.A., Vishnevskiy B.I. i soav. Lekarstvennaya ustoychivost mycobacterium tuberculosis pri razlichnыx lokalizatsiyax zabolevaniya. // Infeksiya i immunitet. №4. 2014. S. –319–330.
CrossRef - Kim, B. H., Jeon, Y. J., Jin, Y. J., Jeong, W. J., Park, S. J., & Ahn, S. H. (2018). Conservative treatment for cutaneous fistula resulted from abscess formation in patients with tuberculous cervical lymphadenitis. Auris Nasus Larynx, 45(5), 1061-1065. https://doi.org/10.1016/j.anl.2018.01.006
CrossRef - Gupta A, Kunder S, Hazra D, Shenoy VP, Chawla K. Tubercular lymphadenitis in the 21st century: A 5-Year single-center retrospective study from South India. Int J Mycobacteriol. 2021 Apr-Jun;10(2):162-165.
CrossRef - Li WX, Zhu Y, Dong Y, Liu L. Diagnosis and Management of Occult Cervical Tuberculous Lymphadenopathy. Ear Nose Throat J. 2022 Jul; 101 (6):359-364.
CrossRef - Albayrak N, Celebi B, Kavas S, Simşek H, Kılıç S, Sezen F, Arslantürk A. Tularemi lenfadeniti şüphesi ile alınan lenf aspiratı örneklerinde Mycobacterium tuberculosis s varlığının araştırılması [Investigation of the presence of Mycobacterium tuberculosis in the lymph node aspirates of the suspected tularemia lymphadenitis cases]. Mikrobiyol Bul. 2014 Jan;48(1):129-34. Turkish.
CrossRef - Vergnon-Miszczycha D, Suy F, Robert F, Carricajo A, Fresard A, Cazorla C, Guglielminotti C, Lucht F, Botelho-Nevers E. Guillain-Barré syndrome associated with Mycobacterium bovis lymphadenitis. Infection. 2015 Oct; 43 (5):603-8.
CrossRef - Xu Z, Xia A, Li X, Zhu Z, Shen Y, Jin S, Lan T, Xie Y, Wu H, Meng C, Sun L, Yin Y, Chen X, Jiao X. Rapid loss of early antigen-presenting activity of lymph node dendritic cells against Ag85A protein following Mycobacterium bovis BCG infection. BMC Immunol. 2018 Jun 25;19(1):19.
CrossRef - Streltsov E.P., Aglyamova T.A., Nugmanov R.T., Solyanik S.N., Knyazeva O.Yu. Extrapulmonary tuberculosis. Difficulties in diagnosing an immunocompromised person. Prakticheskaya meditsina. № 4, (105). 2017, pages 81-84. https://cyberleninka.ru/article/n/vnelegochnyy-tuberkulez-trudnosti-diagnostiki-u-immunokomprometirovannogo-litsa
- Sinitsyna A.K., K Sinelnikova E., Lozovskaya M.E., Krivokhizh V.N., Gavrilov P.V., Osipova M.A. THE POSSIBILITIES OF ULTRASONOGRAPHY IN EARLY DIAGNOSIS OF LYMPH NODE TUBERCULOSIS. Diagnostic radiology and radiotherapy. 2016; (1):58-63. https://doi.org/10.22328/2079-5343-2016-1-58-63
CrossRef - Mordyk A.V., Yakovleva A.A., Nikolaeva I.N., Leontiev V.V. Extrapulmonary tuberculosis problem in epidemiologic situation. Pacific Medical Journal. 2015;(3):19-21.
- Ohene SA, Bakker MI, Ojo J, Toonstra A, Awudi D, Klatser P. Extra-pulmonary tuberculosis: A retrospective study of patients in Accra, Ghana. PLoS One. 2019, 9;14(1):e0209650. doi: 10.1371/journal.pone.0209650.
CrossRef - Gopalaswamy R, Dusthackeer VNA, Kannayan S, Subbian S. Extrapulmonary Tuberculosis—An Update on the Diagnosis, Treatment and Drug Resistance. Journal of Respiration. 2021; 1(2):141-164. https://doi.org/10.3390/jor1020015
CrossRef - Gupta S, Srivastava A, Kumar A, Mohan N. Relevance of CBNAAT in the early diagnosis of suspected tubercular swelling. MGM Journal of Medical Sciences 2023, 10(3): p 500-504, DOI: 10.4103/mgmj.mgmj_87_23
CrossRef - Solonko I.I., Gurevich G.L., Skryagina E.M., Dyusmikeeva M.I. EXTRAPULMONARY TUBERCULOSIS: CLINICAL EPIDEMIOLOGICAL CHARACTERISTICS AND DIAGNOSTICS. Tuberculosis and Lung Diseases. 2018;96(6):22-28. (In Russ.) https://doi.org/10.21292/2075-1230-2018-96-6-22-28
CrossRef







