Manuscript accepted on :10-02-2025
Published online on: 06-03-2025
Plagiarism Check: Yes
Reviewed by: Dr. Arif Ansori
Second Review by: Dr. Deepthi
Final Approval by: Dr. Patorn Piromchai
Marta Setiabudy1* , Anak Agung Gede Indraningrat1
, Anak Agung Gede Indraningrat1 , Kadek Suryawan3
, Kadek Suryawan3 , Ni Wayan Widhidewi1
, Ni Wayan Widhidewi1 , Anak Agung Ayu Lila Paramasatiari1
, Anak Agung Ayu Lila Paramasatiari1 , Made Dharmesti Wijaya2
, Made Dharmesti Wijaya2 , Putu Indah Budi Apsari1
, Putu Indah Budi Apsari1 and Putu Arya Suryanditha1
and Putu Arya Suryanditha1
1Department of Microbiology and Parasitology, Faculty of Medicine and Health Sciences, Universitas Warmadewa, Denpasar, Indonesia
2Department of Pharmacology, Faculty of Medicine and Health Sciences, Universitas Warmadewa, Denpasar, Indonesia
3Department of Clinical Microbiology, Sanjiwani Gianyar Regional General Hospital, Gianyar, Indonesia
Corresponding Author E-mail:marta.sp.mk@gmail.com
Abstract
Schefflera elliptica leaves contain secondary metabolites such as flavonoids, tannins, and saponins that have the potential as antibiofilm agents. Biofilms produced by Staphylococcus aureus are often the cause of chronic infections, especially on wounds and medical devices such as catheters. Antibiofilm activity of plant extracts is promising as an adjuvant or alternative therapy. The purpose of this study was to determine the antibiofilm activity of ethyl acetate extract of S. elliptica leaf against clinical isolates of S. aureus. This study is an analytical study with a laboratory experimental design to determine antibiofilm activity. The research samples were 18 clinical isolates of S. aureus obtained from patients undergoing examination at the hospital from June 2022 to December 2022. The research was carried out using the stages of sample preparation, extract preparation, biofilm assay with microtiter plate, and antimicrobial test with disc diffusion test method. The results of this study showed that the extract of S. elliptica leaf was able to reduce the average biofilm formation by clinical isolates of S. aureus significantly (p<0.001), both at doses of 10 µL (reduced by 12%) and 20 µL (reduced by 56.7%). Despite having antibiofilm activity, a disc diffusion test with a dose of 20 ul extract of S. elliptica leaf showed no or minimal antimicrobial activity against clinical isolates of S. aureus. Overall, the results of this study indicate that the extract of S. elliptica leaf has the potential as an antibiofilm agent against S. aureus. The results of this study are expected to be the basis of further research for the development of antibiofilm from natural materials.
Keywords
Antibiofilm; Clinical Isolate; Extract; Schefflera eliptica; Staphylococcus aureus
| Copy the following to cite this article: Setiabudy M, Indraningrat A. A. G, Suryawan K, Widhidewi N. W, Paramasatiari A. A. A. L, Wijaya M. D, Apsari P. I. D, Suryanditha P. A. Schefflera eliptica Leaf Extract as Antibiofilm Agent Against the Clinical Isolates of Staphylococcus aureus in Wound, Blood, and Sputum. Biomed Pharmacol J 2025;18(1). |
| Copy the following to cite this URL: Setiabudy M, Indraningrat A. A. G, Suryawan K, Widhidewi N. W, Paramasatiari A. A. A. L, Wijaya M. D, Apsari P. I. D, Suryanditha P. A. Schefflera eliptica Leaf Extract as Antibiofilm Agent Against the Clinical Isolates of Staphylococcus aureus in Wound, Blood, and Sputum. Biomed Pharmacol J 2025;18(1). Available from: https://bit.ly/4bw5D28 |
Introduction
Herbal plants in Bali have long been recognized for their medicinal properties, particularly their antibacterial effects. The traditional Balinese medicine system, known as Usadha, utilizes a variety of local plants that have been documented for their therapeutic benefits, including their antibacterial effects against various pathogens. Ethnobotanical studies highlight the traditional applications of various plants, emphasizing their roles in treating ailments such as diarrhea and infections, thus underscoring the importance of plants in local healthcare practices.1
One notable plant is Leaves of Schefflera elliptica, also known as Tulak wood plant in Indonesia, which has been traditionally used in Bali for its purported ability to repel negative influences and used in folk medicine for their potential antimicrobial and anti-inflammatory properties. Recent studies have begun to explore its phytochemical content and its antibacterial and antioxidant activities, revealing promising results that support its traditional uses.2
The study aligns with broader research indicating that many herbal plants contain bioactive compounds that exhibit antibacterial properties, which can be crucial in combating antibiotic resistance.3 This plant is easily found in Indonesia, particularly in Bali. Recent studies have highlighted the bioactive compounds in this plant, such as flavonoids, tannins, and saponins, suggesting its potential application in combating bacterial infections.2,4
Schefflera elliptica (S. elliptica) leaf extract is known to have the ability to act as an antimicrobial in certain doses, particularly in Staphylococcus aureus (S. aureus).4 This antimicrobial agent can work by inhibiting biofilm formation, disrupting the extracellular matrix of biofilms, or killing bacterial cells that reside within the biofilm.
Biofilms produced by S. aureus are causative agents of chronic infections, especially in wounds and medical devices such as catheters.5 Biofilm-associated persistent infections are not easy to treat because they are related to the nature of resistance to many drugs; moreover, in multidrug-resistant bacteria such as methicillin-resistant S. Aureus (MRSA).6 The low efficiency of various treatments and the toxicity of antibiotics available in vivo prompted researchers to research effective natural antimicrobial and antibiofilm agents.
The antimicrobial effect of a plant extract may extend to antibiofilm activity, as many antimicrobial agents are capable of inhibiting not only planktonic bacterial growth but also the formation and persistence of biofilms. This dual action highlights the potential of plant extracts as effective agents against both free-living and biofilm-associated bacteria. For instance, Piper betle exhibits significant antibacterial activity against oral pathogens, suggesting that various herbal plants can serve as sources for new antibacterial agents.7 Chinese eucalyptus oil gel was also known as an antimicrobial agent.8
Antibiofilm activity from plant extracts is very promising as an adjuvant or alternative therapy. Natural product-based antibiofilm extracts are more efficient and have fewer side effects than other chemically synthesized antibiotics.9,10 Another study revealed that plant extract from Paederia foetida leaves has antibacterial and antibiofilm activity against Escherichia coli and Mycobacterium smegmatis.11 The result is in line with the larger trend of treating medical conditions with traditional herbal treatments, especially in places where resistance is rampant and access to synthetic antibiotics may be restricted.12
While the antibacterial potential of S. elliptica has been confirmed, no studies have been reported regarding the activities of the plant extract against the clinical isolate of S. aureus. Such information is crucial to elevate the potential of S. elleptica to combat biofilm formation, especially against clinical isolates.
The purpose of this study is to determine the antimicrobial and antibiofilm activity of the ethyl acetate extract of S. elliptica against the clinical isolate of S. aureus. Clinical isolates are isolates obtained from samples of patients infected with bacteria.
Materials and Methods
Research design
The purpose of this analytical investigation was to ascertain the antibiofilm and antibacterial activity of S. elliptica leaf extract in the clinical isolate of S. aureus using a laboratory experimental approach.
Population and Sample
The target population of this study was the clinical isolate of patients with infection or colonization of S. aureus. The population was the clinical isolate of patients with infection or colonization of S. aureus who were treated at Sanjiwani Hospital, Gianyar, Bali, Indonesia. The study sample was patients with S. aureus infection who were hospitalized from June 2022 to December 2022. The inclusion criteria of this study were S. aureus isolate taken from the culture of patients infected with the germ. The exclusion criteria were the isolates that did not produce biofilm.
Collection of Schefflera elliptica
Healthy S. elliptica leaves were collected from Gerih Village, Bali, Indonesia. The green mature leaves were selected as they are believed to contain a high concentration of phytochemicals with potential bioactive properties. To determine the plant’s identity during the study, the leaves were sent to the National Research and Innovation Agency (BRIN), Candikuning, Tabanan, Bali.
Extraction of Schefflera elliptica
Extraction was the initial process used to isolate the desired natural compounds from raw materials. Common techniques for extraction include solvent-based methods, distillation, pressing, and sublimation, depending on the principles underlying each method.13 The method chosen to extract S. elliptica in this study was maceration. Although it takes a longer time and has low efficiency, it is stable for thermolabile components.13
The making of simplicia started from the determination of the plant, and then 1 kg of leaves was washed and dried by aeration for 2 days. Then, the leaves were dried in the oven at a temperature of 40-60°C for 6 hours. The dried leaves were blended into powder and then sifted using a 60-mesh sieve until as much as 100 g of powder was obtained. The powder was macerated with 1,000 ml of ethyl acetate solvent for 3×24 hours by changing the solvent every day. The filtrate was stored in a container, and the residue was re-macerated for the next 24 hours. The filtrate that had been stored was evaporated on a rotary evaporator to remove the solvent until it formed a viscous extract. This viscous extract was used for antibiofilm testing.2,4
Sample preparation
Preparation of Mueller Hinton Agar (MHA) Media and Bacterial Suspension were done initially. MHA powder was weighed and then dissolved in 1 L of aqueduct on Erlenmeyer, stirred and boiled on a hotplate, then sterilized on an autoclave at 121°C for 15 minutes. Next, the sufficiently cooled medium is poured into a petri dish and placed in an incubator at a temperature of 37°C.14 After the culture was carried out and confirmed to be pure, the process continued with a biofilm test.
Biofilm assay
Biofilm assay using microtiter plate (MTPs) was chosen since it is practical, has high throughput, and low cost compared to other methods; however, it also has limitations, such as the variability of the result.15,16
The steps included preparing the MTPs to place the suspension. The process began by inoculating the bacteria from the culture in a 1% glucose liquid and saline buffer phosphate (PBS) with a pH of 7 as much as 3 ml and transferring a suspension of 200 microliters into the wells in all the MTPs. The step was conducted for all samples, with each sample triplicated. In each microtiter plate, there was a positive control (1% liquid glucose and saline buffer phosphate (PBS) with the addition of chloramphenicol) and a negative control (1% glucose liquid and saline buffer phosphate (PBS) with the addition of ethyl acetate without extraction). Optimization has been carried out.
S. elliptica leaf extract, as many as 10 ul and 20 ul, respectively, are added to each bacterial suspension as described above and continue with incubation. Based on previous research on the antibacterial activity of ethanol and ethyl acetate extract of S. elliptica leaf showed inhibition of S. aureus at concentrations of 50 μg, 100 μg and 200 μg.4 The selection of dosage was adjusted according to the optimization results since there was no previous research on antibiofilm activity. Later, the results of the formation of biofilms will be compared from all the microtiter plates.
Negative controls just used the medium and bacteria, while positive controls used the antibiotic chloramphenicol. There are positive and negative controls for every MTP. A readout followed the biofilm test. After that, rinse each well with Aquabidest and discard the leftover solution. For five minutes, 200 microliters of 0.1% crystal violet were used to stain the biofilm cells that were affixed to the well. After washing, the plate is dried once more. Using an ELISA microplate reader, assess the intensity of the crystal violet fluorescence after adding 200 microliters of 30% acetic acid to each well and letting it dissolve for five to fifteen minutes.17–19
Antimicrobial Activity test
Antimicrobial susceptibility testing was done using a disc diffusion test.20,21 The suspension of S. aureus bacteria that had been normalized with a density of 0.5 McFarland (about 10⁸ CFU/mL) was inoculated evenly on the surface of MHA media.
A total of 20 μL of S. elliptica leaf extract was placed on a sterile filter disc, and then the disc was placed on the agar surface that had been inoculated with bacteria. Antibiotic positive discs (chloramphenicol) and negative control discs (without extracts) are also placed on top of agar for comparison of results.
After the disc is placed in the bowl, the plate is incubated at 37°C for 18-24 hours to allow the growth of bacteria and the formation of an inhibition zone around the disc containing S. elliptica extract.
After the incubation period, the inhibition zone is measured using a ruler with precision down to the millimeter (mm). The formed inhibitory zone showed the presence of antimicrobial activity from S. elliptica leaf extract against S. aureus. The larger the diameter of the inhibition zone, the stronger the antibacterial activity of the extract.
Statistical Analysis
Two phases of analysis are then performed on the gathered data. First, each variable’s features were described using descriptive statistical analysis. Relative frequencies (numbers and percentages) are used to represent the data variables. Before doing the next step, it is necessary to conduct a data normality test. The second step was comparing the production of biofilms in bacterial suspensions with and without the addition of two different amounts of S. elliptica leaf extract. A paired T-test was used to compare the average of each treatment in order to perform the analysis.
SPSS software for Windows version 29 was used for all data analysis. The 95% confidence interval value determines the precision value, and the meaning limit is set at p<0.05.
Results
The collected sample consisted of 18 isolates of S. aureus. Samples were obtained from patients of various ages, and the highest age range was 46-65 years, as many as 44.4%. The most common specimen was from wound (wound bed swab, tissue, and pus) with 8 samples (44.4%), followed by blood with 6 samples (33.3%), and sputum 4 (22.2%). The most dominant diagnoses were skin infection and pneumonia, as much as 6 (33.3%).
There were 4 (22%) isolates of Methicillin-Resistant Staphylococcus aureus (MRSA). This amount is in the average range of MRSA infections in hospitals in Indonesia, which ranges from 0.3 to 52% depending on the setting.22 In general, MRSA infections are considered more difficult to treat because they have more virulence factors and the potential for more serious complications.23
Biofilm tests on samples showed that 100% of the 18 isolates formed a biofilm after incubation for 48 hours at 37°C in a 1% glucose solution. By measuring optical density using an ELISA reader, the average biofilm formed was 0.093. The lowest score was 0.087, and the highest was 0.117. The result is in line with several studies that state that S. aureus bacteria are biofilm producers.17,18
Antibiofilm activity
The results of this study showed that S. elliptica leaf extract was able to reduce the formation of biofilm by S. aureus, both at doses of 10 μL and 20 μL. Testing was carried out using crystal violet, which measured the thickness of the biofilm formed on the glass surface after the extract treatment and was measured using an ELISA reader. The results can be seen in Figure 1.
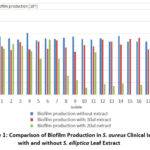 |
Figure 1: Comparison of Biofilm Production in S. aureus Clinical Isolates with and without S. elliptica Leaf Extract |
At a dose of 10 μL, there was an average decrease in biofilm formation by 12%, while at a dose of 20 μL, there was an average decrease in biofilm formation by 56.7%, as seen in Table 1.
Table 1: Descriptive Statistics of Biofilm Production
|
Treatment |
Biofilm Production |
|||
|
Minimum |
Maximum |
Mean |
Std. deviation |
|
|
Without Extract |
0.087 |
0.117 |
0.092 |
0.007 |
|
Extract 10 ul |
0.070 |
0.093 |
0.081 |
0.007 |
|
Extract 20 ul |
0 |
0.090 |
0.040 |
0.031 |
The average decrease in biofilm formation was proven to be significant (p<0.001), as noted in Table 2. At higher doses of extract, a reduction in biofilm formation occurred more substantially, suggesting that higher doses provided a more effective effect in inhibiting biofilm formation by S. aureus. In many antibiofilm studies, the use of higher doses or more concentrated extracts increases the activity of its antibiofilm.24
Table 2: The Significance Level of Difference
|
Paired T-test |
Mean |
95% Confidence Interval |
Significance |
||
|
lower |
upper |
One-Sided p |
Two-Sided p |
||
|
Without extract – Extract 10ul |
11.111 |
5.755 |
16.467 |
<.001 |
<.001 |
|
Without extract – Extract 20 ul |
52.611 |
36.279 |
68.943 |
<.001 |
<.001 |
Antimicrobial activity
The results of the Disc Diffusion test showed that S. elliptica leaf extract with a dose of 20 μL had minimal antimicrobial activity against the clinical isolate of S. aureus. As many as 61.1% of the samples did not show an inhibition zone on the growth medium. The remaining 33.3% showed a minimum inhibition zone of 6-8 mm, and 1 sample (5.6%) showed a moderate inhibition zone, which was 11 mm. Although an inhibitory zone was formed, these results were relatively smaller compared to the positive control disc, which suggests that the effectiveness of the extract at this dose was quite limited.
In S. aureus ATCC, the extract also showed no clear inhibitory zone, as seen in Figure 2.
 |
Figure 2: Absence of a distinct inhibition zone in S. aureus ATCC compared to the positive control. |
Discussion
Antibiofilm activity
S. aureus is known as an opportunistic pathogen that can form biofilms on both intracellular and intracellular surfaces, which contributes to resistance to antibiotic therapy and increases its virulence. Biofilm formation makes treatment more challenging, particularly for patients with chronic wounds and those who use devices like IV catheters, central venous catheters, pacemaker implants, and urinary catheters. Furthermore, if the wound area is not adequately cleaned, biofilm will build and impede the healing process.
The formation of biofilms by S. aureus in the clinical setting is very difficult to overcome and is closely related to chronic infections, such as diabetic wounds, endocarditis and urinary tract infections.25,26 The infection will be more complicated if MRSA causes the infection, and the prevalence is considered high in big cities in Indonesia. It might be higher in the following years without interventions.22
Natural compounds have been instrumental in the creation of new pharmaceutical drugs including as antibiofilm and antibiotic.27,28 The search for natural antimicrobial as well as antibiofilm agents, such as medicinal plants, has become very relevant in efforts to overcome the problem of antibiotic resistance.29–31 Many natural ingredients have been tested and are known to have antibiofilm properties, such as aloe vera, and Launaea nudicaulis (L.) Hook, Myrtus communis L. leaves essential oil and Equisetum hyemale 32–35.
This research aimed to assess the efficacy of S. elliptica leaf extract as a plant-based antibiofilm agent against a clinical isolate of S. aureus. The method chosen to extract S. elliptica in this study was maceration, which took a longer time and had low efficiency but considered stable for thermolabile components in the leaves.13 This research is carried out quite simply, but it is hoped that it can be a foundation for development in the future.
This study revealed that the administration of S. elliptica leaf extract can reduce the formation of biofilms, and this was also directly proportional to the dose used. The bioactive content in S. elliptica bring the stronger potential abilities, such as flavonoids, alkaloids, and terpenoids, which are known to have antimicrobial and antibiofilm activity.2,4,36 These compounds can interfere with the mechanism of biofilm formation by affecting the expression of genes related to bacterial cell adhesion and the production of extracellular matrix biofilms.37
The administration of S. elliptica leaf extract in two different doses showed a significant difference in S. aureus’s ability to inhibit biofilm formation. A dose of 20 μL results in a greater decrease in biofilm, indicating that higher concentrations of S. elliptica leaf extract has a stronger antibiofilm potential. This was in line with research conducted on Pseudomonas aeruginosa PAO1 using Artocarpus heterophyllus Lam. or jackfruit which showed antibiofilm effects at doses of 2.5 mg/ml and 1.25 mg/ml compared to other smaller doses (ranging from 5 to 0.009 mg/ml).38
This decrease in biofilm formation in S. aureus may also be related to the ability of S. elliptica leaf extract to decrease the interaction and communication between bacterial cells, which is essential for the process of aggregation and stable formation of biofilm layers, known as quorum sensing.35,39
Although the results obtained show good antibiofilm potential,40 the effect of a more significant higher dose is related to toxicity to the host cell. Further research is needed to evaluate the toxicity and safety profile of S. elliptica extract, both at high doses and at long-term use.
Antimicrobial activity
The results of the Disc Diffusion test showed that S. elliptica leaf extract with a dose of 20 μL had minimal antimicrobial activity against the clinical isolate of S. aureus. Although S. elliptica leaf extract contains bioactive compounds such as flavonoids and terpenoids that have been shown to have antimicrobial activity, its effectiveness in inhibiting bacterial growth may be affected by the lower concentration of the extract.28 A dose of 20 μL might be too low to achieve a minimum effective concentration in inhibiting bacterial growth. Tests must be conducted at higher doses or using more powerful extraction methods to increase the effectiveness. A previous study with pomegranate peel extract showed a wider inhibition zone at higher doses.41
Previous studies with the same extract showed antimicrobial effects in certain doses. However, it was not done in clinical isolates.4,36 Some of the bioactive compounds in S. elliptica leaf extracts may not be effective enough in inhibiting S. aureus directly at the doses used, or those compounds may take longer to show significant antimicrobial effects, especially in clinical isolates.
Another reason could be that the disc diffusion test is a qualitative method that provides an overview of antimicrobial activity. However, the results obtained do not always reflect the true antibacterial potential when compared to another test, such as the epsilometer test42 or quantitative tests, such as MIC (Minimum Inhibitory Concentration).43,44
Limitation
This study has limitations, including variability in extraction techniques affecting bioactive component efficacy, genetic and phenotypic differences among S. aureus strains, a limited number of isolates, and a potentially narrow concentration range for determining optimal efficacy.
Conclusion
S. elliptica leaf extract has the potential as an antibiofilm agent against clinical isolates of S. aureus. The higher dose (20 μL) provides a more significant effect in reducing biofilm formation, which suggests that S. elliptica leaves may be an alternative candidate in the development of plant-based antibiofilm therapy. Further research on the mechanism of action of S. elliptica leaf extract, as well as its toxicity and clinical effectiveness tests, is urgently needed to ascertain its therapeutic benefits in the treatment of biofilm-related infections. Antimicrobial tests using the Disc Diffusion method showed that S. elliptica leaf extract had the potential to inhibit the growth of S. aureus, but its antibacterial effect at a dose of 20 μL was still limited, as seen from the inhibition zone formed, which was quite minimal. Further research with higher dose variations and the use of quantitative test methods such as MIC is needed to further evaluate the antibacterial potential of S. elliptica leaf extract.
Acknowledgement
The Warmadewa University Faculty of Medicine and Health Science, as well as everyone who helped to finish this project, have the authors’ sincere gratitude. Their knowledge and experience have been extremely helpful in determining the focus and direction of our effort. For their cooperation and commitment to this endeavour, we are also grateful to the laboratory analysts and members of our research team.
Funding Source
With decision No. 1023/Unwar/FKIK/PD-13/IX/2024, the Research Unit of the Faculty of Medicine and Health Sciences, Universitas Warmadewa, Denpasar, Indonesia, provided the monetary support for the study.
Conflict of Interest
The author(s) do not have any conflict of interest
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Author Contributions
Each author mentioned has significantly and directly contributed intellectually to the project and has given their approval for its publication.
Marta Setiabudy: Conceptualization, Methodology, Writing original draft.
Anak Agung Gede Indraningrat: Methodology, Review and editing.
Kadek Suryawan: Data Collection
Ni Wayan Widhidewi: Funding Acquisition, Resources.
Anak Agung Ayu Lila Paramasatiari: Supervision
Made Dharmesti Wijaya: Project Administration.
Putu Indah Budi Apsari: Data analysis
Putu Arya Suryanditha: Visualization
References
- Dasgupta S.C. Bioactive compounds from medicinal plants and its therapeutic uses in the traditional healthcare system. In: Jha S, Halder M, eds. Medicinal Plants: Biodiversity, Biotechnology and Conservation. Sustainable Development and Biodiversity. 2023;33:525-537.
CrossRef - Kirtanayasa I.G.Y.A, Indraningrat A.A.G, Candra I.P. Phytochemical, Antibacterial and Antioxidant Activities of Schefflera elliptica Leaves. Biology, Medicine, & Natural Product Chemistry. 2023;12(1):329-334.
CrossRef - Khameneh B, Iranshahy M, Soheili V, Fazly Bazzaz B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrobial Resistance &Infection Control. 2019;8(1):1-28.
CrossRef - Kristina N.P.S, Aryasa I.W.T, Apriyanthi D.P.R. Antibacterial Activity of Ethanol Extract of Tulak Leaves (Schefflera elliptica (Blume) Harms) Against Staphylococcus aureus and Escherichia coli. Journal of Applied Pharmaceutical Science. 2023;16(1):41-51.
CrossRef - Liu Y, Zhang J, Ji Y. Environmental factors modulate biofilm formation by Staphylococcus aureus. Sci Prog. 2020;103(1):1-14.
CrossRef - Carmona-Orozco M.L, Echeverri F. Induction of biofilm in extended-spectrum beta-lactamase Staphylococcus aureus with drugs commonly used in pharmacotherapy. Microbial Pathogenesis. 2024;195:1-11.
CrossRef - Lubis R.R, Marlisa, Wahyuni D.D. Antibacterial activity of betle leaf (Piper betle l.) extract on inhibiting Staphylococcus aureus in conjunctivitis patient. American Journal of Clinical and Experimental Immunology. 2020;9(1):1-5.
- Dubey D, Vibha, Murti Y, Wal P, Ved A, Kumar P, Sharma A.K, Dwivedi H, Singh A, Singh M.P, Kulshreshtha M. Formulation and evaluation of Chinese eucalyptus oil gel by using different gelling agents as an antimicrobial agent. Pharmacological Research – Modern Chinese Medicine. 2023;9:1-6.
CrossRef - Mishra R, Panda A.K, De Mandal S, Shakeel M, Bisht S.S, Khan J. Natural Anti-biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Frontiers in Microbiology. 2020;11:1-23.
CrossRef - Jafri H, Ahmad I. Application of natural products against fungal biofilm formation. In: A Complete Guidebook on Biofilm Study. 2022;1:95-130.
CrossRef - Priyanto J.A, Prastya M.E, Sinarawadi G.S, Datu’salamah W, Avelina T.Y, Yanuar A.I.A, Azizah E, Tachrim Z.P, Mozef T. The antibacterial and antibiofilm potential of Paederia foetida leaves extract. Journal of Applied Pharmaceutical Science. 2022;12(10):117-124.
CrossRef - Majhi A, Roy S, Mukherjee S, Banerjee S, Bera K, Das M, Ghosh S. A review on antimicrobial activity of some plant. Journal of Medical Plants Studies. 2024;12(1):221-224.
CrossRef - Zhang Q.W, Lin L.G, Ye W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Medicine (UK). 2018;13(1):1-26.
CrossRef - Ogodo A.C, Agwaranze D.I, Daji M, Aso R.E. Microbial techniques and methods: Basic techniques and microscopy. In: Analytical Techniques in Biosciences: From Basics to Applications; 2022;1:201-220.
CrossRef - Thibeaux R, Kainiu M, Goarant C. Biofilm Formation and Quantification Using the 96-Microtiter Plate. In: Methods in Molecular Biology. 2020;2134:207-214.
CrossRef - Kragh K.N, Alhede M, Kvich L, Bjarnsholt T. Into the well—A close look at the complex structures of a microtiter biofilm and the crystal violet assay. Biofilm. 2019;1:1-9.
CrossRef - Omidi M, Firoozeh F, Saffari M, Sedaghat H, Zibaei M, Khaledi A. Ability of biofilm production and molecular analysis of spa and ica genes among clinical isolates of methicillin-resistant Staphylococcus aureus. BMC Res Notes. 2020;13(1):1-7.
CrossRef - Kwiecinski J.M, Jacobsson G, Horswill A.R, Josefsson E, Jin T. Biofilm formation by Staphylococcus aureus clinical isolates correlates with the infection type. Infect Dis. 2019;51(6):446-451.
CrossRef - Grossman A.B, Burgin D.J, Rice K.C. Quantification of Staphylococcus aureus Biofilm Formation by Crystal Violet and Confocal Microscopy. In: Methods in Molecular Biology. 2021;2341:69-78.
CrossRef - Wenzler E, Maximos M, Asempa T.E, Biehle L, Schuetz A.N, Hirsch E.B. Antimicrobial susceptibility testing: An updated primer for clinicians in the era of antimicrobial resistance: Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2023;43(4):264-278.
CrossRef - Gajic I, Kabic J, Kekic D, Jovicevic M, Milenkovic M, Mitic Culafic D, Trudic A, Ranin L, Opavski N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics. 2022;11(4):1-26.
CrossRef - Syahniar R, Kharisma D.S, Khatami M, Duarsa D.B.B. Methicillin-resistant Staphylococcus aureus among clinical isolates in Indonesia: A systematic review. Biomedical and Pharmacological Journal. 2020;13(4):1871-1878.
CrossRef - Kubde V, Dangre G, Mudey A. Healthcare setting and methicillin resistant Staphylococcus aureus. Internatinal Journal of Current Research and Review. 2020;12(14 Special Issue):123-128.
CrossRef - Ray S, Jin J.O, Choi I, Kim M. Cell-Free Supernatant of Bacillus thuringiensis Displays Anti-Biofilm Activity Against Staphylococcus aureus. Applied Biochemistry and Biotechnology. 2023;195(9):5379-5393.
CrossRef - Howden B.P, Giulieri S.G, Wong Fok Lung T, Baines S.L, Sharkey L.K, Lee J.Y.H, Hachani A, Monk I.R, Stinear T.P. Staphylococcus aureus host interactions and adaptation. Nat Rev Microbiol. 2023;21(6):380-395.
CrossRef - Linz M.S, Mattappallil A, Finkel D, Parker D. Clinical Impact of Staphylococcus aureus Skin and Soft Tissue Infections. Antibiotics. 2023;12(3):557:1-27.
CrossRef - Abdelghafar A, Yousef N, Askoura M. Combating Staphylococcus aureus biofilm with Antibiofilm agents as an efficient strategy to control bacterial infection. Research Journal of Pharmacy and Technology. 2020;13(11):5601-5606.
- Shamsudin N.F, Ahmed Q.U, Mahmood S, Shah S.A.A, Khatib A, Mukhtar S, Alsharif M.A, Parveen H, Zakaria ZA. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules. 2022;27(4).
CrossRef - Alves P.S, de Figuerêdo J.S, Santos F.P.S, Furtado P.V.O.S, Andrade T.J.A.S, Júnior J.S.C, Lima N.M, Feitosa C.M. Natural products from plants with antimicrobial action. In: Promising Antimicrobials from Natural Products. 2022;1:183-198.
CrossRef - Tyagi R, Bhattacharjee S, Kumar N. Harnessing nature: Natural products to combat antibacterial drug resistance. In: Frontiers in Combating Antibacterial Resistance: Current Perspectives and Future Horizons. 2024;1:33-46.
CrossRef - Tsopmene U.J, Tokam Kuaté C.R, Kayoka-Kabongo P.N, Bisso B.N, Metopa A, Mofor C.T, Dzoyem J.P. Antibiofilm Activity of Curcumin and Piperine and Their Synergistic Effects with Antifungals against Candida albicans Clinical Isolates. Scientifica (Cairo). 2024;2024.
CrossRef - De C, Pontes B, Rocha Da Silva B, Luís S, Pereira S. Antimicrobial and Antibiofilm of Aloe Vera on Bacteria. International Journal of Development Research. 2021;11(10):51340-51345
- Caputo L, Capozzolo F, Amato G, De Feo V, Fratianni F, Vivenzio G, Nazzaro F. Chemical composition, antibiofilm, cytotoxic, and anti-acetylcholinesterase activities of Myrtus communis leaves essential oil. BMC Complement Medical Therapy. 2022;22(1):1-16.
CrossRef - Elkady F.M, Badr B.M, Hashem A.H, Abdulrahman M.S, Abdelaziz A.M, Al-Askar A.A, AbdElgayed G, Hashem H.R. Unveiling the Launaea nudicaulis (L.) Hook medicinal bioactivities: phytochemical analysis, antibacterial, antibiofilm, and anticancer activities. Front Microbiol. 2024;15(October):1-14.
CrossRef - Santos Alves C.F.D, Bonez P.C, Casagrande C, Clerici D.J, Verdi C.M, Urquhart C.G, Missel M.V, Barin T, Campos EMMAD, Santos RC V. Antibiofilm activity of Equisetum hyemale: interference with quorum sensing. Journal of Herbal Medicine. 2023;42:1-9.
CrossRef - Aryasa I.W.T, Artini N.P.R. Green Synthesis of Silver Nanoparticles using Kayu tulak Leaf (Schefflera Elliptica Harms) Infusion as a Bio-reductant and Its Antibacterial Activity. Jurnal Kimia Sains dan Aplikasi. 2022;25(6):212-217.
CrossRef - Nwafor I.R, Alhassan Y, Udoh J.I, Odanibeh D, Oyaniyi J, Efoli-Bam V.K, Azubuike E.O, Ojobor J.F.C, Nwokafor C.V. Plant-derived Bioactive Compounds and Their Mechanistic Roles in Combating Microbial Biofilms. Microbiology Research Journal International. 2024;34(9):74-85.
CrossRef - Soni M, Pathoor N.N, Viswanathan A, Veeraragavan G.R, Ganesh P.S. Exploring the antimicrobial and antibiofilm activities of Artocarpus heterophyllus Lam. against Pseudomonas aeruginosa PAO1. World Acad Sci J. 2024;6(5).
CrossRef - Subramaniam G, Khan G.Z, Sivasamugham L.A, Wong L.S, Kidd S, Yap C.K. Antimicrobial and anti-biofilm activities of plant extracts against Pseudomonas aeruginosa – a review Journal of Experimental Biology and Agricultural Sciences. 2023;11(5):780-790.
CrossRef - Asma S.T, Imre K, Morar A, Herman V, Acaroz U, Mukhtar H, Arslan-Acaroz D, Shah S.R.A, Gerlach R. An Overview of Biofilm Formation–Combating Strategies and Mechanisms of Action of Antibiofilm Agents. Life. 2022;12(8):1-31.
CrossRef - Alnehia A, Al-Odayni A.B, Al-Sharabi A, Al-Hammadi A.H, Saeed W.S. Pomegranate Peel Extract-Mediated Green Synthesis of ZnO-NPs: Extract Concentration-Dependent Structure, Optical, and Antibacterial Activity. Journal of Chemistry. 2022;2022:1-11.
CrossRef - Homeida H.E, Khalid A, Elamin N.M.H, Osman H.A, Dawod O.Y. Comparison of Epsilometer Test and Disc Diffusion Methods for Antibiotic Susceptibility Testing of Pseudomonas Aeruginosa. Nanotechnol Perceptions. 2024;20(S3):755-761.
CrossRef - Chiu C.T, Lai C.H, Huang Y.H, Yang C.H, Lin J.N. Comparative analysis of gradient diffusion and disk diffusion with agar dilution for susceptibility testing of Elizabethkingia anophelis. Antibiotics. 2021;10(4),450:1-9.
CrossRef - Turnidge J.D, Ferraro M.J, Jorgensen J.H. Susceptibility test methods: general considerations. In: Manual of Clinical Microbiology. 10th ed. Washington, DC: American Society for Microbiology; 2022; 1:1115-1121.
CrossRef







