Sri Wahjuni* , Ni Made Puspawati
, Ni Made Puspawati and Ni Ketut Puspa Sari
and Ni Ketut Puspa Sari
Department of Chemistry Faculty of Mathematics and Natural Sciences Udayana University, Jimbaran, Badung, Bali, Indonesia
Corresponding Author E-mail: sriwahjuni@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/3033
Abstract
Introduction: Kecombrang (Etlingera elatior) flower ethanol extract has been reported to have antihyperglycemic activity and encapsulation of the ethanol extract into nano chitosan-tripolyphospate are believed to have a better delivery system for their bioactive substances. This research aimed to investigate the effectiveness of nano chitosan-Etlingera elatior ethanol extract in reducing blood sugar and malondialdehyde (MDA) levels, as well as boosting superoxide dismutase (SOD) levels in hyperglycemic rats induced by streptozotocin. Methods: This study used a randomized post-test only control group design to observe blood glucose, malondialdehyde (MDA), and superoxide dismutase (SOD) levels as markers of oxidative stress. The chemical constituents of the extract were analysed with liquid chromatography mass spectrometry (LCMS/MS). The nano chitosan-Etlingera elatior ethanol extract used has the characteristic a zeta potential value of -16.2.80 mV and a particle size of 312.7 nm. Results: The antihyperglycemic test conducted on rats induced with streptozotocin revealed that the oral route of nano chitosan-based extract improved oxidative stress parameters. The oral route of the nano chitosan extract at a dose of 50 mg/Kg BW/day provided the best result in reducing blood glucose and MDA levels while boosting SOD level in hyperglycemic rats. Conclusion: The nano chitosan-Etlingera elatior ethanol extract is effective in decreasing blood glucose and malondialdehyde as well as increasing superoxide dismutase level in hyperglycemic rats induced by streptozotocin. Nano extracts created by encapsulating bioactive substances have significant potential for development as a delivery system for bioactive compounds in the medical field.
Keywords
Anti Hyperglycemic; Etlingera elatior; Nano Chitosan; Stress Oxidative
Download this article as:| Copy the following to cite this article: Wahjuni S, Puspawati N. M, Sari N. K. P. Effects of Encapsulated Nano-Chitosan Derived from Kecombrang (Etlingera elatior) Ethanol Extract on Blood Glucose, Malondialdehyde (MDA), and Superoxide Dismutase (SOD) Levels in Hyperglycemic Wistar Rats. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Wahjuni S, Puspawati N. M, Sari N. K. P. Effects of Encapsulated Nano-Chitosan Derived from Kecombrang (Etlingera elatior) Ethanol Extract on Blood Glucose, Malondialdehyde (MDA), and Superoxide Dismutase (SOD) Levels in Hyperglycemic Wistar Rats. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/407lfoX |
Introduction
Diabetes mellitus is a chronic disease that disrupts carbohydrate metabolism, particularly the body’s ability to process glucose effectively. This condition is characterized by consistently high blood sugar levels, which can occur due to defects in insulin secretion, insulin action, or a combination of both. The sustained elevation of glucose levels triggers oxidative stress, arising from an imbalance between the production of free radicals and the body’s antioxidant defenses. Among these free radicals, reactive oxygen species (ROS) are the most common within biological systems 1,2. MDA is often found to be elevated in patients with poorly controlled diabetes, reflecting the oxidative damage due to high blood glucose levels 3,4. In diabetic conditions, there is often an upregulation of antioxidant enzymes like SOD as a compensatory response to increased ROS production 5,6.
Indonesia boasts a wealth of biodiversity and is abundant in antioxidant compounds. Its diverse range of vegetables and fruits, consumed through the generations, includes many phenolic and flavonoid compounds with strong antioxidant properties. Flavonoids have the ability to counteract ROS by directly scavenging them, inhibiting the activity of enzymes responsible for ROS production, and protecting healthy cells from harm, such as lipid peroxidation and DNA damage. Through these actions, flavonoids help to minimize oxidative stress in the body. 7. Flavonoids are believed to repair pancreatic cells damage, which in turn boosts insulin secretion and enhances the activity of antioxidant enzymes 8. Flavonoids support antioxidant enzymes in managing blood sugar levels and preventing complications related to high glucose by eliminating free radicals, lowering oxidative stress, and neutralizing RO) 9. Marella compiled numerous studies and identified 419 species from 133 plant families with antidiabetic properties, including Etlingera. Among these, Etlingera elatior contains apigenin, a flavonoid derivative found in substantial amounts in various plants and fruits, which exhibits hypoglycemic activity 10.
Flavonoids are involved in numerous biological activities and offer several health benefits. However, their effectiveness is limited due to poor absorption in the digestive system and extensive metabolism in the intestines. Furthermore, flavonoids are quickly excreted from the body. Enhancing their bioavailability can lead to greater health benefits by optimizing intestinal absorption, improving transport mechanisms, increasing metabolic stability, and shifting the site of absorption from the large intestine to the small intestine 11. This condition may be achieved by using nanoencapsulation.
Nanomaterials are solid colloidal elements composed of macromolecular constituents and can attend as drug delivery systems. These materials allow for the dissolution, entanglement, and encapsulation of active compounds 12. Utilizing nanosystems for drug delivery enhances the dispersion of drugs in the bloodstream and accelerates their effects 13. Chitosan polymer is a type of nanomaterial commonly utilized for drug delivery systems due to its biocompatibility with the human body 14. When chitosan is combined with tripolyphosphate through ionic crosslinking, it demonstrates minimal swelling. This crosslinked form of chitosan is also highly customizable, allowing adjustments to its properties such as hydrophilicity, density, and crystallinity based on specific functional requirements 15.
The process of producing nano chitosan with ethanol extract from Etlingera elatior in nanotechnology may improve the solubility and bioavailability of bioactive compounds, potentially delivering significant and stable effects even at lower doses. This research aims to investigate the effectiveness of nano chitosan- Etlingera elatior ethanol extract in reducing blood sugar and MDA levels, as well as boosting SOD levels as the marker of stress oxidative in hyperglycemic rats induced by streptozotocin.
Materials and Methods
Design study
This study employed an experimental method using a randomized posttest-only control group design. The preparation for the study and administration of treatments was conducted at the Faculty of Mathematics and Natural Sciences, Universitas Udayana. The research was carried out over a period of approximately three months, from June 2024 to August 2024. The study protocol received approval from the Animal Ethics Committee of the Faculty of Veterinary Medicine, Universitas Udayana, under ethics certificate number 70/UN14.2.9/PT/2022.
Animals study
In this study, 27 male Wistar rats (Rattus norvegicus), weighing between 150 and 200 grams and aged ± 3 months, were split into three groups after a week of acclimation. K0 was the positive control group that received 40 mg/kg of streptozotocin (STZ). P1, the first treatment group, received 50 mg/kg of ethanol extract from Etlingera elatior flowers every day in addition to STZ 40 mg/kg. P2 was the treatment group that got 50 mg/kg of nanochitosan-Etlingera elatior flower ethanol extract every day in addition to STZ 40 mg/kg induction. One of the methods utilized to administer the extract orally was Sonde (Instechlabs).
Rats utilized for study are kept in a clean, dry environment with enough air circulation in the laboratory. The rats were kept in enclosures with a 12-hour light-dark cycle and given aseptic food and water. The room’s environmental parameters were consistently maintained at 22 ± 2◦ C and 50 ± 10% relative humidity.
Induction of diabetes to experimental animals
We modified the previously published technique to induce hyperglycemia in rats. In summary, rats were administered 40 mg/kg of STZ intraperitoneally in 0.1 M cold citrate buffer (Sigma-Aldrich) with pH 4.5. In this investigation, only diabetic rats with blood glucose levels > 250 mg/dl at fasting were used.16
Preparation of Etlingera elatior flower ethanol extract
Dry powder kecombrang flower (Etlingera elatior) with water content of 7.55% was extracted with ethanol 96% (Sigma-Aldrich) at room temperature for 48 h, the solvent was then evaporated at 45 °C. The extract was then filtered using 125 mm Ø filter paper (Whatman).
Preparation of nano chitosan encapsulation
The thick ethanol extract of Etlingera elatior was encapsulated into nano-chitosan-tripolyphosphate as described previously in the paper to yield nano chitosan-kecombrang flower ethanol extract which subsequently characterized.17 One gram of the extract was weighed, 50 milliliters of 96% ethanol were used to dissolve it, and 100 milliliters of distilled water were added. One gram of tripolyphosphate (Sigma-Aldrich) and one gram of chitosan (Sigma-Aldrich) were dissolved in 100 mL of distilled water and 1% glacial acetate, respectively. For approximately two hours, the three solutions were combined and agitated with a magnetic stirrer. Nanoparticles of chitosan, tripolyphosphate, and extractor seed extract was separated by centrifugation. After being separated, the resulting solids were frozen at 4–4°C for a maximum of 2 days. After drying, storage is moved to a refrigerator (above 300°C).
Measurement of blood glucose levels
The blood glucose levels were measured from rats’ tail vein blood using GLUCO M glucose test kit (OneMed Health Care). This was done three times: once after STZ induction, once after kecombrang flower ethanol extract treatment, and once after nano chitosan-kecombrang flower ethanol extract treatment. A glucometer was used to test blood glucose levels on the fourth day following STZ induction and the seventeenth day following the treatment.
Measurement of malondialdehyde (MDA)
Following the manufacturer’s protocol, two treatments were conducted utilizing the thiobarbituric acid reactive substance (TBARS) method with a QuantiChrom TBARS Assay kit (DTBA-100 BioAssay Systems). The MDA levels were found to be mean. The homogenate was incubated in equal parts of thiobarbituric (TBA) reagent for one hour at 100°C. The reaction mixtures were measured fluorometrically utilizing 450 nm excitation and emission wavelengths using a microplate reader (BIOTEK Reader 800TS) after chilling and loading them into duplicate 96-well microplates.
Measurement of superoxide dismutase (SOD)
CuZn/Mn SOD Activity Assay Kit (Hydroxylamine Method, Elabscience Biotechnology Co.) with two treatments. Microplate reader was used to measure the SOD at a wavelength of 450 nm.
Identification of active compounds
Through phytochemical testing and liquid chromatography tandem mass spectrometry Xevo type G2TOF (LC-MS/MS, Thermo Fisher Scientific. The extract of ethanol Up to 5 pl of an ethanol eluent-prepared Etlingera elatior sample was injected into the LCMS/MS instrument using a stationary phase/C18 column (Octadecyl silane). The outcome was displayed as a chromatogram, and the mass of the chemical obtained from each retention period was subsequently determined by analyzing it with MassLynx v 4.1. On the http://www.massbank.ip/ and www.chesmspider.com website, the compound’s name and structure were verified. The Etlingera elatior ethanol was identified by LC-MS/MS, which produced a chromatogram with seven peaks in terms of retention time (3,991; 3.991; 991; 7,703; 8,603; 8,664; 9,883; 10,815 minutes).
Tissue collection and euthanasia samples
Liver, an organ included in the evaluation, was removed from each group and homogenized separately using a homogenizer at a 10% weight/volume ratio. In 7.4 pH phosphate buffer saline (Sigma-Aldrich), specimens were homogenized for the purpose of estimating the presence of various protein contents. The neck dislocation method was then used to end the lives of the Wistar rats that had been chosen for sample on the day 21st. The Helsinki Declaration’s research ethical guidelines—which govern the use of experimental animals—are followed. Following the study procedure, post-mortem animal care is frequently carried out, in which the dead animals are suitably enclosed in paper or plastic bags, burned in an incinerator, and buried.
Statistical analysis
SPSS version 20.0 for Windows was used to analyze data. Data was presented descriptively using graphs. The results of the significance analysis using One Way ANOVA. To determine which groups differed from the control group, a further test was conducted using the Least Significant Difference (LSD) test.
Results
Decrease in blood glucose levels
Figure 1 shows the blood glucose level profile of wistar rats. Take out the ethanol The administration of nanochitosan-tripolyphosphate-Etlingera elatior was started on the fifteenth day. The average blood glucose level for group K0 was 323.7 mg/dL. The average blood glucose level for Group P1 was 136.33 mg/dL. The average blood glucose level for Group P2 was 99.4 mg/dL. Assume that there is a comparison in the decline in rats’ blood glucose levels. In that instance, delivering 50 mg/kgBW of Etlingera elatior ethanol extract nanoparticles were more successful than administering 50 mg/kgBW of Etlingera elatior ethanol extract in terms of lowering blood glucose levels.
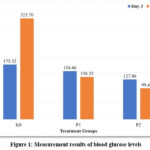 |
Figure 1: Measurement results of blood glucose levels.Click here to View Figure |
Decrease in malondialdehyde levels
Figure 2 displays MDA levels. Following therapy, the four groups’ mean MDA levels differ considerably (p < 0.05). The findings showed that, in comparison to the K0, the MDA levels in P1 and P2 were lower. Compared to P1, the MDA levels in P2 decreased more significantly. This suggests that nanochitosan-Etlingera elatior flower ethanol extract have antihyperglycemic properties.
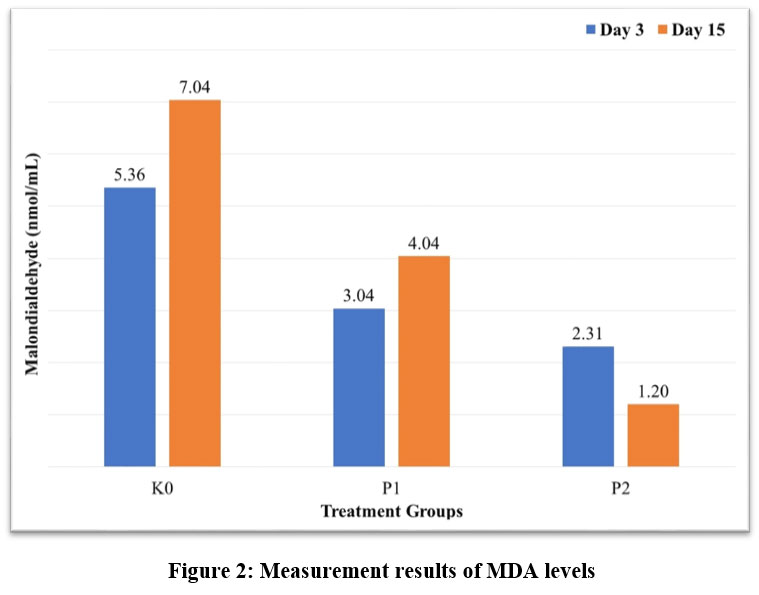 |
Figure 2: Measurement results of MDA levelsClick here to View Figure |
Increase in superoxide dismutase (SOD)
The mean SOD levels in the four groups differed considerably (p<0.05). Based on the test results, it can be inferred that the P2 had higher SOD levels than the K0. This suggests that the antihyperglycemic properties of the nanochitosan-Etlingera elatior flower ethanol extract are improved.
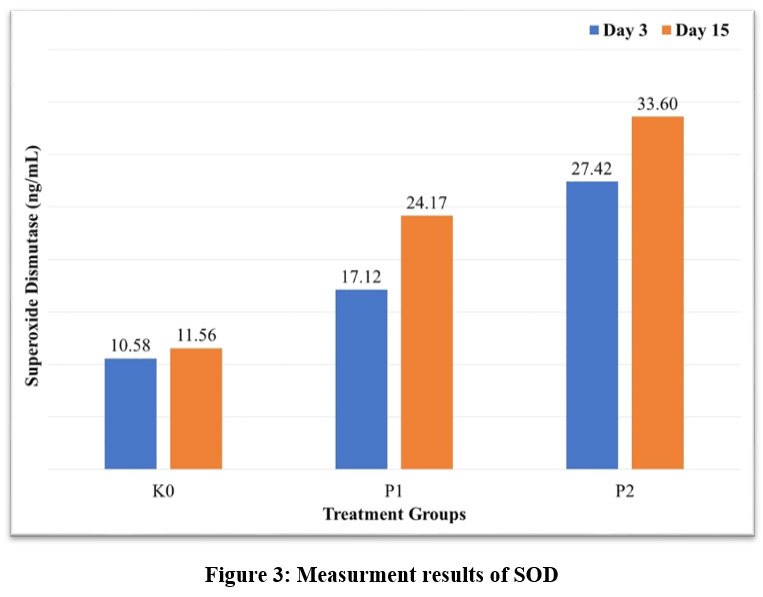 |
Figure 3: Measurment results of SODClick here to View Figure |
There may be 11 different types of chemicals in the ethanol extract from Etlingera elatior, as indicated by the chromatogram data shown in the Figure 3, which show 11 peaks at various retention durations. Following examination, just seven peaks could be found. The molecule is suspected to be Hemigossypol; I, according to the database-based LC-MS/MS identification results of Etlingera elatior extract ethanol. There may be a chemical called hemigossypol. This study suggested that the flavonoid-derived Hemigossypol I molecule might have antihyperglycemic properties.
Discussion
Decrease in blood glucose levels
Based on the findings, Etlingera elatior ethanol extract nanoparticles effectively reduced blood glucose levels in Wistar rats. A previous study using Etlingera elatior aqueous extract also showed that active compounds in Etlingera elatior seeds, including alkaloids and flavonoids, can significantly lower blood glucose levels in alloxan-induced type 2 diabetes rats 18. Another study investigating the effects of Etlingera elatior ethanol extract in streptozotocin-Nicotinamide (STZ-NA) induced diabetic rats revealed promising results in modulating diabetes-related complications, particularly in controlling both hyperglycemia and hypertension. The findings demonstrated a significant reduction in blood glucose levels, suggesting the extract’s potential to improve glycemic control. Additionally, the extract contributed to a reduction in blood pressure, indicating its potential role in managing diabetic hypertension 19. This reduction was likely due to secondary metabolites with antihyperglycemic and antioxidant properties. In hyperglycemic conditions, increased oxidative stress and decreased endogenous antioxidant levels contribute to the progression of diseases such as diabetes. Therefore, supplementary intake of natural antioxidants can help counteract this by inhibiting lipid peroxidation and protecting pancreatic beta cells from oxidative damage. Phytochemical analysis of the extract revealed the presence of polyphenolic compounds, alkaloids, and flavonoids, which are known to have a role in glucose metabolism and regulation 20.
In contrast to several other natural component preparations that have similar mechanisms as common antidiabetic drugs, including increased insulin secretion and sensitization, the mechanisms of antihyperglycemic and antioxidant properties in Etlingera elatior can be mediated by regulation of several enzymes such as catalase, superoxide dismutase, and malondialdehyde. Furthermore, the extract can inhibit α-glucosidase, slowing glucose absorption in the intestines and reducing postprandial blood glucose levels 20. Etlingera elatior extract can also modulate the polyol pathway by inhibiting aldose reductase, an enzyme linked to diabetic complications such as neuropathy and retinopathy, contributing to improved glucose management 20,21.
Based on the active component content, Etlingera elatior contains a wide variety of bioactive compounds that have proven to have antidiabetic effects. Based on the previous study, cyanidin-3-O-glycosides are in high levels among the anthocyanins group, providing strong antioxidant activity linked to the plant’s antidiabetic effects 19. Flavonols like quercetin and kaempferol also contribute by inhibiting α-glucosidase and reducing oxidative stress. Flavones such as apigenin and luteolin offer antioxidant and anti-inflammatory properties, further supporting diabetes management. Additionally, although not specifically identified, flavanols are abundant in the plant’s leaves, flowers, stems, and rhizomes, enhancing its antidiabetic potential 22.
The nanoparticle formulation used in this study enhances the bioavailability and targeted distribution of active compounds, leading to more efficient absorption and utilization. This formulation also allows for the gradual release of active ingredients, improving therapeutic outcomes. This mechanism also aligned with previous studies that reported silver nanoparticle-based carriers for antidiabetic drugs and pterostilbene extract can significantly improve drug bio-availability and substantially reduce side effects 23,24. Therefore, compared to conventional extracts, the nanoparticle-based extract demonstrates increased efficacy. The synergistic action between the nanoparticle formulation and bioactive compounds further amplifies its antihyperglycemic and antioxidant effects, offering a more comprehensive therapeutic approach for managing hyperglycemia.
Decrease in malondialdehyde levels
Based on the findings, the MDA level of Wistar rats given Etlingera elatior ethanol extract was significantly lower than the control group. This result also aligned with a study investigating the antioxidant activity of the flowers and leaves of Etlingera elatior (Jack) RM Sm, known as honje, using sheep blood as a model. The results indicated that the ethanol extract of Etlingera elatior flowers and the ethyl acetate extract of Etlingera elatior leaves showed the most significant reductions in MDA levels (27.24% and 31.91% at 20 ppm, respectively). The highest increase in SOD activity was observed with the ethyl acetate extract of Etlingera elatior flowers and the ethanol extract of Etlingera elatior leaves (133.99% and 144.29% at 20 ppm, respectively) 25,26. The decrease in malondialdehyde (MDA) levels, which serves as a key indicator of lipid peroxidation and oxidative stress, observed with the administration of Etlingera elatior extract, can be largely attributed to its strong antioxidant capabilities. The extract is rich in phenolic compounds and flavonoids, which play a crucial role in neutralizing free radicals and reactive oxygen species (ROS). By scavenging these harmful molecules, Etlingera elatior helps protect cellular lipids from oxidative damage, which in turn leads to a reduction in MDA production. This protective mechanism highlights the extract’s ability to mitigate oxidative stress, making it a valuable candidate for preventing or reducing oxidative damage in various pathological conditions where lipid peroxidation is a contributing factor.
Additionally, these antioxidant compounds inhibit lipid peroxidation by neutralizing free radicals, lowering MDA levels in biological systems 18,27. Previous studies have shown that supplementation with Etlingera elatior extract effectively reduces serum lipid hydroperoxides and protein carbonyl content, both of which are key indicators of lipid peroxidation. In addition to these reductions, the extract has been shown to enhance the activity of endogenous antioxidant enzymes, including superoxide dismutase (SOD) and glutathione peroxidase (GPx), providing further protection against oxidative stress. This increase in antioxidant enzyme activity also leads to a significant decrease in MDA levels, indicating the extract’s role in mitigating oxidative damage and supporting the body’s defense against free radical-induced cellular damage. Essential phytochemicals like quercetin and kaempferol contribute to these antioxidant effects by scavenging free radicals and regenerating other antioxidants, boosting the overall antioxidant capacity of the extract 28–30.
Increase in superoxide dismutase (SOD)
Based on the findings, the MDA level of Wistar rats given Etlingera elatior ethanol extract was significantly higher than the control group. This result also aligned with a previous study using rats exposed to lead acetate in drinking water (500 ppm) for 14 days, alone or in combination with different Etlingera elatior ethanol extract (50, 100, and 200 mg/kg). Exposure to lead acetate significantly increased lipid hydroperoxides, indicating elevated oxidative stress and lipid peroxidation, while simultaneously reducing total antioxidant capacity and the activity of key antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione S-transferase (GST). These changes reflect the damaging impact of lead-induced oxidative stress on cellular function. However, supplementation with Etlingera elatior extract markedly reversed these effects by lowering serum lipid hydroperoxide levels and significantly enhancing total antioxidant capacity. The activity of essential antioxidant enzymes was also restored, with notable improvements in superoxide dismutase, glutathione peroxidase, and malondialdehyde (MDA) levels, suggesting a protective effect of the extract against oxidative damage. These results indicate that Etlingera elatior may serve as a potent therapeutic agent in mitigating oxidative stress and protecting against lead-induced cellular damage 26.
Study limitation
This study has several limitations. First, the use of STZ-induced hyperglycemic Wistar rats as the sole model limits the clinical relevance of the findings, necessitating further research involving broader populations, including humans. Second, the relatively short duration of the study and therapy restricts the ability to assess and achieve long-term effects on key parameters such as glucose, MDA, and SOD levels. Third, the study’s design included only a single dose (50 mg/kg BW/day) and a limited number of groups, constraining the evaluation of dose-response relationships and differences between therapy doses. Additionally, pharmacokinetic and pharmacodynamic analyses of the nanoencapsulated formulation were not performed, leaving a gap in understanding its absorption, distribution, metabolism, and excretion. Lastly, while bioactive compounds were identified through LC-MS/MS, their specific biological roles remain unconfirmed, and long-term stability under various storage conditions was not assessed, further limiting the translation of findings to clinical applications.
Conclusion
The process of creating nano extracts involves encapsulating the bioactive compounds found in the ethanol extract of Etlingera elatior using a combination of chitosan and tripolyphosphate polymers serves as a significant structural framework for the nano-delivery system, which enhances the stability, bioavailability, and controlled release of these bioactive substances. However, several limitations were identified, including the use of a single animal model (Wistar rats) limiting clinical applicability, a short duration that hinders assessment of long-term effects, a limited number of groups reducing dose-response evaluation, and the absence of pharmacokinetic and pharmacodynamic analysis of the nanoencapsulated extract.
There are several recommendations for future studies, i.e nano extract of Etlingera elatior is expected to be developed as a nano herbal therapy for hyperglycemia by targeting the accessible radical pathway, with a focus on monitoring glucose, MDA, and blood SOD biomarkers, the encapsulation of Etlingera elatior nano extract into chitosan-tripolyphosphate nanoparticles via ionic gelation leads to changes in physical-chemical properties, including functional groups and zeta potential, which influence the particle size of bioactive compounds, and administration of Etlingera elatior nano extract has shown to decrease glucose and MDA levels, while increasing SOD, in streptozotocin-induced hyperglycemic Wistar rats.
Acknowledgement
Gratitude is expressed to all parties who helped and contributed to this research, thanks to the Dean of the Faculty of Mathematics and Natural Sciences, Udayana University for the opportunity given to the author.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethical Consideration
In this study, test animals were used, so the author validated the animal ethics approval certificate by the animal ethics committee of the Faculty of Veterinary Medicine, Udayana University with certificate number No: 70/UN14.2.9/PT/2022 dated September 2, 2024. In this study, no clinical trials were conducted on humans.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
Sri Wahjuni: First Author, Conceptualization, Methodology, Writing – Original Draft.
Ni Made Puspawati: Co Author, Data Collection, Analysis, Writing – Review & Editing.
Ni Ketut Puspasari:Co Author, Analysis, Editor
References
- Elsayed Azab A, A Adwas Almokhtar, Ibrahim Elsayed AS, A Adwas A, Ibrahim Elsayed Ata Sedik, Quwaydir FA. Oxidative stress and antioxidant mechanisms in human body. J Appl Biotechnol Bioeng. 2019;6(1):43-47.
CrossRef - Liu J, Han X, Zhang T, Tian K, Li Z, Luo F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: from mechanism to therapy. J Hematol Oncol. 2023;16(1):116.
CrossRef - Angie E, Girsang E, Ikhtiari R. Linking MDA Levels and Blood Glucose in Streptozotocin-Induced Rat Diabetes: Implications for Diabetic Complications and Therapeutic Strategies. J Penelit Pendidik IPA. 2024;10(6):2898-2905.
CrossRef - Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6(3):456-480.
CrossRef - Lei S, Liu Y, Liu H, Yu H, Wang H, Xia Z. Effects of N-acetylcysteine on nicotinamide dinucleotide phosphate oxidase activation and antioxidant status in heart, lung, liver and kidney in streptozotocin-induced diabetic rats. Yonsei Med J. 2012;53(2):294-303.
CrossRef - Jomova K, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Valko M. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch Toxicol. 2024;98(5):1323-1367.
CrossRef - Kafshgar MH, Khorram M, Khodadoost M, Khavari S. Reinforcement of chitosan nanoparticles obtained by an ionic cross-linking process. Iran Polym J (English Ed. 2011;20(5):445-456.
- Vimalkumar C, Hosagaudar V, Suja S, Vilash V, Krishnakumar N, Latha P. Comparative preliminary phytochemical analysis of ethanolic extracts of leaves of Olea dioica Roxb., infected with the rust fungus Zaghouania oleae (E.J. Butler) Cummins and non-infected plants. J Pharmacogn Phytochem. 2014;3(4):69-72.
- Akolade JO, Oloyede HOB, Onyenekwe PC. Encapsulation in chitosan-based polyelectrolyte complexes enhances antidiabetic activity of curcumin. J Funct Foods. 2017;35(C):584-594.
CrossRef - Marella S, Maddirela DR, Kumar EGT V, Tilak TK, Badri KR, Chippada A. Mcy protein, a potential antidiabetic agent: evaluation of carbohydrate metabolic enzymes and antioxidant status. Int J Biol Macromol. 2016;86:481-488.
CrossRef - Thilakarathna SH, Rupasinghe HPV. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5(9):3367-3387.
CrossRef - Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53(46):12320-12364.
CrossRef - Sahu T, Ratre YK, Chauhan S, Bhaskar LVKS, Nair MP, Verma HK. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J Drug Deliv Sci Technol. 2021;63:102487.
CrossRef - Mikušová V, Mikuš P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int J Mol Sci. 2021;22(17).
CrossRef - Sang Z, Qian J, Han J. Comparison of three water-soluble polyphosphate tripolyphosphate, phytic acid, and sodium hexametaphosphate as crosslinking agents in chitosan nanoparticle formulation. Carbohydr Polym. 2020;230:115577.
CrossRef - Jamal Gilani S, Nasser Bin-Jumah M, Al-Abbasi FA, Fustin ameliorates hyperglycemia in streptozotocin induced type-2 diabetes via modulating glutathione/Superoxide dismutase/Catalase expressions, suppress lipid peroxidation and regulates histopathological changes. Saudi J Biol Sci. 2021;28(12):6963-6971.
CrossRef - Azri FA, Selamat J, Sukor R, Yusof NA. Etlingera elatior-Mediated Synthesis of Gold Nanoparticles and Their Application as Electrochemical Current Enhancer. Molecules. 2019;24(17):3141.
CrossRef - Noordin L, Wan Ahmad WAN, Muhamad Nor NA, Abu Bakar NH, Ugusman A. Etlingera elatior Flower Aqueous Extract Protects against Oxidative Stress-Induced Nephropathy in a Rat Model of Type 2 Diabetes. Evid Based Complement Alternat Med. 2022;2022:2814196.
CrossRef - Widyarini T, Indarto D, Soetrisno S, Purwanto B. Modulation effects of Etlingera elatior ethanol extract as anti-inflammatory on chronic kidney disease in mice with hypertension and diabetes. J Popul Ther Clin Pharmacol = J la Ther des Popul la Pharmacol Clin. 2022;29(4):e140-e149.
CrossRef - Zumaidar Z, Asmilia N, Saudah S, Husnah M. In Vitro Alpha-Glucosidase Inhibitory Effect of Etlingera Elatior Ethanol Extract Growing in Gayo Highland, Aceh Province, Indonesia. F1000Research. 2024;13:489.
CrossRef - Nurjannah L, Azhari A, Wulandari AP, Amin S, Supratman U. Screening and evaluation of antidiabetic activities of endophytic fungi associated with Etlingera elatior. Biodiversitas. 2023;24(6):3481-3487.
CrossRef - Fitrianita A, Yardi Y, Musir A. Uji Efek Antihiperglikemia Ekstrak Etanol 70% Daun Kecombrang (Etlingera Elatior) pada Tikus Sprague Dawley dengan Penginduksi Aloksan. J Ilm Farm. 2018;14(1):9-16.
CrossRef - Zhao X, Shi A, Ma Q, Nanoparticles prepared from pterostilbene reduce blood glucose and improve diabetes complications. J Nanobiotechnology. 2021;19(1):1-18.
CrossRef - Paul S, Sarkar I, Sarkar N, Silver nanoparticles in diabetes mellitus: therapeutic potential and mechanistic insights. Bull Natl Res Cent. 2024;48(1).
CrossRef - Rachmatiah T, Kimura W, Kusmiati K. Aktivitas Antioksidan Ekstrak Etanol, Etil Asetat Bunga dan Daun Honje (Etlingera elatior (Jack) R.M. Sm) pada Darah Domba Terinduksi tert- Butil Hidroperoksida (t-BHP). Sainstech Farma. 2021;14(2):102-108. doi:10.37277/sfj.v14i2.1076
CrossRef - Jackie T, Haleagrahara N, Chakravarthi S. Antioxidant effects of Etlingera elatior flower extract against lead acetate – Induced perturbations in free radical scavenging enzymes and lipid peroxidation in rats. BMC Res Notes. 2011;4(1):67.
CrossRef - Ghasemzadeh A, Jaafar HZE, Rahmat A, Ashkani S. Secondary metabolites constituents and antioxidant, anticancer and antibacterial activities of Etlingera elatior (Jack) R.M.Sm grown in different locations of Malaysia. BMC Complement Altern Med. 2015;15:335.
CrossRef - Safrina U, Wardiyah, Cartika H. Evaluation of Total Flavonoid, Total Phenolic, and Antioxidant Activity of Etlingera elatior (Jack) R.M.Sm Flower, Fruit, and Leaf. Maj Obat Tradis. 2022;27(1):51-59.
CrossRef - Utami YP, Yulianty R, Djabir YY, Alam G. Antioxidant Activity, Total Phenolic and Total Flavonoid Contents of Etlingera elatior (Jack) R.M. Smith from North Luwu, Indonesia. Trop J Nat Prod Res. 2024;8(1):5955-5961.
CrossRef - Ghasemzadeh A, Jaafar HZE, Rahmat A, Ashkani S. Secondary metabolites constituents and antioxidant, anticancer and antibacterial activities of Etlingera elatior (Jack) R.M.Sm grown in different locations of Malaysia. BMC Complement Altern Med. 2015;15(1):1-10.
CrossRef








