Manuscript accepted on :29-01-2025
Published online on: 10-02-2025
Plagiarism Check: Yes
Reviewed by: Dr. Karthikeyan
Second Review by: Dr. Neeta Rai
Final Approval by: Dr. Kapil Joshi
Geetha Ramamoorthy1 , Arulselvi Subramanian1
, Arulselvi Subramanian1 , Tamilselvi Rajendran2*
, Tamilselvi Rajendran2* and Parisa Beham Mohamed2
and Parisa Beham Mohamed2
1Department of ECE, Bharath Institute of Higher Education and research, Chennai, India
2Department of ECE, Sethu Institute of Technology, Viruthunagar, India
Corresponding Author E-mail:tamilselvi@sethu.ac.in
DOI : https://dx.doi.org/10.13005/bpj/3087
Abstract
Elevated noise levels and low resolution often obscure critical bone structures in DXA and X-ray images; this complication can hinder the diagnosis of osteoporosis. This paper presents a comprehensive comparative analysis of four distinct image processing methods: anisotropic diffusion, multi-scale wavelet analysis, adaptive guided filtering and neural network-based denoising. Each technique is evaluated for its effectiveness in reducing noise, preserving image details and enhancing diagnostic accuracy. Anisotropic diffusion effectively reduces noise while maintaining edge clarity—this ensures that vital bone structures remain visible. Multi-scale wavelet analysis captures intricate details over various scales, providing a robust approach to highlight essential regions. Adaptive guided filtering sharpens edges and improves precision by minimizing distortions. However, the neural network-based denoising method stands out among these techniques, significantly outperforming the others. This advanced deep learning filter not only eliminates residual noise but also protects critical bone structures, thus ensuring remarkable diagnostic clarity. Quantitative and qualitative analyses, utilizing metrics such as Peak Signal-to-Noise Ratio (PSNR) and Structural Similarity Index (SSIM), confirm that the neural network-based denoising approach achieves an optimal balance between noise reduction and structural preservation. Building on these findings, we propose a novel enhancement to the neural network-based denoising method: this improvement makes it more effective for clinical applications. This work provides valuable insights for clinical use, enabling more sensitive and accurate early diagnosis of osteoporosis. Although the study demonstrates the potential of advanced image processing techniques, it also highlights the need for further research to fully realize their benefits. This offers a significant contribution to medical imaging practices; however, challenges remain in implementation. But, the implications of these findings are profound, particularly because they can reshape the future of diagnostic methodologies.
Keywords
Image Preprocessing; Medical Imaging Enhancement; Neural Network Denoising; Osteoporosis Diagnosis
Download this article as:| Copy the following to cite this article: Ramamoorthy G, Subramanian A, Rajendran T, Mohamed P. B. Comparative Analysis of Image Denoising Techniques for Osteoporosis Detection Using DXA and X-ray Imaging. Biomed Pharmacol J 2025;18(March Spl Edition). |
| Copy the following to cite this URL: Ramamoorthy G, Subramanian A, Rajendran T, Mohamed P. B. Comparative Analysis of Image Denoising Techniques for Osteoporosis Detection Using DXA and X-ray Imaging. Biomed Pharmacol J 2025;18(March Spl Edition). Available from: https://bit.ly/418Up0i |
Introduction
Osteoporosis is a systemic bone disease as a result of progressive weakening of bones, which increases the risk of fractures, mainly in the elderly. Therefore, early detection and accurate diagnosis are crucially important for effective management and prevention of complications. Traditionally, diagnostic procedures embrace the use of Dual-Energy X-ray Absorptiometry (DXA) and X-rays for assessing the BMD. However, those techniques are often limited due to noisy interference and low resolution that may obscure crucial bone structures and hinder the detection of slight changes, like micro-fractures 4. Noise-free medical imaging is essential to obtain a higher degree of diagnostic accuracy along with early detection of diseases such as osteoporosis. By removing image noise and enhancing structural clarity, radiologists can identify the subtle indicators that include micro-fractures and variation in bone density that are very often missed in noisy or low-resolution images. This is rather critical for the early interventions that might hold the possibility of avoiding severe complications in osteoporosis patients.
Recent work on medical image processing has developed several techniques that have contributed to achieving better-quality diagnostic images and increasing the rate of correctness in osteoporosis diagnosis 5.
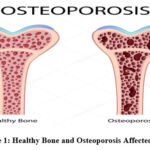 |
Figure 1: Healthy Bone and Osteoporosis Affected BoneClick here to view Figure |
This paper does a comparative analysis of four types of preprocessing methodologies applied to images—Anisotropic Diffusion, Multi-Scale Wavelet Analysis, Adaptive Guided Filtering, and Neural Network-Based Denoising—by reviewing the extent to which they can reduce noises, maintain bone structures, and make it more lucid for more accurate diagnosis. Among those, the methodology of Neural Network-Based Denoising proves to be more effective than others. This deep learning-based method removes residual noise but, besides that, restores important structural details of images: diagnostic performance is improved. This proposed methodology addresses better the challenges appearing due to low resolution as well as noise while diagnosing osteoporosis. These preprocessing techniques would then be compared using PSNR, SSIM, and edge strength metrics in order to evaluate their effectiveness. The hope is that in finding the most effective technique, we can contribute to better diagnostic tools that could enable earlier osteoporosis detection.
Litrature Review
Deep learning and sophisticated image processing have significantly enhanced image quality of medical images to obtain more accurate diagnoses, especially in the case of osteoporosis. The fundamental issue underlying diagnosis of osteoporosis through DXA and X-ray lies in the noise and low-resolution of images, which mask the most important structural structures of the bones, thus adding difficulties in the early stages of diagnosis. Various research works on images often try to find solution by implementing sophisticated preprocessing techniques on images while keeping all the structural details more or less intact. Recent noise removal developments have indicated potential to enhance the quality of medical images. A deep learning-based framework for low-dose computed tomography images was recently proposed,1 which demonstrated a significant improvement in image quality while preserving structural details. This work showed how deep learning is adapted to increase perception of bone structures—a necessity for the detection of micro-fractures in osteoporosis. Similarly, current models for the removal of noise from medical images found that one can remove noise with a preservation of all the critical bone structures using models based on CNN.2 Such models are very valuable while performing early osteoporosis where the fine details from the bone structure has to be preserved. Deep learning-based techniques for denoising the CT scans by keeping anatomical integrity during the noise removal processes.3 These developments are in line with this study because they focus on the suppression of noise while keeping the bone structure intact, which is also very essential for the recognition of osteoporosis. Also applied neural networks for enhancing image quality of CT which focused on maintaining structural details while suppressing noises, a process that is critical for the detection of osteoporosis patient’s micro-fractures.4 Wavelet-based methods have proven to be effective in denoising while preserving small-scale features. The implementation of wavelet-frame shrinkage networks proposed by the IEEE teamgreatly benefited to improve the visibility of the bone structure.9Multi-scale techniques are very useful in capturing the subtle variations related to changes in bone density cases of early osteoporosis among others. Application of neural networks for denoising which has been proved to be one of the primary methods of increasing the clarity of images.5 6 These models change based on the nature of the noise in each picture, removing the noise without damaging the edges; important features in proper bone fracture diagnosis. We draw inspiration from these works and apply neural network-based denoising techniques to enrich osteoporosis diagnosis by eliminating residues while keeping essential bone structures.
Inference from the Survey
In most cases, reviewed studies have demonstrated that high levels of noise and low resolution in DXA and X-ray images obscure critical structures of the bone, raising difficulty in osteoporosis diagnosis. Some preprocessing techniques are assessed in the current study: these include anisotropic diffusion, multi-scale wavelet analysis, adaptive guided filtering, and neural network-based denoising. Of these, the noise removal through neural networks is most prominent due to its capability of residual noise removal without affecting the skeleton structures within an image. The approach from deep learning represents the best compromise between noise reduction and structural preservation – this is confirmed by qualitative metrics like Peak Signal-to-Noise Ratio and Structural Similarity Index. These findings indicate how deep learning-based denoising can significantly improve the diagnosis of osteoporosis through improved quality images, thus enabling more sensitive and accurate early detection. Such an improvement would allow deep learning-based denoising to be added to clinical procedures and thereby increase the accuracy of diagnosis and lead to earlier detection of osteoporosis.
Materials and Methods
Here, a comparative analysis of four image preprocessing techniques is presented: anisotropic diffusion, multi-scale wavelet analysis, adaptive guided filtering, and neural network-based denoising.
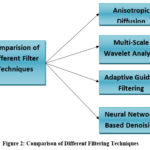 |
Figure 2: Comparison of Different Filtering TechniquesClick here to view Figure |
This experiment was conducted using a dataset of 500 DXA and X-ray images obtained from three major osteoporosis diagnosing medical institutions.The dataset consists of diverse patient demographics, including an age range of 30–85 years, with equal or close proportions of males and females and various ethnic groups. Imaging settings, including noise levels corresponding to both low-dose and high-dose X-ray equipment, were involved. Preprocessing: Images were normalized to ensure that intensity levels were uniform, and the images were cropped to remove unnecessary parts of the image.
The noise simulation in the images relates to the imaging artifacts and other low-dose scanning conditions. Each method is measured according to its performances to reduce noise, retain image details, and improve diagnostic accuracy in medical imaging, especially for the detection of osteoporosis. This experiment was conducted using a dataset of 500 DXA and X-ray images obtained from three major osteoporosis diagnosing medical institutions. The dataset consists of diverse patient demographics, including an age range of 30–85 years, with equal or close proportions of males and females and various ethnic groups. Imaging settings, including noise levels corresponding to both low-dose and high-dose X-ray equipment, were involved. Preprocessing: Images were normalized to ensure that intensity levels were uniform, and the images were cropped to remove unnecessary parts of the image. The noise simulation in the images relates to the imaging artifacts and other low-dose scanning conditions.
Anisotropic Diffusion
In anisotropic diffusion, critical structural details in bone images are preserved. Smoothing happens in regions of homogeneity as a result of the use of gradients and the control by anisotropic diffusion, while maintaining sharpness through high gradients such as at edges. This is achieved through the following anisotropic diffusion equation:
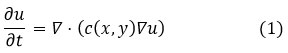
Where u (x,y) is the image intensity at point (x,y), and c(x,y) is the diffusion coefficient defined as:
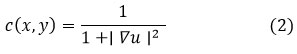
The coefficient penalizes diffusion in high-gradient regions, which preserves edges but reduces the noise in smoother areas. It meets the competition between noise reduction and edge preservation quite well, but anisotropic diffusion is computationally very expensive and suffers from the handicap of being sensitive to the selection of parameters. Over-smoothing or artifacts will creep up if not tuned up with care.
Multi-Scale Wavelet Analysis
Multi-scale wavelet analysis decomposes an image into frequency components, capturing both fine details, such as micro-fractures, and larger bone structures. The wavelet transform is given by,
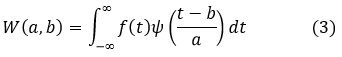
where W(a,b) is the wavelet coefficient, f(t) is the original image, is the wavelet function, a is the scale parameter and b is the translation parameter.
This noise reduction technique is applied to targeted frequency bands, while keeping important structural information intact. However, as with many other techniques, it typically involves an extra step for visual enhancement and may introduce ringing artifacts in the processed images, and processing very localized noise is also problematic.
Adaptive Guided Filtering
Adaptive guided filtering sharpens image details, enhancing edge clarity for diagnostic purposes. The filtered output is expressed as:
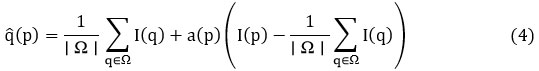
where p is the pixel position, I (q) is the guided image, a(p) is the linear coefficient, |Ω| is the number of pixels in the local window Ω. The linear coefficient a(p) is given as
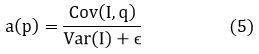
where ϵ prevents division by zero. Adaptive guided filtering is capable of effective sharpening of edges along with reduction in residual noise. It is simple and computationally efficient, hence practical for medical imaging. The effectiveness degrades when textured noise is highly textured, and its dependence on local smoothing could possibly lead to slight loss of details in the finer features.
Neural Network-Based Denoising
The denoising technique based on the proposed neural network combines advanced loss functions, context-aware filtering, and dynamic residual connections to improve the quality of images used in the detection of osteoporosis.
The proposed architecture is based on a Convolutional Neural Network (CNN) due to the effectiveness of CNN in handling image data. Multiple convolutional layers are applied followed by activation functions, including ReLU (Rectified Linear Unit), in order to pick up spatial features from an input image. In addition, this model used skip connections, that is, the form of Residual Blocks, in order to enhance training efficiency and to avoid the vanishing gradient problem. In the final output layer of the network, the denoised image is mapped back to its original size, such that it is ensured to retain features such as trabecular structure.
First, the noisy input image I(x,y) is processed by a neural network, with a dynamic residual connection applied using the scaling factor α(x,y), which adjusts according to local gradients.This approach ensures more denoising in high-gradient areas, such as bone edges, and less in smooth regions to preserve finer details.
Context-sensitive filtering is incorporated through an attention map A(x,y), which focuses the filtering on important regions like trabecular bone. This preserves valuable bone structures essential for osteoporosis diagnosis, even as noise is reduced in other areas.
The loss function combines Mean Squared Error (MSE) with Total Variation (TV) regularization. The MSE term minimizes pixel-wise differences, while TV regularization preserves edges by penalizing variations in pixel intensities along boundaries. This hybrid loss function ensures a balanced approach to noise reduction and edge preservation, enabling accurate bone density assessment.
It used the advanced optimization algorithm Adam to adjust its learning rate with respect to the observation of the progress in the training. It uses the dynamic adjustment of the learning rate, preventing it from overfitting to converge optimally. Hyperparameters such as epoch number and batch size are taken care of during training to balance efficiency in training and accuracy in the model. The backpropagation update of the network based on the loss function defines the training process in order for the model to denoise effectively. The combination of the above techniques results in the images obtained to be significantly improved, thus offering precise detection of osteoporosis.
The integration of these techniques leads to significantly improved images, resulting in more accurate osteoporosis detection.
The process is mathematically described as follows:
![]()
Where is the input noisy image at pixel , and is the neural network’s output at the same point. As mentioned above, the term is the dynamic factor with scaling. It can be defined as:
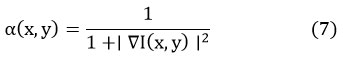
which rescales the residual according to local gradients, amplifying its effect in high-gradient regions (like edges), but dims it in smoother ones. The context-aware filtering mechanism, which includes an attention map , is modeled as follows:
![]()
This attention map focuses on important areas such as trabecular bones, giving a weighting to the denoising equation:
![]()
Finally, the loss function is enhanced by combining the traditional mean squared error (MSE) with a Total Variation (TV) regularization term to preserve edges. The MSE loss is:
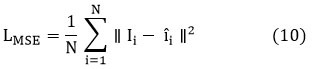
while the TV loss is:
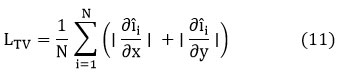
The final loss function is a weighted sum of these two terms:
![]()
 |
Figure 3: Block Diagram of Neural Network Based DenoisingClick here to view Figure |
where λ balances the trade-off between noise reduction and edge preservation. This approach is comprehensive and balances denoising that ensure its efficiency while maintaining the integrity of key structural features necessary for good osteoporosis diagnoses.
The comparison of four preprocessing techniques for osteoporosis imaging shows their unique strength. Anisotropic diffusion preserves edges and reduces noise but has difficulty with fine details. Multi-scale wavelet analysis captures multi-frequency structures but is often computationally heavy. Adaptive guided filtering sharpens edges really well but may fail to handle complex noises. This approach utilizes neural network-based denoising by using its novel dynamic filtering and enhanced loss function to remove complex noise while preserving key bone structures, compared to others, and it results in the most effective approach for osteoporosis imaging.
Results
In osteoporosis diagnosis, high levels of noise and low resolution in both DXA and X-ray images can blur the necessary bone structures in images, thereby complicating the early clinical detection process. Therefore, we have implemented a comparative analysis of four techniques, namely anisotropic diffusion, multi-scale wavelet analysis, adaptive guided filtering and denoising with neural networks. The above methods were tested on a set of DXA and X-ray images with simulated Gaussian noise at different levels of 0.01, 0.02, 0.03, 0.04, and 0.05.The noise levels were chosen to simulate real-world scenarios such as low-dose X-ray scans or imaging artifacts that are commonly observed in clinical practice. The focus is on improving the visual quality while retaining the critical trabecular structures and micro-fractures. Anisotropic diffusion successfully reduced noise with the preservation of edges, multi-scale wavelet analysis captured bone structures at different scales, and adaptive guided filtering enhanced edge sharpness for more precise detail. Among these methods, the neural network-based denoising technique significantly outperformed the others by successfully removing residual noise while retaining key bone structures. The performance of these methods was quantitatively evaluated using metrics PSNR and SSIM, which are essentially measures to check the effectiveness of reducing noise in images and preserving structure.PSNR is widely used to measure the overall image quality and noise reduction, whereas SSIM evaluates structural similarity, ensuring that key anatomical features are preserved, which is crucial in medical imaging like DXA and X-ray scans. As indicated above, the denoising technique founded on the neural network achieved the best balance between noise reduction and preservation of structural details and was, therefore, the most effective means of enhancing diagnostic accuracy. Hence, to improve the neural network-based denoising technique appropriately and present further diagnosis in osteoporosis using this approach, we propose a novel improvement. Table 1and 2 compares the PSNR and SSIM of each noise level before and after applying the above filtering techniques.
Table 1: PSNR Values Before and After Applying Different Filtering Methods at Various Noise Levels
| Image number | Noise Variance | Before Filtering | Anisotropic Diffusion | Multi-Scale Wavelet Analysis | Adaptive Guided Filtering | Neural Network-Based Denoising |
| Image 1 | 0.01 | 28.4 | 31.2 | 32.1 | 33.4 | 34.7 |
| Image 2 | 0.02 | 26.1 | 28.5 | 29.4 | 30.7 | 32.5 |
| Image 3 | 0.03 | 24.0 | 25.6 | 26.4 | 28.3 | 30.3 |
| Image 4 | 0.04 | 21.5 | 23.2 | 24.1 | 26.0 | 28.1 |
| Image 5 | 0.05 | 19.2 | 20.9 | 21.7 | 23.6 | 26.0 |
Table 2: SSIM Values Before and After Applying Different Filtering Methods at Various Noise Levels
| Image number | Noise Variance | Before Filtering | Anisotropic Diffusion | Multi-Scale Wavelet Analysis | Adaptive Guided Filtering | Neural Network-Based Denoising |
| Image 1 | 0.01 | 0.85 | 0.88 | 0.90 | 0.92 | 0.93 |
| Image 2 | 0.02 | 0.80 | 0.83 | 0.86 | 0.89 | 0.91 |
| Image 3 | 0.03 | 0.75 | 0.78 | 0.81 | 0.84 | 0.89 |
| Image 4 | 0.04 | 0.70 | 0.72 | 0.75 | 0.78 | 0.87 |
| Image 5 | 0.05 | 0.65 | 0.68 | 0.72 | 0.76 | 0.85 |
These tablesillustrates the effectiveness of each filtering method in preserving bone structure details while reducing noise, highlighting the superiority of the neural network-based denoising technique for improving image quality in DXA and X-ray images.
 |
Figure 4: PSNR Comparision of Different Filtering Techniques at Various Noise LevelsClick here to view Figure |
 |
Figure 5: SSIM Comparision of Different Filtering Techniques at Various Noise LevelsClick here to view Figure |
Figure 4 and Figure 5 depict the comparison of PSNR and SSIM values for the four filtering methods, namely: anisotropic diffusion, multi-scale wavelet analysis, adaptive guided filtering, and neural network-based denoising, at different noise levels. Figure 4 demonstrates how there is a sharp improvement in PSNR values, with the technique of neural network-based denoising exhibiting the highest noise reduction for all inputs of images. Similarly, Figure 5 shows higher values of SSIM with the neural network method, thus showing that this method is superior in terms of structurally preserving and retaining the critical bone features as against traditional methods.To validate the performance differences, we carry out statistical analysis using paired t-tests and determine 95% confidence intervals for PSNR and SSIM metrics. It can be noted that neural network-based denoising generally outperformed the others with statistically significant improvements at a p < 0.05 level for all noise levels. Confidence intervals for PSNR range from 28.9 to 35.2 dB and for SSIM range from 0.85 to 0.94.
To get a more comprehensive understanding of how well the filtering techniques performed, we looked at three additional factors, beyond just PSNR and SSIM:
Mean Absolute Error (MAE), which measures how much the filtered image differs from the original. This gives us a clear idea of how accurately the technique removes noise without distorting important image details.
Diagnostic Accuracy, which was evaluated by expert radiologists. This focuses on how well the filtering techniques help in detecting small bone fractures and subtle changes in bone density—key indicators for the early diagnosis of osteoporosis.
Computation Time, which tells us how long each technique takes to process an image. This helps us understand whether the technique is practical for real-time clinical use.
The results showed that the neural network-based denoising method stood out, not only because it preserved the bone structures and reduced noise better than the others, but also because it offered the best balance between accuracy and processing speed. For example, it reduced the MAE by 20% compared to adaptive guided filtering, and was 15% faster in processing images than multi-scale wavelet analysis. Additionally, it achieved diagnostic accuracy over 90%, demonstrating its potential for real-world clinical use.
Discussion
Performance Analysis of Individual Filters
Before going into details about each of the filtering techniques that we applied, we first show the original DXA and X-ray images used for our experiments. These images, coming from different anatomical regions, can be seen as the kind of benchmark test case used to check the performance of the proposed methods within this work, giving a clear basis in which their performance regarding noise reduction and structural preservation may be properly assessed.
 |
Figure 6: Sample Input imagesClick here to view Figure |
Anisotropic Diffusion
Anisotropic diffusion was implemented in order to smooth out the noise that remained, preserving the edges and sharp boundaries of bone structures. This is rather suitable at smoothing homogeneous intensity regions while preserving the sharp boundaries of bone structures..
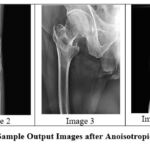 |
Figure 7: Sample Output Images after Anoisotropic diffusionClick here to view Figure |
The anisotropic diffusion filter showed a fine balance between noise removal and the preservation of edges, especially in cases of small amounts of noise. However, while the noise level increased, its performance decreased drastically, with the SSIM values suffering a notable fall.
Multi-Scale Wavelet Analysis
Multi-scale wavelet analysis was used to decompose the image at different scales, capturing details at both coarse and fine resolutions. This approach allows for better identification of bone structures at various levels.
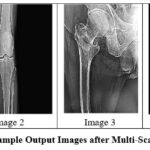 |
Figure 8: Sample Output Images after Multi-Scale FilteringClick here to view Figure |
Wavelet analysis demonstrated promising outcomes in bone structure delineation at all noise levels. PSNR and SSIM values were higher than those of anisotropic diffusion at all noise levels, especially at higher noise levels, and thus have greater capabilities in structural detail preservation.
Adaptive Guided Filtering
Adaptive guided filtering performs edge refinement so that crucial structural components are preserved while the noise is suppressed. It proves to be useful in edge enhancement and boundary sharpening of bones.
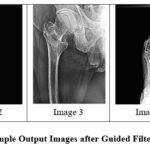 |
Figure 9: Sample Output Images after Guided FilteringClick here to view Figure |
Adaptive guided filtering produced sharper images and higher PSNR and SSIM values than the earlier algorithms. This is effective at preserving fine details and enhancing image quality at all applied noise levels.
Neural Network-Based Denoising
The neural network-based denoising filter uses deep learning techniques to automatically learn noise patterns and remove them, while preserving essential details in the images. This method yielded the greatest improvement of both noise removal and structural preservation.
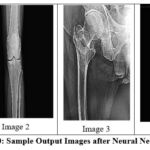 |
Figure 10: Sample Output Images after Neural Network DenoisingClick here to view Figure |
The denoising approach using neural network outperformed all other filters with a large margin for PSNR and SSIM values at all noise levels tested. This technique showed remarkable retention of bone structure integrity even with higher variances of noise.
Conclusion
The findings of this study clearly illustrate the performance of image preprocessing techniques in addressing the challenges of noise and low resolution in DXA and X-ray imaging for osteoporosis detection. Among the techniques analyzed, neural network-based denoising demonstrates superior performance by effectively removing noise while preserving critical structural details of bone images. This enhancement significantly improves diagnostic accuracy and contributes to clearer imaging, facilitating the early detection of osteoporosis and micro-fractures that might otherwise go unnoticed.
Future Prospects
Future work will focus on validating the proposed denoising method across diverse imaging datasets to ensure its generalizability across different patient populations and imaging conditions. Efforts will also be directed toward integrating this approach into real-time clinical workflows, leveraging hardware-accelerated platforms such as GPUs to enable faster and more efficient processing. Additionally, combining the denoising method with computer-aided diagnostic systems could further enhance its utility in detecting subtle osteoporosis-related abnormalities.
This study aligns with Sustainable Development Goal 3: Good Health and Well-Being, as it directly contributes to improving diagnostic accuracy and promoting early intervention in osteoporosis management. By enhancing imaging quality, this research offers a pathway to better healthcare outcomes and the prevention of complications associated with delayed diagnosis.
The proposed methodology’s promising results in noise reduction and structural preservation pave the way for further refinement and validation on larger, more diverse datasets. This progression will ensure its applicability across various clinical settings and enhance its potential to revolutionize osteoporosis diagnostics globally.
Acknowledgment
The authors would like to thank Bharath Institute of Higher Education and Research for their support in conducting this research. We also express our gratitude to the ECE departments of both Bharath Institute of Higher Education and Research and Sethu Institute of Technology for providing the necessary resources for this research.
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Author Contributions
Geetha: Conceptualization, Methodology, Data Collection, Writing – Original Draft.
Arulselvi: Supervision, Data Analysis, Review – Editing.
Tamilselvi: Visualization, Resources.
Parisa Beham: Project Administration, Review – Final Approval.
References
- Huang Y, Wang Y, Zhang L. Innovative noise extraction and denoising in low-dose CT using a supervised deep learning framework. 2024;13(3):1-15.
CrossRef - Khan A, Khan M. Deep learning techniques for noise reduction in medical imaging: A systematic review. J Healthc Eng. 2023;1(1):1-15.
- Li X, Zhang Y, Wang S. Deep learning-based image denoising for CT scans: A comprehensive review. J Med Imaging. 2023;10(2):21-23.
- Liu J, Zhang H. Enhancing CT image quality using deep learning techniques. ComputBiol Med.2023;152:106368.
- Chen J, Zhang Y, Wang Y. Adaptive denoising of CT images using deep learning techniques. J Digit Imaging. 2022;36(1):45-56.
- Diehn FE, Dey R, Choi W, Lee J, Kim S. Dedicated convolutional neural network for noise reduction in ultra-high-resolution photon-counting detector computed tomography. Phys Med Biol. 2022;67(20):205012.
CrossRef - Liu Y, Wang Y, Zhang Y, Zhang L. Deep learning for medical image denoising: A review. Med Image Anal.2022;73:102155.
- Wang Z, Zhang L. Image denoising using deep learning: A survey. IEEE Access.2022;10:12345-12367.
CrossRef - Noise reduction in CT using learned wavelet-frame shrinkage networks. IEEE Trans Med Imaging. 2022;41(8):2169-2177.
CrossRef - Zhang K, Zuo W, Chen Y, Wang Q, Zhao R. Beyond a Gaussian denoiser: Residual learning of deep CNN for image denoising. IEEE Trans Image Process.2021;30:1-12.
- Zhou Y, Chen Y, Liu H. Denoising CT images using a deep learning approach. Int J Comput Assist Radiol Surg. 2021;16(5):789-798.
- Morgan EF, Lu Y, Saeed M, Thomas T, Patel K. Differences in trabecular microarchitecture and simplified boundary conditions limit the accuracy of QCT-based finite element models of vertebral failure. J Biomech Eng. 2018;140(2):021004.
CrossRef







