Manuscript accepted on :27-01-2025
Published online on: 10-02-2025
Plagiarism Check: Yes
Reviewed by: Dr. Patel Maulik
Second Review by: Dr. Pravinkumar Darji
Final Approval by: Dr. Prabhishek Singh
Vanshika Sautha1 , Mansi Butola1*
, Mansi Butola1* , Meenu Chaudhary3
, Meenu Chaudhary3 , Praveen Kumar4
, Praveen Kumar4 , Vikash Jakhmola2
, Vikash Jakhmola2 , Siddhant Dhyani1
, Siddhant Dhyani1 and Arif Nur Muhammad Ansori5
and Arif Nur Muhammad Ansori5
1Department of Pharmaceutics, Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun, Uttarakhand, India
2Department of Pharmaceutical Chemistry, Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun, Uttarakhand, India
3School of Pharmaceutical Sciences, Shri Guru Ram Rai University, Dehradun, Uttarakhand, India
4Himalayan Institute of Pharmacy and Research, Dehradun, Uttarakhand, India
5Universitas Airlangga, Surabaya, Indonesia.
Corresponding Author E-mail: mansibutola1995@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3070
Abstract
Since its beginnings in the 1980s, 3D printing has transformed several research areas, including the pharmaceutical sector. The primary objective is to manufacture complex, customized products using a cost-effective, on-demand manufacturing process. In the past decade, 3D printing has gained the interest of several research groups for the development of various drug delivery systems. Advantages of 3D printing technologies over traditional manufacturing procedures include the modification of pharmaceuticals with customized dosages, the capability to produce complex solid dosage forms, on-demand manufacturing, and cost efficiency. Nonetheless, although 3D printing technology has several potential medical and economic advantages, some technological and regulatory obstacles limit its wide application in pharmaceutical products. Thus, further innovation and refinement in 3D printing processes must address existing limitations and provide patient-specific healthcare with customized drugs on demand. This review presents several 3D printing processes useful for pharmaceutical manufacturing, their application in the development of various dosage forms, and the treatment of various disorders, demonstrating the potential of this technology for regular commercial production.
Keywords
Customized dosage; Commercial production; Delivery systems; Economic advantages; Potential applications
Download this article as:| Copy the following to cite this article: Sautha V, Butola M, Chaudhary M, Kumar P, Jakhmola V, Dhyani S, Ansori A. N. M. Overview of 3d Printing Technology with Pharmaceutical Applications, Challenges and Future Aspects. Biomed Pharmacol J 2025;18(March Spl Edition). |
| Copy the following to cite this URL: Sautha V, Butola M, Chaudhary M, Kumar P, Jakhmola V, Dhyani S, Ansori A. N. M. Overview of 3d Printing Technology with Pharmaceutical Applications, Challenges and Future Aspects. Biomed Pharmacol J 2025;18(March Spl Edition). Available from: https://bit.ly/4hoYBP0 |
Introduction
Using a digital design platform, three-dimensional printing (3DP), commonly referred to as additive manufacturing (AM), creates 3D items quickly, effectively, and layer by layer 1. The advent of 3D printing in the pharmaceutical sector has revolutionized drug manufacturing, enabling the conversion of non-digitalized medicinal products into digital 3D content. Over the past 20 years, there has been a significant advancement in the field of three-dimensional (3D) printing. It has aided in supplying appropriate information for the manufacturing processes of different industries, including pharmaceuticals, medicine, environmental monitoring, aviation, and autos, as well as in research. Emanuel Sachs and associate researchers at the Massachusetts Institute of Technology in Boston, USA, invented 3D printing technology in the late 1980s (US patent number 5204055) 2. In, 2015, the US Food and Drug Administration (FDA) authorized the first 3D-printed drug product, Spritam (levetiracetam), for treating partial-onset seizures, myoclonic seizures, and generalized tonic-colonic seizures, marking a watershed moment in additive manufacturing 3. When compared to traditional manufacturing methods, the use of 3D printing is very cost-effective, with a drug-loading efficiency of up to 1000 mg in a single dose and the opportunity for personalized distribution based on the patient’s needs. With the benefits of boosting production efficiency and lowering costs and defect counts by preventing human error, 3D printing technology is expanding quickly across industrial manufacturing sectors. Converting the introductory factors (medicine and excipients) into a “curable essay” or printable material is the primary problem of 3D printing technology. Even the fabrication of things consisting of a single material or a combination of materials is possible with this approach; each substance may be deposited using a different print head or by using additional deposition stages. Customized treatment solutions are provided for individual patients or groups by 3D printing, since it may generate individualized medications to treat specific patient groups. 3D printing can be a successful therapeutic option for the treatment of complicated disorders such as cancer, dentistry, cardiovascular diseases, orthopaedics, diabetes, etc 4. Other advantages and some disadvantages are shown in Fig 1. Creating intricate dosage forms with several doses of medications with a narrow therapeutic index to assist long-acting medication therapy may be facilitated by 3D printing. Customized dosage forms in the form of polypills loaded with several doses of one drug or several doses of several drugs are made possible by 3D printing. Additionally, 3D printing enhances the PK performance and solubility of poorly water-soluble medications. The creation of solid pharmaceutical dosage forms has been greatly impacted by 3D printing. These days, 3D printing can be used at every stage of the drug development process, from preclinical research and clinical trials to providing direct patient care.
 |
Figure 1: Advantages and disadvantages of 3D printing technology |
3D printing in medicine: technology overview
3D printing techniques are additive manufacturing methods that include the layer-by-layer material deposition onto a substrate to create the final 3D product, which is created with the use of computer software. All 3D printing methods include two fundamental stages. One relates to product design using computer software, while the other involves deposition of objects using a 3D printer. The design of a 3D object may be performed using numerous software applications like AutoCAD, Fusion 360, 3D Slash, SketchUp, and Solid Works. The following steps involve slicing the developed design using slicer software such as KISSlicer, Cure, OctoPrint, Slic3r, and Simplify3D. This 3D software will transform a 3D design file into 3D printer readable G code. The slicer software established the printing settings for the sliced item, including the number of layers, initial offset height between the print head and platform, infill percentage, printing speed, total printing duration, and layer spacing. The generated G code is then uploaded to the 3D printer, which after receiving a print command, will execute the process to create an object of the specified dimensions and configurations. The product deposition process via a 3D printer differs according to the specific kind of 3D printer used. Different 3D printing technologies are shown in Figure 2 and their diagrammatic representation is shown in Figure 3.
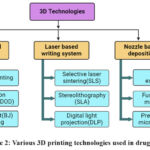 |
Figure 2: Various 3D printing technologies used in drug designing |
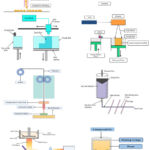 |
Figure 3: (a) Inkjet printing; (b) Drop on-demand printing; (c) Binder jet printing (d) Stereolithography; (e) Digital light projection; (f) Semi-solid extrusion; (g) Fused deposition modeling; (h) Pressure assisted micro syringe |
Inkjet printing: For precise and repeatable structure development, inkjet printing also referred to as mask-less or tool-less uses the movement of the inkjet nozzle or substrate. With the use of a powder base, inkjet printing produces exact dosages of active medicinal compounds by spraying ink droplets onto a non-powder substrate, which then solidifies. Many materials can be used, including resins, ceramics, and even edible ink, using Inkjet 3DP. Until the finished product is produced, the technology precisely deposits material droplets in a predetermined pattern using an inkjet print head, LbL 5.
Drop-on-demand printing: Ink droplets with a diameter of 10–50 μm and a volume of 1–70 pL are generated during DOD printing 6. In DOD printing, thermal heads and piezoelectric crystals are employed as printer heads. The liquid ink in the DOD printing thermal head is locally heated to as high as 300 °C. It creates bubbles that force the ink out so that printing can occur. However, only volatile liquids can be used with this thermal head. Additionally, employing such a high temperature and high vapor pressure might damage medications and pharmaceutical substances. They can be classified into drop-on-deposition and drop-on-solid types.
Binder jet printing: Using an industrial printer to carefully apply a small coating of powdered materials like ceramics, foundry sand, metal, or composite along with a liquid bonding agent, binder jetting is used to create expensive and distinctive parts and machinery 7. For example, Spritam is produced by Binder jetting.
Selective laser sintering: By heating and fusing powder particles at their surfaces, it uses a laser beam to produce solid things 8. Carl Deckard created the SLS technology in 1984. It was based on a 100 W neodymium-doped yttrium aluminium garnet (Nd: YAG) laser. Most commercial SLS printers today use carbon dioxide (CO2) lasers, which offer more power at lower cost and allow a wide range of powdered thermoplastic materials to be used. Compared to other 3DP formats, SLS offers several benefits, including excellent resolution and the ability to dispense and consume pointlets right away without the need for post-processing steps like drying or UV curing.
Stereolithography (SLA): Stereo (solid) and lithography (photo) are the two words from which the term SLA is derived which suggests the form of writing with light. Despite being the first and oldest, SLA can produce items with smooth surfaces and intricate geometries because of its high printing resolution. SLA is a laser-based 3D printing technique that uses UV-sensitive liquid resins to create models, patterns, and prototypes through photochemical reactions in a layered format. High resolution and speed of printing, along with less localized heating, are benefits of this printing technique that make it appropriate for printing thermolabile medications. Initially employed for Nano and Micro patterns for micro-electro-mechanical systems, stereolithography’s high-resolution capabilities allow for high-quality microneedle patch production. Using this printing method in pharmaceutics, miscible components like excipients and APIs can be incorporated into the polymeric matrix when crosslinking takes place 9.
Digital light projection: The DLP technology projects light onto the photopolymerizable material using tiny pixels (rectangular cubes) from an entire cross-sectional layer of objects using a digital projector screen. Upon light projection, the entire cross-sectional region solidifies. The final 3D object has a rough surface finish because it is made up of tiny rectangular cubes known as voxels. When it comes to building 3D objects, the DLP approach is faster than SLA and allows easy modification in the thickness of the polymerized layer. Example- Using a top-down DLP printer (Gizmo® 3D printer), fluticasone-eluting oesophageal-targeted 3D rings (pre-loaded and post-loaded) dosage forms and drug-eluting devices are made 10.
Semi-solid extrusion: A material extrusion technique called semi-solid extrusion (SSE) uses successive layers of paste or gel to form three-dimensional objects. The goal of the first clinical trial utilizing an SSE 3D printer in a hospital was to produce chewable Printlets™ a customized medication for kids suffering from Maple Syrup Urine Disease11. SSE 3D printing operates at room temperature throughout the entire process, highlighting its benefits that can be extended to a wider range of active medications and excipients, including hydrogels and epoxy resins. This technique is commonly utilized in tissue engineering and regenerative medicine to fabricate scaffolds.
Fused deposition modeling: Another term for it is fused filament fabrication. A solid-based rapid prototyping method called fused deposition modeling creates a model by layer-by-layer extrusion of material. It works by heating and extruding a semi-liquid thermoplastic ink, which is then gradually settled with the use of a detachable scaffold to create a three-dimensional object. Several materials, including pastes, polymers, silicones, suspensions, and other semisolids, are used in extrusion printing. This approach has several benefits, including low cost, easy accessibility, and simplicity, but it also has drawbacks, including inadequate drug loading, poor drug dissolution speed, and heat degradation of components 12.
Pressure-assisted micro syringe (PAM): PAM is a nozzle-based deposition technique that releases semi-liquid and viscous materials by extrusion using a micro syringe and pressurized air. Using PAM technology, a microstructure as small as 5–10 μm can be produced 13. Room temperature is required for this 3DP technology to function in a continuous flow.
Types of materials used in 3d printing
Biodegradable polymers: Biodegradable polymers are ecologically conscious or bio-based polymers that show great promise in addressing the problem of plastic waste. In the environment, these polymers spontaneously break down. Good mechanical strength, thermal behaviour, and barrier qualities are just a few of the various characteristics they exhibit. When it comes to technology and the environment, 3D printing with biodegradable polymers has many advantages. Among the many advantages of 3D printing are its adaptability and simplicity, which allow for complex designs and accurate prints 14. Examples include polypropylene, polyhydroxyalkanoates, acrylonitrile butadiene styrene (ABS), polyamide, polycarbonate, polycaprolactone (PCL), polylactic acid (PLA), polyvinyl alcohol (PVA), nylon.
Natural fillers as reinforcing material: It is highly advised to use polymers as 3D printing matrices since they provide high resistance to chemicals, low cost, and simple processability. However, one of their primary production process constraints is their low functionality and low mechanical and tensile qualities, which refer to the end product’s modulus, strength, and impact resistance. Using reinforcing materials is one of the most promising approaches among the various solutions proposed to address these problems. These substances can absorb moisture, have poorer thermal stability, degrade more quickly, be less compatible with polymeric matrices, and have batch-to-batch fluctuations in chemical composition. All these limitations have an impact on filler quality 15. Examples include chitosan, cellulose, and keratin.
Metals: The novel process of “metal 3D printing” uses wires or molten metal powders to build objects in layers, allowing for localized geometry modification according to internal tensions. Numerous studies have examined the use of 3D printing for metal materials, each with a distinct purpose and set of restrictions. Malekjafarian 16 attempted an experimental investigation into the buckling capacity of metal materials 3D printed by laser, but encountered difficulties since there was no set buckling criterion. A metal-plated 3D-printed electrode was created for the electrochemical detection of carbohydrates 17. Titanium, zinc, stainless steel, and chromium-cobalt alloys are a few of the metals used in 3D printing 18.
Applications of 3D printing in cardiovascular diseases
Cardiovascular diseases (CVD) are the foremost cause of death and a significant contributor to global disability, affecting 523 million individuals and resulting in about 18.6 million deaths per year19. The clinical diagnosis and therapy of CVD mostly depend on less invasive imaging techniques, including computed tomography (CT), magnetic resonance imaging (MRI), and echocardiography. Although commonly used in routine practice, translating 2D images of cardiovascular anatomy and pathology into 3D representations poses significant challenges, particularly when visualizing complex cardiovascular structures and conditions, such as congenital heart disease, which typically includes multiple cardiac components exhibiting a range of abnormalities. 3D printing has overcome these limitations by delivering realistic models, hence presenting significant benefits over existing image visualization. Various 3D printing technologies used to treat cardiovascular diseases are Antiplatelet tubular grafts 20, Artificial blood vessels 21, Cardiovascular stents 22, and Nanocomposites 23.
Cancer
Cancer is said to be the cause of many reported fatalities worldwide as well as treatment-related suffering. The remarkable capabilities of Additive Manufacturing technology provide better options for treating malignant tumors. This technology’s models aid in understanding a patient’s behaviour related to their illness. Three-dimensional printing has a significant impact on research that uses cancer models for diagnosis and treatment. In research on drug screening, cancer metastasis, and prognosis, 3D-printed tailored tumor models including physical, bio-printed, and tumor-on-chip models display better correlation. Various 3D printing technologies used in cancer are cardiovascular implants 24, Scaffolds 25, Fe-CaSiO3 Bio-scaffolds26, nanoparticle-loaded hydrogel-based scaffold 27.
Dentistry
Due to its rapid fabrication, excellent accuracy, and personalized customization, whole denatures and implant teeth are more readily accessible. Furthermore, the use of 3D printing in dentistry might facilitate the delivery of cost-effective and customized treatments to patients, while simplifying the complex procedures associated with the fabrication of dental fabrication of dental equipment. Before the widespread use of 3D printing technology, restoration was often manufactured via milling. Currently, 3D-printed restorations have several benefits. Certain investigations indicate that the edge and internal gap measurements of 3D printed restorations are significantly lower than those of milled restorations. Dental crowns are often produced using conventional plaster models, however, crowns manufactured using 3D printing technology have gained popularity as well. Recent research indicates that the fit of crowns produced by 3D printing is lower than that created from plaster models, suggesting that 3D printing technologies are innovative but under-researched. Various 3D printing technologies in dentistry are chlorhexidine-coated mouthguard28, monolithic zirconia crown29, occlusal device30, dental implants31, dental crown32.
Diabetes
Diabetes is a metabolic condition defined by increased blood sugar/glucose levels. Prediabetes is a metabolic disease characterized by increased blood sugar levels above the usual range. High blood glucose primarily impacts big blood vessels, resulting in macrovascular difficulties, if untreated, it subsequently affects small blood vessels, leading to microvascular challenges33. Increased blood sugar levels can reduce an individual’s immune response, making diabetes patients more prone to infections compared to healthy individuals. In recent years, 3D printing and 3D bioprinting have gained attention as leading technologies in the diagnosis and treatment of diabetes by creating analytical devices for sensing and detecting high blood glucose levels and other oral medications34. Various 3D printing technologies in diabetes treatment are microneedle biosensing device, electrochemiluminescence biosensor35, self-nano emulsified based 3 D printed tablets36, printed glipizide tablets37, microneedle patches38, orthopaedic insoles39, bio printed scaffolds40.
Orthopaedics
The aging of the world’s population raises the danger of orthopaedic conditions such as fractures and metabolic disorders, which impair function and cause discomfort in many older people. As an advanced digital manufacturing technique, 3D printing has completely changed the biomedical area. Orthopaedic implants customized to the patient’s surgical needs are designed and manufactured by clinic physicians using CT/MRI patient imaging data. It can be applied to orthopedic surgery, mainly for prosthetics, tissue engineering scaffolds, tailored equipment, and surgical planning. Using 3D printing for surgical preparation shortens operating times by helping a surgeon diagnose more accurately, mimic surgery, and comprehend the anatomy of a surgical site. The high cost of production, the length of time required for data processing and printing, and the lack of strength resulting from layer-by-layer manufacturing are the drawbacks of 3D printing in orthopedics 41. Various 3D printing technologies in orthopedics are titanium implants42, spinal implant43, pelvic implant44, antimicrobial hydrogel45, finger splint46.
3D printing of drug delivery systems
The most common method of drug administration is oral, using solid dose forms. Oral tablets provide dosage accuracy, chemical and microbiological stability, controlled release characteristics, and convenience of administration. Oral tablets are conveniently portable for the patient, facilitating on-demand administration. despite these benefits, traditional tablet manufacturing techniques involve a time-consuming and laborious process. 3D printing technology facilitates the production of custom-designed oral tablets characterized by significant architectural, structural, and compositional complexity, potentially leading to an important change in tablet manufacturing from a uniform approach to personalized medicine. Various drug-loaded oral tablets prepared by 3D printing technology are Amitriptyline Hydrochloride 47, Indomethacin 48, Ondansetron 49, Clindamycin palmitate hydrochloride 50, Carvedilol and haloperidol51.
The first 3D-printed capsular devices were produced in 2015 by Melocchi. Hydroxypropyl cellulose-containing filaments were produced using hot-melt extrusion and then 3D printed. The produced samples were swellable, erodible capsules designed for oral pulsatile drug release52. Fused deposition modelling and inkjet printing were used to manufacture capsules from various polymer compositions. The capsules included three components: two hollow sections with a closed cylindrical end and a rounded open end; the central section functioned as a junction and a partition. The hollow sections differed in geometry and wall thickness. The samples included model APIs, and the findings indicated that the device successfully released the model APIs in pulses within 2 hours. Acetaminophen and furosemide53, Metoprolol and nadalolol 54, Isoniazid and acetaminophen55, Norfloxacin56 are a few examples of drug-loaded capsules prepared by using 3D printing technology.
3D printing technologies have significant potential to produce advanced drug implants and customized macro-porosity implants with personalized drug release characteristics. Presently, commercially marketed implants often lack the personalization of treatment and consideration of several factors, including anatomical variances, age, gender, and disease conditions. A study group found that 3D-printed implants may exhibit complex drug release patterns compared to traditionally manufactured drug implants. The manufacturing of 3D printing technology has enabled the production of smaller, less invasive, and more site-specific implants in contrast to traditional methods 57. Various 3D printed implants are Doxycycline58, Ciprofloxacin 59, Diclofenac sodium 60, Lidocaine 61.
Microneedle technology is a method of active transdermal administration of drugs designed to replace conventional syringe injections. The microneedle array is used to transverse the stratum corneum and administer the drug with minimum invasive action. These arrays consist of micro-sized needles with heights varying from 25 to 2000 μm. A significant benefit of 3D printing is its ability to incorporate drug substances with complex solubility profiles into microneedles optimized for patient requirements. The manufactured microneedles provide precise dosing while maintaining mechanical strength compared to conventional microneedles. Additionally, microneedles with small tips at the nanoscale allow effective skin penetration and can facilitate targeted cell delivery. Many researchers carried out studies on 3D printed microneedles, Insulin62, Diclofenac63, 5-fluororacil, curcumin and cisplatin 64 , Fluorescein 65.
Present methodologies did not consider the specific anatomy of each patient, along with their medical problems, age, and gender specificity, which limits the effective therapy, since each patient is unique and requires different dosages of drugs and hormones. Moreover, factors such as variability in drug absorption associated with the menstrual cycle, menopause, and pregnancy varied across individuals. Therefore, patient-specific methods are essential for delivering customized shapes, sizes, and drug releases to enhance effectiveness and increase compliance. The 3D-printed delivery system may provide customized geometry, ensure a secure fit, enhance effectiveness, improve patient adherence, and prevent post-surgical complications66. Estrogen/progesterone67, Paclitaxel and cidofovir68, Chloramphenicol and metronidazole69 are studies performed for the preparation of 3D-printed vaginal drug delivery systems.
Challenges
Void formation: One of the main drawbacks of 3D printing is the formation of voids between layers of material. Because additive manufacturing can produce extraordinarily high levels of additional porosity, the interfacial adhesion between printed layers can be reduced, lowering mechanical performance. One of the key faults that lead to poorer and anisotropic mechanical properties in methods that use filaments of materials like FDM is the production of voids, which is more common in these processes. By reducing the height of each layer in the powder-bed printing of alumina/glass composite, the high porosity of additive manufacturing can be significantly decreased 70. The decreased height can lessen the creation of interlayer voids by increasing laser penetration through the top layer and facilitating the diffusion of ceramic powders between layers.
3D printer-related challenges
Geometric restrictions: Geometric flexibility can be achieved with 3D printing, but there are drawbacks as well. Certain geometrical possibilities restrict the printing method 71.
Dependency on direction: One characteristic of layered manufacturing processes is thought to be directional dependency. The strength and load-bearing capacity of the material are significantly impacted by the printing direction 72. For most 3D-printed shapes, a linear filament deposit is necessary. Since filament has a direction, directional dependency is a problem.
Quality of 3D printer: The size and drug concentration of the intended printed drug items must be considered when selecting a 3D printer for printing pharmaceuticals. The tolerance ranges of various printer machines can affect how accurately a pharmacological product is printed.
Contamination: Contamination concerns arise when drug formulation components are reused in 3D printing operations because of the possibility of meeting ink that has been used previously. Printer components can also be a source of contamination. For instance, FDM printers come equipped with brass nozzles that contain lead; these nozzles need to be updated to stainless steel nozzles for use in medical applications 73.
Raw materials: Physicochemical stability and printability of APIs and excipients, along with the kind of 3D printer to be utilized, are the determining factors in their selection. Rheological characteristics and mechanical resilience are the primary variables that can affect how printable the raw materials are. One important component of print viability has been identified as mechanical resilience. The type of material will determine the standards for that material. Common requirements for solid materials are filament diameter and diametric tolerances for filaments, particle size, distribution, and pertinent rheological performance for powders. Viscosity and viscoelasticity are significant properties of fluid materials; similarly, composition, glass transition temperature, chemical structure, purity, water content, and molecular weight, are significant properties of polymers or monomer mixtures 74.
Mechanical properties: The fundamental principle of 3D printing is the stacking of various polymers or powders, which leads to a rough surface and inadequate mechanical strength of the objects75. The mechanical characteristics of dosage forms, which are impacted by variables including adhesive viscosity, surface tension, and nozzle fineness, are critical for quality control because they impact tablet performance, repeatability, and postprocessing appropriateness. It is possible to enhance the mechanical behaviour of products by the optimization of the printing apparatus, including computer control programs, adhesive nozzle refinement, and printing process parameter adjustments.
Cost: The high expense of creating computer models and printing them, together with the need for materials, 3D printing knowledge, segmentation software, and medical-grade printers, prevents 3D printing from being widely used.
Anisotropic microstructure: Anisotropic behaviour is a problem for additive manufacturing because layer-by-layer printing produces different microstructures within each layer than the borders between layers. When new layers are added to metals and alloys that are 3D printed using heat fusion (SLS or SLM), the borders of the earlier layers are reheated. This causes the microstructure to change and causes anisotropic behaviour because of thermal gradients 76.
Challenges relating to liability and regulations
Liability issues: Different companies are involved in 3D-printed object development, but accountability and liability issues arise regarding responsibility in case of failure. To determine who is accountable for an accident, a precise legal framework needs to be established.
Absence of norms and guidelines: Because there are no set rules governing the use of 3D printing in construction, it is difficult to follow all construction norms and guidelines. There are attempts to amend these rules to incorporate 3D printing. To incorporate 3D printing into the construction guidelines, a few Chinese enterprises are collaborating with the National Construction Standards agency 77.
Differential in both conception and implementation / Divergent from design to execution: The primary tool for designing an item that can be 3D printed is computer-aided design (CAD) software. Boundaries and solid geometry combine to form the CAD system. However, translating CAD data to a 3D-printed component frequently leads to errors and flaws, especially in curved surfaces 78. Cutting the part into enough layers, creating supporting materials, planning, and determining the part’s ideal orientation are all essential to minimizing divergence from concept to execution.
Interoperability: Data interoperability guarantees that using the same information to express the intended model, all disciplines will collaborate effectively. Because 3D printing relies on digital processes for all operations, compatibility between apps used for structural analysis, architectural design, and printing must be ensured79.
Regulatory landscape: Since there are now no regulations governing 3D printing in healthcare, quality control must be established 80. Focusing on the design, production, and use of 3D-printed medical devices, the FDA published its final advice in 2017 outlining technical factors for regulatory control. The pharmaceutical sector faces issues in identifying critical parameters, assessing the efficacy of 3D-printed medications, investigating their release in vitro and in vivo, and managing formulation quality as printing technology develops. The development of regulatory policies has the potential to greatly speed up the transition of printing technology in pharmaceutical formulations from theoretical investigation to workable solutions.
Switching to a personalized medication: Some 3D printing techniques alter the drug’s physical state, which affects its characteristics. Thus, to anticipate drug content, 3D printing pharmaceuticals must be assayed. Certain polymer filaments can have their diameter changed by heating, which could result in uneven tablet printing or even malfunctioning printers. Lubricant and proper nozzle sizing are two ways to address inconsistent tablet printing outcomes81. When certain polymers require higher temperatures, drug degradation may become an issue.
Environmental challenges: According to the study, which contrasts additive manufacturing with conventional manufacturing techniques, highly specialized raw materials are required for additive manufacturing technology to function. There are extra environmental implications since the material preparation process needs to take extra steps because of a particular requirement. Firstly, the environmental impact of additive manufacturing techniques appears to be influenced by electrical energy. Because of variations in material characteristics and the requirement for certain raw materials, the recovery of waste materials generated by AM technology is still unknown 82.
Future Aspects
Customized medicine: The use of 3D printing makes it possible to create personalized medicine dosages for each patient. Treating complex disorders such as cancer, cardiovascular diseases, and paediatric care requires personalization, which involves delivering tailored doses depending on age, genetics, and medical history. 3D-printed pharmaceuticals are probably going to be a main choice for customized treatments in the future 83.
Advancement in Biocompatible Substances: A broader spectrum of biocompatible materials, including biodegradable polymers and smart materials for implants and medication delivery systems, will be developed in the future of 3D printing in medicine. Healthcare applications will be further enhanced using these advanced substances to create scaffolds for tissue engineering and regenerative medicine.
On-Demand Pharmaceutical Manufacturing: The capacity to print medications on demand eliminates the need for large supply chains by enabling local, decentralized manufacture at pharmacies or hospitals. When supply systems are disrupted during health emergencies like pandemics, such as in rural or isolated areas, this can be quite helpful.
Integration with Nanotechnology: The combination of nanotechnology with 3D printing to create targeted medication delivery systems has the potential to completely transform medical therapy. These will provide more accuracy and control over the delivery of drugs, particularly in cancer treatments where it is essential to target cells 23.
Sustainable and Economical Manufacturing: Compared to traditional manufacturing, 3D printing uses less energy and produces less waste materials, which means that technology will provide more environmentally friendly ways to produce drugs. The method will be a desirable alternative for the development of rare and orphan drugs due to its cost-effectiveness, particularly in small-batch production.
Innovations in Regulatory and Quality Control: It is predicted that authorities such as the FDA will modify and enhance their protocols for approving 3D printed pharmaceuticals as technology advances. This will speed up the transfer from research to clinical applications by guaranteeing that printed pharmaceuticals fulfil safety and efficacy standards 84.
Various clinical trials conducted on 3D printing technology are given in Table 1 and, Table2 enlisting various patents of 3D printed pharmaceutical products, devices, and organs.
Table 1: Clinical trials
| S. No | Trial ID | Sponsor | Purpose/Aim | Ref. |
| 1. | NCT05273060 | Mayo Clinic | To collect data on safety and contrast 3D printing with conventional planning for nasal repair. | 85 |
| 2. | NCT05741892 | Insel Gruppe AG, University Hospital Bern | The study’s objective is to evaluate the efficacy of fracture reduction in cases of comminuted femur- and tibia-shaft fractures when surgical guides are created and 3D printed at the point of treatment. | 86 |
| 3. | NCT05845099 | Ain Shams University | This research is a controlled clinical experiment that randomly assigns individuals to one of two groups—conventionally made CRD or 3D-printed CAD/CAM CRD—and compares patient satisfaction and oral microbiota growth in each group. | 87 |
| 4. | NCT06035211 | University Hospital, Bordeaux | This clinical trial evaluates the impact of presenting and manipulating a three-dimensional model of a patient’s tumored kidney before nephron-sparing surgery. | 88 |
Table 2: Patented 3D printed pharmaceutical products, devices, and organs
| S. No | Patent no. | Inventor | Title | Status | Ref. |
| Pharmaceutical Products | |||||
| 1. | WO2020145898A1 | Seng Han LIM, Wei Jiang GOH | Three-dimensional printing of personalized pills | Active | 89 |
| 3. | CN109125912B | Lei Yifeng | 3D printing microneedle patch for intelligent blood sugar regulation | Active | 90 |
| 4. | ES2966388T3 | Morten | Procedure for 3D printing, use of a 3D printing suspension and a 3D printer | Active | 91 |
| 5. | US11305480B2 | Charles W. Hull | Methods and apparatus for 3D printed hydrogel materials | Active | 92 |
|
3D printed devices |
|||||
| 6. | CN108724712B | G.Wei | 3D printing of porous implants | Active | 93 |
| 7. | CN108724713B | G.Wei | 3D printing of mesh implants for bone delivery | Active | 94 |
| 8. | JP7045386B2 | Wallace Anthony | 3D printing of optical devices | Active | 95 |
| 9. | US10543638B2 | DionysiosDouroumis | 3D printed stent | Active | 96 |
|
3D printed organs |
|||||
| 10. | EP2970896B1 | Benjamin R | Engineered liver tissues, arrays thereof, and methods of making the same | Active | 97 |
| 11. | AU2017359330B2 | Kapil BHARTI | 3D vascularized human ocular tissue for cell therapy and drug discovery | Active | 98 |
| 12. | EP3215603B1 | Kelsey Nicole Retting | Engineered three-dimensional skin tissues, arrays thereof, and methods of making the same | Active | 99 |
Conclusion
Numerous 3D printing systems have been developed and classified into subgroups according to their operational principles. 3D technology facilitates the production of complex and sophisticated dosage forms, providing more control over their shape and microstructure. Moreover, 3D printing represents an innovative and highly promising techniques for on-demand manufacturing and the personalization of dosage forms, potentially enhancing patient compliance and drug efficacy, minimizing side effects, addressing stability concerns of drugs with limited shelf life, and ultimately facilitating patient-specific healthcare through customized medicinal products. However, despite several possible medical and economic benefits, certain technical challenges limit the widespread use of 3D printing technology in product commercialization, particularly the limited selection of biocompatible materials available for 3D printers.
This review discusses potential applications of 3D printing technology, outlining the various techniques that have been developed, with the challenges associated with these methods and their implementation in drug delivery systems. 3D printing will transform conventional manufacturing by improving health applications, including drug delivery.
Acknowledgment
The authors expressed their gratitude to Mr. Jitender Joshi, President, Prof. (Dr.) Dharam Buddhi, Vice Chancellor of Uttaranchal University Dehradun, and Prof. (Dr.) Vikash Jakhmola, Director, Uttaranchal Institute of Pharmaceutical Sciences, for their encouragement and guidance in publishing this review work.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement- This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
- Data collection and manuscript: VS, MB, and SD.
- Reviewed and corrected: MC, PK, VJ and AN.
References
- Xiao YQ, Kan CW. Review on Development and Application of 3D-Printing Technology in Textile and Fashion Design. Coatings. 2022;12(2):267.
CrossRef - Sachs EM, Haggerty JS, Cima MJ, Williams PA. Three-dimensional printing techniques. Massachusetts Institute of Technology. US5204055A. April 20,1993.
- Beg S, Almalki WH, Malik A, et al. 3D printing for drug delivery and biomedical applications. Drug Discov Today. 2020;25(9):1668-1681.
CrossRef - Alhnan MA, Okwuosa TC, Sadia M, Wan KW, Ahmed W, Arafat B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm Res. 2016;33(8):1817-1832.
CrossRef - Gupta MS, Kumar TP, Davidson R, Kuppu GR, Pathak K, Gowda DV. Printing Methods in the Production of Orodispersible Films. AAPS PharmSciTech. 2021;22(3):1-17.
CrossRef - Konta AA, García-Piña M, Serrano DR. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering. 2017;4(4):79.
CrossRef - Ricci JL, Clark EA, Murriky A, Smay JE. Three-dimensional printing of bone repair and replacement materials: Impact on craniofacial surgery. Journal of Craniofacial Surgery. 2012;23(1):304-308.
CrossRef - Fina F, Gaisford S, Basit AW. Powder Bed Fusion: The Working Process, Current Applications and Opportunities. AAPS Advances in the Pharmaceutical Sciences Series. 2018;31:81-105.
CrossRef - Okafor-Muo OL, Hassanin H, Kayyali R, ElShaer A. 3D Printing of Solid Oral Dosage Forms: Numerous Challenges with Unique Opportunities. J Pharm Sci. 2020;109(12):3535-3550.
CrossRef - Prasher A, Shrivastava R, Dahl D, et al. Steroid Eluting Esophageal-Targeted Drug Delivery Devices for Treatment of Eosinophilic Esophagitis. Polymers. 2021;13(4):557.
CrossRef - Díaz-Torres E, Rodríguez-Pombo L, Ong JJ, et al. Integrating pressure sensor control into semi-solid extrusion 3D printing to optimize medicine manufacturing. Int J Pharm X. 2022;4:100133.
CrossRef - Kempin W, Domsta V, Grathoff G, et al. Immediate Release 3D-Printed Tablets Produced Via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharm Res. 2018;35(6):1-12.
CrossRef - Vozzi G, Flaim C, Ahluwalia A, Bhatia S. Fabrication of PLGA scaffolds using soft lithography and microsyringe deposition. Biomaterials. 2003;24(14):2533-2540.
CrossRef - Randhawa A, Dutta SD, Ganguly K, Patel DK, Patil TV, Lim KT. Recent Advances in 3D Printing of Photocurable Polymers: Types, Mechanism, and Tissue Engineering Application. Macromol Biosci. 2023;23(1):2200278.
CrossRef - Sabbatini B, Cambriani A, Cespi M, Palmieri GF, Perinelli DR, Bonacucina G. An Overview of Natural Polymers as Reinforcing Agents for 3D Printing. ChemEngineering. 2021;5(4):78.
CrossRef - Malekjafarian A, OBrien EJ, Micu LA Investigation of Buckling Capacity of Metal Materials Manufactured by Laser 3D Printing. Procedia Manuf. 2017;7:696-700.
CrossRef - Kumar KPA, Ghosh K, Alduhaish O, Pumera M. Metal-plated 3D-printed electrode for electrochemical detection of carbohydrates. Electrochem commun. 2020;120:106827.
CrossRef - Gadagi B, Lekurwale R. A review on advances in 3D metal printing. Mater Today Proc. 2021;45:277-283.
CrossRef - Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):E139-E596.
- Domínguez-Robles J, Diaz-Gomez L, Utomo E, et al. Use of 3d printing for the development of biodegradable antiplatelet materials for cardiovascular applications. Pharmaceuticals. 2021;14(9):921.
CrossRef - Esmaeili S, Shahali M, Kordjamshidi A, et al. An artificial blood vessel fabricated by 3D printing for pharmaceutical application. Nanomed J. 2019;6(3):183-194.
CrossRef - Veerubhotla K, Lee CH. Design of biodegradable 3D-printed cardiovascular stent. Bioprinting. 2022;26:e00204.
CrossRef - Junior NPDJ, da Silva L, de Almeida EC, et al. Polycaprolactone/ atorvastatin nanocomposite – A supplier for 3D printing and drug delivery systems. Mater Lett. 2024;357:135792.
CrossRef - Hagan CT, Bloomquist C, Warner S, et al. 3D printed drug-loaded implantable devices for intraoperative treatment of cancer. Journal of Controlled Release. 2022;344:147-156.
CrossRef - Liu C, Wang Z, Wei X, Chen B, Luo Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021;131:314-325.
CrossRef - Ma H, Li T, Huan Z, et al. 3D printing of high-strength bioscaffolds for the synergistic treatment of bone cancer. NPG Asia Materials. 2018;10(4):31-44.
CrossRef - Colak B, Ertas YN. Implantable, 3D-Printed Alginate Scaffolds with Bismuth Sulfide Nanoparticles for the Treatment of Local Breast Cancer via Enhanced Radiotherapy. ACS Appl Mater Interfaces. 2024;16(13):15718-15729.
CrossRef - Liang K, Carmone S, Brambilla D, Leroux JC. 3D printing of a wearable personalized oral delivery device: A first-in-human study. Sci Adv. 2018;4(5):eaat2544.
CrossRef - Zhu H, Zhou Y, Jiang J, Wang Y, He F. Accuracy and margin quality of advanced 3D-printed monolithic zirconia crowns. Journal of Prosthetic Dentistry.
CrossRef - Piedra-Cascón W, Krishnamurthy VR, Att W, Revilla-León M. 3D printing parameters, supporting structures, slicing, and post-processing procedures of vat-polymerization additive manufacturing technologies: A narrative review. J Dent. 2021;109:103630.
CrossRef - Zhang L, Liu H, Yao H, Zeng Y, Chen J. Preparation, Microstructure, and Properties of ZrO2(3Y)/Al2O3 Bioceramics for 3D Printing of All-ceramic Dental Implants by Vat Photopolymerization. Chinese Journal of Mechanical Engineering: Additive Manufacturing Frontiers. 2022;1(2):100023.
CrossRef - Bora PV, Ahmed AS, Alford A, Pitttman K, Thomas V, Lawson NC. Characterization of materials used for 3D printing dental crowns and hybrid prostheses. Journal of Esthetic and Restorative Dentistry. 2024;36(1):220-230.
CrossRef - Amin R, Marashi SMH, Noori SMR, et al. Medical, pharmaceutical, and nutritional applications of 3D-printing technology in diabetes. Diabetes Metab Syndr. 2024;18(4):103002.
CrossRef - Liu Y, Yu Q, Luo X, Yang L, Cui Y. Continuous monitoring of diabetes with an integrated microneedle biosensing device through 3D printing. Microsystems & Nanoengineering. 2021;7(1):1-12.
CrossRef - Kumar A, Jain D, Bahuguna J, et al. Machine learning assisted and smartphone integrated homogeneous electrochemiluminescence biosensor platform for sample to answer detection of various human metabolites. Biosens Bioelectron. 2023;238:115582 .
CrossRef - Ahmad J, Garg A, Mustafa G, Mohammed AA, Ahmad MZ. 3D Printing Technology as a Promising Tool to Design Nanomedicine-Based Solid Dosage Forms: Contemporary Research and Future Scope. Pharmaceutics. 2023;15(5):1448.
CrossRef - Cui M, Yang Y, Jia D, et al. Effect of novel internal structures on printability and drug release behavior of 3D printed tablets. J Drug Deliv Sci Technol. 2019;49:14-23.
CrossRef - Wu M, Zhang Y, Huang H, et al. Assisted 3D printing of microneedle patches for minimally invasive glucose control in diabetes. Mater Sci Eng C Mater Biol Appl. 2020;117:111299.
CrossRef - Muir BC, Li JS, Hudak YF, Kaufman GE, Cullum S, Aubin PM. Evaluation of novel plantar pressure-based 3-dimensional printed accommodative insoles – A feasibility study. Clinical Biomechanics. 2022;98:105739.
CrossRef - Glover K, Mathew E, Pitzanti G, Magee E, Lamprou DA. 3D bioprinted scaffolds for diabetic wound-healing applications. Drug Deliv Transl Res. 2023;13(8):2096-2109.
CrossRef - Wong TM, Jin J, Lau TW, et al. The use of three-dimensional printing technology in orthopaedic surgery: A review. Journal of Orthopaedic Surgery. 2017;25(1):2309499016684077.
CrossRef - Taniguchi N, Fujibayashi S, Takemoto M, et al. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Materials Science and Engineering: C. 2016;59:690-701.
CrossRef - Hu X, Kenan S, Cheng M, Cai W, Huang W, Yan W. 3D-Printed Patient-Customized Artificial Vertebral Body for Spinal Reconstruction after Total En Bloc Spondylectomy of Complex Multi-Level Spinal Tumors. Int J Bioprint. 2022;8(3):82-95.
CrossRef - Chen G, Muheremu A, Yang L, et al. Three-dimensional printed implant for reconstruction of pelvic bone after removal of giant chondrosarcoma: a case report. Journal of International Medical Research. 2020;48(4):0300060520917275.
CrossRef - Wu X, Liu S, Chen K, et al. 3D printed chitosan-gelatine hydrogel coating on titanium alloy surface as biological fixation interface of artificial joint prosthesis. Int J Biol Macromol. 2021;182:669-679.
CrossRef - Chhikara K, Gupta S, Saharawat S, Sarkar S, Chanda A. Design, Manufacturing, and Trial of a 3D Printed Customized Finger Splint for Patients with Rheumatoid Arthritis. Rheumato. 2023;3(1):51-62.
CrossRef - Sen K, Manchanda A, Mehta T, Ma AWK, Chaudhuri B. Formulation design for inkjet-based 3D printed tablets. Int J Pharm. 2020;584:119430.
CrossRef - Chang SY, Li SW, Kowsari K, et al Binder-Jet 3D Printing of Indomethacin-laden Pharmaceutical Dosage Forms. J Pharm Sci. 2020;109(10):3054-3063.
CrossRef - Allahham N, Fina F, Marcuta C, et al. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics. 2020;12(2):110.
CrossRef - Mohamed EM, Ali SFB, Rahman Z, et al. Formulation Optimization of Selective Laser Sintering 3D-Printed Tablets of Clindamycin Palmitate Hydrochloride by Response Surface Methodology. AAPS PharmSciTech. 2020;21(6):1-15.
CrossRef - Wei C, Solanki NG, Vasoya JM, Shah AV, Serajuddin ATM. Development of 3D Printed Tablets by Fused Deposition Modeling Using Polyvinyl Alcohol as Polymeric Matrix for Rapid Drug Release. J Pharm Sci. 2020;109(4):1558-1572.
CrossRef - Maroni A, Melocchi A, Parietti F, Foppoli A, Zema L, Gazzaniga A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J Control Release. 2017;268:10-18.
CrossRef - Melocchi A, Parietti F, Maroni A, Foppoli A, Gazzaniga A, Zema L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int J Pharm. 2016;509(1-2):255-263.
CrossRef - Auvinen VV, Virtanen J, Merivaara A, et al. Modulating sustained drug release from nanocellulose hydrogel by adjusting the inner geometry of implantable capsules. J Drug Deliv Sci Technol. 2020;57:101625.
CrossRef - Hu J, Wan J, Xi J, Shi W, Qian H. AI-driven design of customized 3D-printed multi-layer capsules with controlled drug release profiles for personalized medicine. Int J Pharm. 2024;656:124114.
CrossRef - Suryavanshi P, Chaudhari VS, Banerjee S. Customized 3D-printed hollow capsular device filled with norfloxacin-loaded micropellets for controlled-release delivery. Drug Deliv Transl Res. 2023;13(5):1183-1194.
CrossRef - Rattanakit P, Moulton SE, Santiago KS, Liawruangrath S, Wallace GG. Extrusion printed polymer structures: a facile and versatile approach to tailored drug delivery platforms. Int J Pharm. 2012;422(1-2):254-263.
CrossRef - Benmassaoud MM, Kohama C, Kim TWB, et al. Efficacy of eluted antibiotics through 3D printed femoral implants. Biomed Microdevices. 2019;21(3):1-10.
CrossRef - Qamar N, Abbas N, Irfan M, et al. Personalized 3D printed ciprofloxacin impregnated meshes for the management of hernia. J Drug Deliv Sci Technol. 2019;53:101164.
CrossRef - Arany P, Papp I, Zichar M, et al. In Vitro Tests of FDM 3D-Printed Diclofenac Sodium-Containing Implants. Molecules. 2020;25(24):5889.
CrossRef - Xu X, Goyanes A, Trenfield SJ, et al. Stereolithography (SLA) 3D printing of a bladder device for intravesical drug delivery. Mater Sci Eng C Mater Biol Appl. 2021;120:111773.
CrossRef - Pere CPP, Economidou SN, Lall G, Ziraud C, et al. 3D printed microneedles for insulin skin delivery. Int J Pharm. 2018;544(2):425-432.
CrossRef - Lim SH, Ng JY, Kang L. Three-dimensional printing of a microneedle array on personalized curved surfaces for dual-pronged treatment of trigger finger. Biofabrication. 2017;9(1):015010.
CrossRef - Uddin MJ, Scoutaris N, Klepetsanis P, Chowdhry B, Prausnitz MR, Douroumis D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int J Pharm. 2015;494(2):593-602.
CrossRef - Luzuriaga MA, Berry DR, Reagan JC, Smaldone RA, Gassensmith JJ. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip. 2018;18(8):1223-1230.
CrossRef - Weisman JA, Ballard DH, Jammalamadaka U, et al. 3D printed antibiotic and chemotherapeutic eluting catheters for potential use in interventional radiology: in vitro proof of concept study. Acad Radiol. 2018;26(2):270.
CrossRef - Tappa K, Jammalamadaka U, Ballard DH, et al. Medication eluting devices for the field of OBGYN (MEDOBGYN): 3D printed biodegradable hormone eluting constructs, a proof of concept study. PLoS One. 2017;12(8):e0182929.
CrossRef - Varan C, Wickström H, Sandler N, Aktaş Y, Bilensoy E. Inkjet printing of antiviral PCL nanoparticles and anticancer cyclodextrin inclusion complexes on bioadhesive film for cervical administration. Int J Pharm. 2017;531(2):701-713.
CrossRef - Arany P, Papp I, Zichar M, et al. Manufacturing and Examination of Vaginal Drug Delivery System by FDM 3D Printing. Pharmaceutics. 2021;13(10):1714.
CrossRef - Zhang W, Melcher R, Travitzky N, Bordia RK, Greil P. Three-Dimensional Printing of Complex-Shaped Alumina/Glass Composites. Adv Eng Mater. 2009;11(12):1039-1043.
CrossRef - Bos F, Wolfs R, Ahmed Z, Salet T. Additive manufacturing of concrete in construction: potentials and challenges of 3D concrete printing. Virtual Phys Prototyp. 2016;11(3):209-225.
CrossRef - Paul SC, Tay YWD, Panda B, Tan MJ. Fresh and hardened properties of 3D printable cementitious materials for building and construction. Archives of Civil and Mechanical Engineering. 2018;18(1):311-319.
CrossRef - Melnyk LA, Oyewumi MO. Integration of 3D printing technology in pharmaceutical compounding: Progress, prospects, and challenges. Annals of 3D Printed Medicine. 2021;4:100035.
CrossRef - Cerda JR, Arifi T, Ayyoubi S, et al. Personalised 3D Printed Medicines: Optimising Material Properties for Successful Passive Diffusion Loading of Filaments for Fused Deposition Modelling of Solid Dosage Forms. Pharmaceutics. 2020;12(4):345.
CrossRef - Zheng F, Huang SW. Advances in Study on Three-dimensional Printing in Pharmaceutics. Chin Herb Med. 2016;8(2):121-125.
CrossRef - Carroll BE, Palmer TA, Beese AM. Anisotropic tensile behavior of Ti–6Al–4V components fabricated with directed energy deposition additive manufacturing. Acta Mater. 2015;87:309-320.
CrossRef - El-Sayegh S, Romdhane L, Manjikian S. A critical review of 3D printing in construction: benefits, challenges, and risks. Archives of Civil and Mechanical Engineering. 2020;20(2):1-25.
CrossRef - Oropallo W, Piegl LA. Ten challenges in 3D printing. Eng Comput. 2016;32(1):135-148.
CrossRef - Hager I, Golonka A, Putanowicz R. 3D Printing of Buildings and Building Components as the Future of Sustainable Construction? Procedia Eng. 2016;151:292-299.
CrossRef - Di Prima M, Coburn J, Hwang D, Kelly J, Khairuzzaman A, Ricles L. Additively manufactured medical products – the FDA perspective. 3D Printing in Medicine 2016 2:1. 2016;2(1):1-6.
CrossRef - Awad A, Trenfield SJ, Gaisford S, Basit AW. 3D printed medicines: A new branch of digital healthcare. Int J Pharm. 2018;548(1):586-596.
CrossRef - Chen X, Cao Q, Chen T, Wang D, Fan Y, Xing W. 3D printing for precision construction of ceramic membranes: Current status, challenges, and prospects. Advanced Membranes. 2023;3:100068.
CrossRef - Goyanes A, Det-Amornrat U, Wang J, Basit AW, Gaisford S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. Journal of Controlled Release. 2016;234:41-48.
CrossRef - Khairuzzaman A. Regulatory Perspectives on 3D Printing in Pharmaceuticals. AAPS Advances in the Pharmaceutical Sciences Series. 2018;31:215-236.
CrossRef - Howard BE. Regenerative Medicine Approach to Nasal Reconstruction. Mayo Clinic. NCT05273060. April 2025.
- Gruppe I. 3D Planned Surgery of Acute Fractures Performed With 3D Guides Printed at the Point of Care. University Hospital Bern. NCT05741892. December 1, 2025.
- Srinivasan M, Kalberer N, Fankhauser N, Naharro M, Maniewicz S, Müller F. CAD-CAM complete removable dental prostheses: A double-blind, randomized, crossover clinical trial evaluating milled and 3D-printed dentures. J Dent. 2021;115:103842.
CrossRef - Effect on Pre-operative Anxiety of a Personalized Three-dimensional Kidney Model Prior to Nephron-sparing Surgery- Rein 3D Anxiety. University Hospital, Bordeaux. NCT06035211. January 2026.
- Han S, Jiang W. Three-dimensional printing of personalized pills. WO2020145898A1.July 16, 2020.
- Lei Y, Liu S, Zhang Y, Li Y. 3D printing microneedle patch for intelligent blood sugar regulation and preparation method thereof. Wuhan University WHU. CN109125912B. September 8, 2020.
- Andersen MQ, Jensen MB, Slots C. Procedure for 3D printing, use of a 3D printing suspension and a 3D printer. Ossiform Aps. ES2966388T3. April 22, 2024.
- Hull CW. Methods and apparatus for 3D printed hydrogel materials. 3D Systems Inc. US11305480B2. April 19, 2022.
- Wei G. 3D printing of porous implants. Warsaw Orthopedic Inc. CN108724712B. March 22, 2022.
- Wei G. 3D printing of mesh implants for bone delivery. Warsaw Orthopedic Inc. CN108724713B. November 29, 2022
- Martin WA, Kumar GN, Guillon ML, Kindt-Larsen T. 3D printing of optical devices. Atheneum Optical Sciences LLC. JP7045386B2. March 31, 2022
- Douroumis D, Bradley MSA, Scoutaris N. Stent. University of Greenwich. US10543638B2.January 28, 2020.
- Shepherd BR, Robbins JB, Gorgen VA, Presnell SC. Engineered liver tissues, arrays thereof, and methods of making the same. Organovo Inc. EP2970896B1. April 24, 2024.
- Bharti K, Quinn RL, Song MJ. 3D vascularized human ocular tissue for cell therapy and drug discovery. US Department of Health and Human Services. AU2017359330B2. March 10, 2022.
- Retting KN, O’neill CM, Nguyen DLG, et al. Engineered three-dimensional skin tissues, arrays thereof, and methods of making the same. LOreal SA Organovo Inc. EP3215603B1.September 22, 2021.
Abbreviations
3D: Three-dimensional, 3DP: Three-dimensional printing, ABS: Acrylonitrile butadiene styrene, AM: Additive manufacturing, APIs: Active pharmaceutical ingredients, BJ: Binder jet, CAD: Computer-aided design, CT: Computed tomography, CVD: Cardiovascular diseases, DLP: Digital light projection, DOD: Drop-on-demand, FDA: Food and Drug Administration, FDM: Fused deposition modeling, MRI: Magnetic resonance imaging, PAM: Pressure-assisted micro syringe, PCL: Polycaprolactone, PLA: Polylactic acid, PVA: Polyvinyl alcohol, SLA: Stereolithography, SLS: Selective laser sintering, SSE: Semi-solid extrusion







