Stefano Mastrangelo1 , Alberto Romano1
, Alberto Romano1 , Palma Maurizi1
, Palma Maurizi1 , Daniela Rizzo2
, Daniela Rizzo2 , Giorgio Attinà1
, Giorgio Attinà1 and Antonio Ruggiero1
and Antonio Ruggiero1
1Pediatric Oncology Unit, Fondazione Policlinico Universitario A.Gemelli IRCCS, Universita’ Cattolica Sacro Cuore, Rome, Italy.
2UOC Oncoematologia Pediatrica, P.O. "Vito Fazzi, Lecce, Italy.
Corresponding Author E-mail:antonio.ruggiero@unicatt.it
DOI : https://dx.doi.org/10.13005/bpj/3017
Abstract
Sarcopenia and malnutrition can coexist in pediatric patients with neoplasia, worsening the patient's prognosis. The classification of primary and secondary sarcopenia may be helpful in clinical practice, as it can help with timely initiation of appropriate and tailored dietary treatments to address it. This review summarizes the current state of the art of assessing skeletal muscle function in children and adolescents with cancer and discusses the role of nutritional interventions in the management of children with cancer. It highlights the urgent need for comprehensive nutritional support and interventions to mitigate the impact of malnutritions on both treatment outcomes and patients' well-being.
Keywords
Cancer; Children; Malnutrition; Natural Products; Sarcopenia
Download this article as:| Copy the following to cite this article: Mastrangelo S, Romano A, Maurizi P, Rizzo D, Attinà O, Ruggiero A. Nutritional Challenges in Paediatric Oncology: Screening and Managing Malnutrition and Sarcopenia. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Mastrangelo S, Romano A, Maurizi P, Rizzo D, Attinà O, Ruggiero A. Nutritional Challenges in Paediatric Oncology: Screening and Managing Malnutrition and Sarcopenia. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3ZcVMKh |
Introduction
Cancer is a significant cause of mortality among children and adolescents in the United States, ranking as the second most common cause of death for children aged 1–14 years and the fourth most common cause of death for adolescents aged 15–19 years. This highlights the impact of cancer on young people and the need for continued research and support for pediatric and adolescent cancer patients.
Children who have cancer frequently experience malnutrition. Numerous research investigations indicated that the incidence of this medical condition ranges widely with reports showing that it affects 6–51 % of children admitted to hospitals. Malnutrition in children with tumors is associated with higher energy needs and losses, as well as reduced intake of essential nutrients.1–5 Tumor-secreted proinflammatory cytokines increase metabolism and catabolism, leading to protein loss and faster oxidation of energy sources.6-14 Gastrointestinal issues (i.e., vomiting, nausea, etc) from chemotherapy toxicity can also contribute to increased energy losses. Chemotherapy can result in changes in taste, reduced appetite, and lower nutrient absorption, which may result in decreased appetite.15-28 All of these processes lead to neoplastic cachexia, a metabolic syndrome involving ongoing loss of muscle mass that cannot be fully reversed with typical nutritional aid. 29–32 This results in anorexia, muscle wasting, fatigue, abnormal biochemical levels, functional decline, and inadequate weight changes. 33-39
Among other predisposing factors, malnutrition of protein-energy can lead to sarcopenia. Sarcopenia refers to the gradual loss of mass, strength, and skeletal muscle function. Sarcopenia may occur in young people, such as children with cancer, related to malnutrition, aggravating its side effects and increasing patients’ sensitivity to various complications.
Sarcopenia, in contrast to malnutrition, is a new term in the pediatric literature. Sarcopenia in adults, which is a component of malnutrition, is defined as a decrease in skeletal muscle mass (SMM) and a decrease in muscle strength or physical performance.40 However, sarcopenia associated with decreased SMM in pediatrics has only recently been recognized. A pathological condition called sarcopenia is defined by a gradual and widespread loss of muscle mass – both quantitative and qualitative – as well as a deterioration in physical function. It is a leading cause of mortality, poor quality of life, loss of independence and physical disability.41 After age 50, muscle mass typically declines by 0–1 percent annually in women and 0.5–1 percent in men. This condition is typically associated with age. Nevertheless, certain pathological conditions characterized by systemic inflammation, such as cancer, endocrine disorders and chronic inflammatory diseases, can also lead to early development of sarcopenia due to the catabolic state that inflammatory cytokines induce in skeletal muscle.42,43 Sarcopenia and malnutrition may coexist in pediatric patients with neoplasia, worsening the patient’s prognosis. The co-occurrence of two clinical conditions, malnutrition and sarcopenia syndrome, has been documented in the adult literature, with notable overlap in treatment outcomes and adverse events.44, 45 Similar concepts regarding loss of muscle mass, changes in muscle function, and inadequate nutrient intake leading to deficits in fine motor skills, cognition, and nutrition are shared in the definitions of malnutrition and sarcopenia.
In this review, we want to emphasize the critical importance of early detection of malnutrition in pediatric cancer patients and the subsequent optimization of nutritional interventions within this population. This highlights the immense significance of regularly assessing the nutritional status and identifying the risk factors for the development of malnutrition. Additionally, the review presents practical tools that are highly applicable in daily clinical practice, further aiding in the battle against malnutrition.
Materials and Methods
Pathogenesis
Sarcopenia can be caused by various factors and progress through different mechanisms such as muscle fat content, neuromuscular integrity, proteolysis, and protein synthesis. Understanding these mechanisms and their causes can help in designing intervention trials. Some individuals may have a clear cause for sarcopenia, while others may not. Thus, primary and secondary classifications are used in clinical practice. Age-related sarcopenia is classified as secondary, while sarcopenia with additional causes is classified as primary. The multifactorial nature of sarcopenia in older adults may make classification challenging for each individual.46-56
Classification
The European Working Group on Sarcopenia in the Elderly (EWGSOP) recommends using the terms “presarcopenia”, “sarcopenia”, and “severe sarcopenia” to classify stages of muscle mass loss in the elderly. Presarcopenia, with low muscle mass but no impact on strength or performance, requires precise measurement methods for diagnosis. Sarcopenia is marked by low muscle mass, strength or performance, while severe sarcopenia meets all three criteria. Understanding these stages aids in selecting appropriate treatments and recovery goals. 57,58 EWGSOP has identified acute and chronic sarcopenia as subcategories, with acute cases lasting less than six months and chronic cases lasting longer. Regular evaluation is crucial to monitor progression and enable early intervention to prevent or slow the effects of sarcopenia (Figure 1).
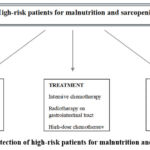 |
Figure 1: Detection of high-risk patients for malnutrition and sarcopenia |
Evaluation
Early and sufficient nutritional intervention can help hospitalized children avoid growth arrest, increase therapy tolerance, improve their quality of life, and shorten their hospital stay.59,60
Screening Tools
To identify children at risk and establish an appropriate nutritional support plan, the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and ESPEN recommend screening for nutritional risks at the time of hospital admission.61,62 Despite the fact that a number of pediatric nutritional risk assessments have been reported in research, screening for nutritional disorders is often not performed and there is no consensus on the “optimal” screening tool.63 Currently, there are seven primary screening tools for childhood nutritional risk: PNRS, SGNA, STAMP, PYMS, STRONGkids, and PNST. Patients at risk of malnutrition must undergo a specific nutritional assessment following the nutritional risk assessment. It is important to examine growth curves, the onset of puberty, psychomotor development, motor skills and swallowing ability, gastrointestinal complaints, weight fluctuations, medication intake, eating habits, dietary habits, allergies and food intolerances.64-66
Physical Examination
The physical examination assesses the patient’s general health and looks for evidence of specific nutritional deficiencies. Although no single laboratory test can provide a comprehensive assessment of nutritional status, laboratory data plays a complementary role in the assessment process. An inflammatory state is often associated with disease-related malnutrition, which may reduce the benefit of nutritional interventions. In acute inflammation or catabolic states, levels of acute phase proteins are increased, whereas albumin, prealbumin, retinol-binding protein and transferrin are reduced.67-73
Anthropometric Measurements
Although weight is a measure of a person’s overall nutritional status, a number of factors, including age, gender, daily intake and fluid intake, can affect a person’s weight. Standing patients older than two years should be weighed using a digital scale or a platform scale with movable weights. For patients who cannot stand, we use wheelchair or bed scales. When weighing a child under two years of age, it is best to place them on a scale on their back and make sure the weight is evenly distributed in the center of the scale.74 A critical metric for tracking long-term nutritional status is height, whether measured as length, height, or another variable. When measuring a child under two years old, he should lie on his back and use an infantometer. If possible, a vertical stadiometer mounted on the wall is used for children aged two and over. The head circumference of babies as young as 36 months can be measured using a flexible tape measure that is wrapped around the head. Head size should be viewed as a measure of nutritional status and brain development.
Weight-for-height, which corresponds to BMI in patients over two years of age, can be used as an evaluation measure in children under two years of age. The formula for calculating BMI is BMI=weight(kg)/height2 (m2). Due to the variability of age and gender, different specific BMI values are available for children. BMI can be used to determine obesity or overweight in children. The World Health Organization (WHO) has approved the use of BMI to measure thinness in adolescents, but underweight in children is defined as “low weight for age” and is not determined by BMI. Furthermore, because BMI ignores variations in body composition, it should not be the sole measure of a child’s nutritional status under clinical conditions. Mid upper arm circumference (MUAC) is a simple measurement that can be taken using a flexible tape measure positioned perpendicular to the extended axis of the arm. The midpoint of the upper arm, which is located between the olecranon and the acromion, is measured and marked. In patients with edema, MUAC is a more accurate measure of body composition than BMI because it is unaffected by fluid intake.
To measure the thickness of the triceps skinfold, place your thumb and index finger between the skin and subcutaneous fat over the MUAC point. Although it can also be helpful in identifying body fat depots in patients, this parameter is widely used in research. A cost-effective, non-invasive method for assessing the functional status of muscles is the handgrip test, which is performed using a portable dynamometer. The patient uses the dynamometer to subject their hand and forearm muscles to a series of movements designed to replicate their maximum strength. Grip strength is a useful tool for detecting malnutrition in children because dietary changes affect muscle function before muscle mass.
A child’s weight, length, height, weight length, or BMI are expressed as a percentile for age and sex on a bell-shaped reference curve constructed from population data. To compare a child’s position with a population of other children similar to him or her in terms of age and gender, a percentile is used to indicate the percentage of the population that remains above or below the measured value. However, according to the WHO, Z-scores would be a better way to express anthropometric measurements because percentiles do not accurately reflect the extent of the patient’s deviation from population norms. Z-scores, which compare each individual anthropometric measurement to data from reference age groups, are more sensitive than percentiles because they express the child’s deviation from the mean in standard deviation (SD).74
Body Composition Asssessment
Body composition can be routinely determined using several methods, including bioimpedance (BIA), dual-energy x-ray absorptiometry (DXA), and whole-body potassium counting (TBK).75,76 Despite the common use of these reference methods, each has certain practical limitations. A non-invasive technique for measuring body composition that can be used in patients of all ages is dual-energy X-ray absorptiometry (DXA). It is a quick, cost-effective and radiation-safe procedure.77 Another safe, noninvasive, and widely used technique for indirectly determining body composition is bioimpedance analysis (BIA). Its basis is the idea that the body’s ability to conduct an alternating electrical current can find a contact resistance (impedance) that is inversely proportional to the concentration of electrolyte and water.
Fat mass, lacking water and electrolytes, shows strong resistance to high impedance, while free-fat mass, composed of well-hydrated cells, exhibits lower impedance. Air within the lungs, parenchymal organs, and bones are not considered to be effective conductors and are therefore disregarded. Both resistance (R) and reactance (Xc) are components of the impedance (Z) that affects the flow of current through the body. Fat mass and extracellular water (ECW) are the main factors that primarily affect R. Xc, which is the capacity of normal cell membranes to take in an electric charge and then later discharge it, serves as a representation of the cellular mass in the body. The early detection of sarcopenic status can help with timely initiation of appropriate and tailored dietary treatments to address it. Different techniques are available in the medical field to assess muscle mass and diagnose sarcopenia.80 Anthropometric measurements like weight, BMI, mid-arm circumference, and triceps skinfold thickness can be easily influenced by illness and therapy and may not provide an accurate evaluation of body composition. Bone densitometry (DXA), bioelectrical impedance analysis (BIA), and air displacement plethysmography (ADP) are more accurate methods for body composition assessment, but their usage is restricted due to the need for specialized staff and equipment.81,82
CT and MRI are the top choices for measuring skeletal muscle mass in adults, with CT being the preferred method despite its high radiation levels. The radiation exposure from standard CT scans is a significant disadvantage when evaluating sarcopenia in children. Although MRI does not involve radiation, it is pricier than CT and might not be as easily accessible for regular monitoring. Yet, kids with chronic illnesses frequently undergo imaging as a component of their routine healthcare, enabling the assessment of muscle mass without any extra expenses or radiation exposure. 83,84
Results and Discussion
Examining the general development is essential when evaluating body composition in pediatrics, as it can influence the evaluation of sarcopenia in children. In puberty, there are significant differences in skeletal muscle mass and fat mass proportions, despite similar lean and fat mass in young boys and girls. During puberty, it has been observed that males typically see a larger boost in lean muscle mass due to hormonal factors like growth and sex hormones (estrogens, testosterone), whereas females tend to accumulate more fat mass. 85,86 At present, there is no definitive tool for assessing the decline in motor function in children undergoing evaluation for sarcopenia. Assessing muscle function in infants with standardized methods is difficult due to several factors influencing their motor performance, including postural control development, coordination, core stability, and ability to perform specific movements.
Illnesses can hinder the capability to perform specific muscle evaluations. Even though studies on sarcopenia in children are ongoing, evaluations in early childhood still do not include assessments of motor function. Handgrip and 6-minute walk tests are frequently employed among older children and teenagers to assess upper body strength and overall exercise capacity. These evaluations align with the suggested tests for detecting muscle function deficiencies in adults diagnosed with sarcopenia. While other tests for strength and performance have been used with children, their effectiveness is limited due to the lack of standardized protocols. 87-92
Measuring the psoas muscle area (SMA) from abdominal computed tomography (CT) images at the L3-L4 and L4-L5 levels is a quick, efficient, and reliable way to determine muscle mass.93,94 The skeletal muscle index (SMI) is calculated by multiplying the patient’s height squared (m2) by the SMA (cm2). Sarcopenia may be present if the SMI is below 55 cm2/m2 for men and 39 cm2/m2 for women. Examining the muscle mass of adult cancer patients, patients undergoing regular axial tomography re-evaluation scans, patients with liver disease, critically ill patients, and surgical patients has become a frequent occurrence. 95-101 Sarcopenia diagnosis has been used in patients with pediatric cancer, inflammatory bowel diseases, type 2 diabetes, end-stage liver disease, and intestinal failure.102-112 Recently, age- and sex-specific curves for total area of psoas muscle (tPMA) have been developed for pediatric patients aged 1 to 16. These curves allow for a quick assessment of sarcopenia and calculation of Z-Scores for PMA.113–119 Lurz et al. found that when examining tPMA at the L3–L4 and L4–L5 levels, the psoas muscle has a rounder shape, allowing for a more accurate contour design. As a result, tPMA at the L4–L5 level seems to be more important in pediatric patients. Additionally, a measurement taken at the L4-L5 reference level gives a reliable assessment of skeletal muscle and adipose tissue, as this level is commonly used to assess visceral adipose tissue.120-125 In more recent times, MRI has been used to measure the thickness of the temporal muscle as a way to detect sarcopenia, especially in patients with brain tumors.126-128
Clinicians should combine nutrition assessment with screening for sarcopenia to properly evaluate these two connected nutritional issues and ultimately enhance patients’ clinical outcomes. Several malnutrition screening tools are accessible, including the Malnutrition Screening Tool (MST), Malnutrition Universal Screening Tool (MUST), the short version of the Mini-Nutritional Assessment (SF-MNA), and Nutrition Risk Screening-2002 (NRS-2002).129-132
Indicators of history and physical examination are often seen in both tools, such as unintentional weight loss, reduced food consumption, digestive issues, and impairment in daily functioning. Additional studies are needed to establish the accuracy and effectiveness of this evaluation tool among various patient groups and environments.
Recent advances in muscle biology have unveiled new insights into the molecular mechanisms of sarcopenia and potential nutritional treatments. While the conventional recommendation for preventing age-related muscle weakness and loss involves a combination of resistance training and amino acid-containing supplements, it’s been found that protein-only supplements have little impact on sarcopenia symptoms. Candidate substances like catechins, soy isoflavones, and ursolic acid show promise in combating sarcopenia. These compounds have a crucial role in regulating proteins responsible for aging and inflammation-related signalling pathways. This regulation is key in the development of sarcopenia and its associated pathogenesis. Understanding these pathways and how they are affected by these compounds can provide valuable insights into potential interventions for sarcopenia and age-related inflammation.
Certain compounds derived from plants or food, known as phytochemicals, have been attracting attention for their remarkable antioxidant properties. Numerous in vivo and in vitro studies have unveiled the potential of phytochemicals, particularly polyphenols, in facilitating muscle recovery and potentially serving as a treatment for muscle atrophy. In various models of muscle damage, whether caused by pathological conditions or exhaustive exercise, compounds like curcumin and sulforaphane (SFN) have demonstrated their effectiveness in preventing or minimizing injuries to skeletal muscle mass. These remarkable compounds activate cytoprotective signaling pathways and trigger an optimal antioxidant response, combating inflammation and promoting the restoration of skeletal muscle. Specifically, curcumin has shown promising results in preventing muscle atrophy by inhibiting protein synthesis, primarily by regulating ubiquitin ligases.
Moreover, curcumin has demonstrated remarkable myogenic and mitochondrial qualities in both in vitro and in vivo studies. Currently, a variety of natural compounds, including terpenoids (such as ursolic acid, celastrol, and tanshinone IIA), polyphenols (like resveratrol, curcumin, and urolithin A), flavonoids (such as quercetin and apigenin), alkaloids (like tomatidine and magnoflorine), and vitamin D, are known for their ability to enhance muscle strength, increase muscle mass, facilitate muscle stem cell differentiation, promote mitochondrial biogenesis, and reduce hydrogen peroxide production and inflammation in skeletal muscles. These compounds have pleiotropic functions, but some of them also have specific targets and can regulate distinct signaling pathways. For instance, ursolic acid, resveratrol, curcumin, berberine, and others stimulate AMPK-mediated PGC-1α expression, inhibit the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and activate mitogen-activated protein kinase (MAPK) pathways associated with protein catabolism, thereby facilitating muscle myogenesis. Despite these promising findings, the efficacy of natural products compared to synthetic drugs is hindered by the limited research on their molecular mechanisms, bioavailability, and clinical trials. Nevertheless, herbal medicines and their purified components, such as polyphenols and alkaloids, continue to hold significant potential in the realm of muscle recovery and overall well-being. Additionally, further research is needed to fully elucidate the mechanisms involved and to identify specific compounds that can effectively target these pathways.133-139 One limitation of using natural products is that the studies often use amounts that exceed what would be consumed through typical food sources. This means that the effects observed may not accurately represent real-life scenarios. Additionally, the issue of bioavailability, such as in the case of curcumin, must be taken into account. However, this is not a concern with SFN found in broccoli, as other factors like preparation and cooking play a role. Lastly, it is crucial to conduct more studies involving human subjects. While research on cell lines and animal models is valuable for understanding the mechanisms of action, it is imperative to fully comprehend how these active compounds affect the human biological system.140
Conclusion
Malnutrition in cancer patients significantly impacts survival rates by lowering disease-free survival, increasing treatment-related mortality, and reducing chemotherapy tolerance. Malnutrition also negatively affects physical, emotional, and social well-being, hindering recovery and making cancer treatment more challenging.
Distinguishing between primary and secondary sarcopenia can be beneficial in a clinical setting, as it can aid in promptly starting suitable and personalized dietary interventions to manage it. This review outlines the current status of evaluating skeletal muscle function in children and adolescents with cancer and explores the impact of nutritional strategies in treating pediatric cancer patients.
Comprehensive nutritional support is crucial to alleviate these negative impacts and improve treatment outcomes and patient well-being.
Acknowledgement
The authors thank “Fondazione per l’Oncologia Pediatrica ONLUS” for their dedicated patient care and scientific support.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required
Clinical Trial Registration
This research does not involve any clinical trials
Author contributions
Stefano Mastrangelo, Antonio Ruggiero: Conceptualization, Methodology, Writing – Original Draft.
Alberto Romano, Palma Maurizi, Daniela Rizzo, Giorgio Attinà: Data Collection, Analysis, Writing – Review & Editing.
Antonio Ruggiero, Giorgio Attinà, Daniela Rizzo, Alberto Romano: Visualization, Supervision, Project Administration.
Antonio Ruggiero, Stefano Mastrangelo: Funding Acquisition, Resources, Supervision
References
- Kim E, Seol EM, Lee HJ. The Association of Body Mass Index on Falls Risk and Mortality in Hospitalized Patients of Different Old-Age Categories Requiring Nutritional Support. Clin Nutr Res. 2024;13(2):96-107. doi: 10.7762/cnr.2024.13.2.96.
CrossRef - Macedo C, Amaral TF, Rodrigues J, Santin F, Avesani CM. Malnutrition and Sarcopenia Combined Increases the Risk for Mortality in Older Adults on Hemodialysis. Front Nutr. 2021;8:721941. doi: 10.3389/fnut.2021.721941.
CrossRef - Mehta NM, Corkins MR, Lyman B, et al. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37(4):460-481. doi:10.1177/0148607113479972
CrossRef - Joosten KFM, Hulst JM. Prevalence of malnutrition in pediatric hospital patients. Curr Opin Pediatr. 2008;20(5):590-596. doi:10.1097/MOP.0b013e32830c6ede
CrossRef - Sala A, Pencharz P, Barr RD. Children, cancer, and nutrition–A dynamic triangle in review. Cancer. 2004;100(4):677-687. doi:10.1002/cncr.11833
CrossRef - Aprile G, Basile D, Giaretta R, Schiavo G, La Verde N, Corradi E, Monge T, Agustoni F, Stragliotto S. The Clinical Value of Nutritional Care before and during Active Cancer Treatment. Nutrients. 2021;13(4):1196. doi: 10.3390/nu13041196.
CrossRef - Altuntas AO, Slavin J, Smith PJ, et al. Accuracy of computed tomography guided core needle biopsy of musculoskeletal tumours. ANZ J Surg. 2005;75(4):187-191. doi:10.1111/j.1445-2197.2005.03332.x
CrossRef - Juby AG, Mager DR. A review of nutrition screening tools used to assess the malnutrition-sarcopenia syndrome (MSS) in the older adult. Clin Nutr ESPEN. 2019;32:8-15. doi: 10.1016/j.clnesp.2019.04.003
CrossRef - Landi F, Camprubi-Robles M, Bear DE, Cederholm T, Malafarina V, Welch AA, Cruz-Jentoft AJ. Muscle loss: The new malnutrition challenge in clinical practice. Clin Nutr. 2019;38(5):2113-2120. doi: 10.1016/j.clnu.2018.11.021
CrossRef - Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. 1980. Clin Orthop Relat Res. 2003;(415):4-18. doi:10.1097/01.blo.0000093891.12372.0f
CrossRef - Felipe TL, Grili PPDF, Vidigal CV, Albergaria BH, da Cruz GF, Marques-Rocha JL, Guandalini VR. Skeletal muscle mass obtained by anthropometric equation and presence of sarcopenia in postmenopausal women. Rev Bras Ginecol Obstet. 2024;46:e-rbgo9. doi: 10.61622/rbgo/2024AO09
CrossRef - Kundu ZS. Classification, imaging, biopsy and staging of osteosarcoma. Indian J Orthop. 2014;48(3):238-246. doi:10.4103/0019-5413.132491
CrossRef - Bernstein M, Kovar H, Paulussen M, et al. Ewing’s sarcoma family of tumors: current management. Oncologist. 2006;11(5):503-519. doi:10.1634/theoncologist.11-5-503
CrossRef - Donaldson SS, Torrey M, Link MP, et al. A multidisciplinary study investigating radiotherapy in Ewing’s sarcoma: end results of POG #8346. Pediatric Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42(1):125-135. doi:10.1016/s0360-3016(98)00191-6
CrossRef - Dunst J, Schuck A. Role of radiotherapy in Ewing tumors. Pediatr Blood Cancer. 2004;42(5):465-470. doi:10.1002/pbc.10446
CrossRef - Newberry C, Dakin G. Nutrition and Weight Management in the Elderly. Clin Geriatr Med. 2021;37(1):131-140. doi: 10.1016/j.cger.2020.08.010
CrossRef - Shapeero LG, Vanel D. Imaging evaluation of the response of high-grade osteosarcoma and Ewing sarcoma to chemotherapy with emphasis on dynamic contrast-enhanced magnetic resonance imaging. Semin Musculoskelet Radiol. 2000;4(1):137-146. doi:10.1055/s-2000-6861
CrossRef - Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi:10.1016/j.ejca.2008.10.026
CrossRef - Lourenço AP, Freitas C, Timóteo MH, Soares M, Figueiredo JP, Osório N, Valado A, Trapali M, Pereira T, Caseiro A. Laboratory Assessment of the Effects of AGA@4life Multidisciplinary Intervention on the Inflammatory Profile, MMPs, and TIMPs in a Geriatric Population. Healthcare (Basel). 2024;12(5):509. doi: 10.3390/healthcare12050509
CrossRef - Paulussen M, Craft AW, Lewis I, et al. Results of the EICESS-92 Study: two randomized trials of Ewing’s sarcoma treatment–cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clin Oncol. 2008;26(27):4385-4393. doi:10.1200/JCO.2008.16.5720
CrossRef - Ferrari S, Palmerini E, Alberghini M, et al. Vincristine, doxorubicin, cyclophosfamide, actinomycin D, ifosfamide, and etoposide in adult and pediatric patients with nonmetastatic Ewing sarcoma. Final results of a monoinstitutional study. Tumori. 2010;96(2):213-218.
CrossRef - Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694-701. doi:10.1056/NEJMoa020890
CrossRef - Ferrari S, Sundby Hall K, Luksch R, et al. Nonmetastatic Ewing family tumors: high-dose chemotherapy with stem cell rescue in poor responder patients. Results of the Italian Sarcoma Group/Scandinavian Sarcoma Group III protocol. Ann Oncol. 2011;22(5):1221-1227. doi:10.1093/annonc/mdq573
CrossRef - Castex M-P, Rubie H, Stevens MCG, et al. Extraosseous localized ewing tumors: improved outcome with anthracyclines–the French society of pediatric oncology and international society of pediatric oncology. J Clin Oncol. 2007;25(10):1176-1182. doi:10.1200/JCO.2005.05.0559
CrossRef - Oberlin O, Rey A, Desfachelles AS, et al. Impact of high-dose busulfan plus melphalan as consolidation in metastatic Ewing tumors: a study by the Société Française des Cancers de l’Enfant. J Clin Oncol. 2006;24(24):3997-4002. doi:10.1200/JCO.2006.05.7059
CrossRef - Skipworth RJE, Stewart GD, Dejong CHC, Preston T, Fearon KCH. Pathophysiology of cancer cachexia: much more than host-tumour interaction? Clin Nutr. 2007;26(6):667-676. doi:10.1016/j.clnu.2007.03.011
CrossRef - Mignini E V, Scarpellini E, Rinninella E, et al. Impact of patients nutritional status on major surgery outcome. Eur Rev Med Pharmacol Sci. 2018;22(11):3524-3533. doi:10.26355/eurrev_201806_15179
- Pereira T, Cipriano I, Costa T, Saraiva M, Martins A; AGA@4life Consortium. Exercise, ageing and cognitive function – Effects of a personalized physical exercise program in the cognitive function of older adults. Physiol Behav. 2019;202:8-13. doi: 10.1016/j.physbeh.2019.01.018
CrossRef - Brinksma A, Huizinga G, Sulkers E, Kamps W, Roodbol P, Tissing W. Malnutrition in childhood cancer patients: a review on its prevalence and possible causes. Crit Rev Oncol Hematol. 2012;83(2):249-275. doi:10.1016/j.critrevonc.2011.12.003
CrossRef - Rinninella E, Annetta MG, Serricchio ML, Dal Lago AA, Miggiano GAD, Mele MC. Nutritional support in acute pancreatitis: from physiopathology to practice. An evidence-based approach. Eur Rev Med Pharmacol Sci. 2017;21(2):421-432.
- Romano A, Capozza MA, Mastrangelo S, et al. Assessment and Management of Platinum-Related Ototoxicity in Children Treated for Cancer. Cancers (Basel). 2020;12(5):1266. doi: 10.3390/cancers12051266.
CrossRef - Fetoni AR, Ruggiero A, Lucidi D, et al. Audiological Monitoring in Children Treated with Platinum Chemotherapy. Audiol Neurootol. 2016;21(4):203-211. doi: 10.1159/000442435.
CrossRef - Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489-495. doi:10.1016/S1470-2045(10)70218-7
CrossRef - Sofia R, Melita V, De Vita A, et al. Cardiac Surveillance for Early Detection of Late Subclinical Cardiac Dysfunction in Childhood Cancer Survivors After Anthracycline Therapy. Front Oncol. 2021;11:624057. doi: 10.3389/fonc.2021.624057.
CrossRef - Triarico S, Agresti P, Rinninella E, et al. Oral Microbiota during Childhood and Its Role in Chemotherapy-Induced Oral Mucositis in Children with Cancer. Pathogens. 2022;11(4):448. doi: 10.3390/pathogens11040448.
CrossRef - Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793-799. doi:10.1016/j.clnu.2008.06.013
CrossRef - Meco D, Attinà G, Mastrangelo S, Navarra P, Ruggiero A. Emerging Perspectives on the Antiparasitic Mebendazole as a Repurposed Drug for the Treatment of Brain Cancers. Int J Mol Sci. 2023;24(2):1334. doi: 10.3390/ijms24021334
CrossRef - Co-Reyes E, Li R, Huh W, Chandra J. Malnutrition and obesity in pediatric oncology patients: causes, consequences, and interventions. Pediatr Blood Cancer. 2012;59(7):1160-1167. doi:10.1002/pbc.24272
CrossRef - Romano A, Del Vescovo E, Rivetti S, et al. Biomarkers Predictive of Metabolic Syndrome and Cardiovascular Disease in Childhood Cancer Survivors. J Pers Med. 2022;12(6):880. doi: 10.3390/jpm12060880.
CrossRef - Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412-423. doi:10.1093/ageing/afq034
CrossRef - Najm A, Niculescu AG, Grumezescu AM, Beuran M. Emerging Therapeutic Strategies in Sarcopenia: An Updated Review on Pathogenesis and Treatment Advances. Int J Mol Sci. 2024;25(8):4300. doi: 10.3390/ijms25084300.
CrossRef - Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16-31. doi:10.1093/ageing/afy169
CrossRef - Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3 JUL(July):1-18. doi:10.3389/fphys.2012.00260
CrossRef - Collocaa G, Di Capua B, Bellieni A, et al. Muscoloskeletal aging, sarcopenia and cancer. J Geriatr Oncol. 2019;10(3):504-509. doi:10.1016/j.jgo.2018.11.007
CrossRef - Gaspar-Silva F, Trigo D, Magalhaes J. Ageing in the brain: mechanisms and rejuvenating strategies. Cell Mol Life Sci. 2023;80(7):190. doi: 10.1007/s00018-023-04832-6
CrossRef - Loeffen EAH, Brinksma A, Miedema KGE, de Bock GH, Tissing WJE. Clinical implications of malnutrition in childhood cancer patients–infections and mortality. Support care cancer Off J Multinatl Assoc Support Care Cancer. 2015;23(1):143-150. doi:10.1007/s00520-014-2350-9
CrossRef - Timeus F, Crescenzio N, Longoni D, et al. Paroxysmal nocturnal hemoglobinuria clones in children with acquired aplastic anemia: a multicentre study. PLoS One. 2014;9(7):e101948. Published 2014 Jul 9. doi:10.1371/journal.pone.0101948
CrossRef - Chiaretti A, Ruggiero A, Barbi E, et al. Comparison of propofol versus propofol-ketamine combination in pediatric oncologic procedures performed by non-anesthesiologists. Pediatr Blood Cancer. 2011;57(7):1163-1167
CrossRef - Pribnow AK, Ortiz R, Báez LF, Mendieta L, Luna-Fineman S. Effects of malnutrition on treatment-related morbidity and survival of children with cancer in Nicaragua. Pediatr Blood Cancer. 2017;64(11). doi:10.1002/pbc.26590
CrossRef - Riccardi A, Mazzarella G, Cefalo G, et al. Pharmacokinetics of Temozolomide given three times a day in pediatric and adult patients. Cancer Chemother Pharmacol. 2003; 52: 459-464
CrossRef - Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27(1):5-15. doi:10.1016/j.clnu.2007.10.007
CrossRef - Lazzareschi I, Ruggiero A, Riccardi R, Attinà G, Colosimo C, Lasorella A. Hypersensitivity reactions to carboplatin in children. J Neurooncol. 2002; 58:33-37
CrossRef - Rogers PCJ. Nutritional status as a prognostic indicator for pediatric malignancies. J Clin Oncol. 2014;32(13):1293-1294. doi:10.1200/JCO.2014.55.0616
CrossRef - Chiaretti A, Aloe L, Antonelli A, et al. Neurotrophic factor expression in childhood low-grade astrocytomas and ependymomas. Childs Nerv Syst. 2004; 20:412-419
CrossRef - Trisciuzzi MT, Riccardi R, Piccardi M, et al. A fast visual evoked potential method for functional assessment and follow-up of childhood optic gliomas. Clin Neurophysiol. 2004; 115: 217-226
CrossRef - Sala A, Rossi E, Antillon F, et al. Nutritional status at diagnosis is related to clinical outcomes in children and adolescents with cancer: a perspective from Central America. Eur J Cancer. 2012;48(2):243-252. doi:10.1016/j.ejca.2011.06.006
CrossRef - Garg M, Gandhi K, Gera P, Jadhav SM, Mohanty B, Gurjar M, Sandupatla B, Gala R, Chaudhari P, Prasad M, Chinnaswamy G, Gota V. Implications of chronic moderate protein-deficiency malnutrition on doxorubicin pharmacokinetics and cardiotoxicity in early post-weaning stage. Life Sci. 2024;350:122765. doi: 10.1016/j.lfs.2024.122765
CrossRef - Brinksma A, Sanderman R, Roodbol PF, et al. Malnutrition is associated with worse health-related quality of life in children with cancer. Support care cancer Off J Multinatl Assoc Support Care Cancer. 2015;23(10):3043-3052. doi:10.1007/s00520-015-2674-0
CrossRef - Omer E, Chiodi C. Fat digestion and absorption: Normal physiology and pathophysiology of malabsorption, including diagnostic testing. Nutr Clin Pract. 2024 Apr;39 Suppl 1:S6-S16. doi: 10.1002/ncp.11130
CrossRef - Hartman C, Shamir R, Hecht C, Koletzko B. Malnutrition screening tools for hospitalized children. Curr Opin Clin Nutr Metab Care. 2012;15(3):303-309. doi:10.1097/MCO.0b013e328352dcd4
CrossRef - Agostoni C, Axelson I, Colomb V, et al. The need for nutrition support teams in pediatric units: a commentary by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2005;41(1):8-11. doi:10.1097/01.mpg.0000163735.92142.87
CrossRef - Ruggiero A, Maurizi P, Larocca LM, Arlotta A, Riccardi R. Childhood CD4+/CD56+ hematodermic neoplasm: case report and review of the literature. Haematologica. 2006;91(12 Suppl):ECR48.
CrossRef - Teixeira AF, Viana KDAL. Nutritional screening in hospitalized pediatric patients: a systematic review. J Pediatr (Rio J). 2016;92(4):343-352. doi:10.1016/j.jped.2015.08.011
CrossRef - Ruggiero A, Rizzo D, Catalano M, Coccia P, Triarico S, Attiná G. Acute chemotherapy-induced nausea and vomiting in children with cancer: Still waiting for a common consensus on treatment. J Int Med Res. 2018;46(6):2149-2156. doi: 10.1177/0300060518765324.
CrossRef - Joosten KFM, Hulst JM. Nutritional screening tools for hospitalized children: methodological considerations. Clin Nutr. 2014;33(1):1-5. doi:10.1016/j.clnu.2013.08.002
CrossRef - Ruggiero A, Rizzo D, Trombatore G, Maurizi P, Riccardi R. The ability of mannitol to decrease cisplatin-induced nephrotoxicity in children: real or not?. Cancer Chemother Pharmacol. 2016;77(1):19-26.
CrossRef - Erkan T. Methods to evaluate the nutrition risk in hospitalized patients. Turk Pediatr Ars. 2014;49(4):276-281. doi:10.5152/tpa.2014.2226
CrossRef - Huysentruyt K, Devreker T, Dejonckheere J, De Schepper J, Vandenplas Y, Cools F. Accuracy of Nutritional Screening Tools in Assessing the Risk of Undernutrition in Hospitalized Children. J Pediatr Gastroenterol Nutr. 2015;61(2):159-166. doi:10.1097/ MPG.0000000000000810
CrossRef - Ruggiero A, Rizzo D, Mastrangelo S, Battaglia D, Attinà G, Riccardi R. Interactions between antiepileptic and chemotherapeutic drugs in children with brain tumors: is it time to change treatment?. Pediatr Blood Cancer. 2010;54(2):193-198
CrossRef - Falsini B, Ziccardi L, Lazzareschi I, et al. Longitudinal assessment of childhood optic gliomas: relationship between flicker visual evoked potentials and magnetic resonance imaging findings. J Neurooncol. 2008; 88: 87-96.
CrossRef - Xu E, Chen C, Fu J, et al. Dietary fatty acids in gut health: absorption, metabolism and function. Anim Nutr. 2021;7(4):1337-1344. doi:10.1016/j.aninu.2021.09.010
CrossRef - Ruggiero A, Barone G, Liotti L, Chiaretti A, Lazzareschi I, Riccardi R. Safety and efficacy of fentanyl administered by patient controlled analgesia in children with cancer pain. Support Care Cancer. 2007 May;15(5):569-73. doi: 10.1007/s00520-006-0193-8.
CrossRef - Jensen GL. Inflammation as the key interface of the medical and nutrition universes: a provocative examination of the future of clinical nutrition and medicine. JPEN J Parenter Enteral Nutr. 2006;30(5):453-463. doi:10.1177/0148607106030005453
CrossRef - Triarico S, Rinninella E, Attinà G, Romano A, Maurizi P, Mastrangelo S, Ruggiero A. Nutritional status in the pediatric oncology patients. Front Biosci (Elite Ed). 2022;14(1):4. doi: 10.31083/j.fbe1401004.
CrossRef - Green Corkins K, Teague EE. Pediatric Nutrition Assessment. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2017;32(1):40-51. doi:10.1177/0884533616679639
CrossRef - Lazzer S, Bedogni G, Agosti F, De Col A, Mornati D, Sartorio A. Comparison of dual-energy X-ray absorptiometry, air displacement plethysmography and bioelectrical impedance analysis for the assessment of body composition in severely obese Caucasian children and adolescents. Br J Nutr. 2008;100(4):918-924. doi:10.1017/S0007114508922558
CrossRef - Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care. 2008;11(5):566-572. doi:10.1097/MCO.0b013e32830b5f23
CrossRef - Ooi PH, Thompson-Hodgetts S, Pritchard-Wiart L, Gilmour SM, Mager DR. Pediatric Sarcopenia: A Paradigm in the Overall Definition of Malnutrition in Children? J Parenter Enter Nutr. 2020;44(3):407-418. doi:10.1002/jpen.1681
CrossRef - Carvalho do Nascimento PR, Poitras S, Bilodeau M. How do we define and measure sarcopenia? Protocol for a systematic review. Syst Rev. 2018;7(1):1-9. doi:10.1186/s13643-018-0712-y
CrossRef - Shuhada NA, Aziz A, Mohd NI, et al. Assessing the nutritional status of hospitalized elderly. Clin Interv Aging. 2017;12:1615-1625. doi:10.2147/CIA.S140859
CrossRef - Cooper PC, Fielding PR, Mayer J, et al. Tools in the assessment of sarcopenia Europe PMC Funders Group. Calcif Tissue Int. 2013;93(3):201-210. doi:10.1007/s00223-013-9757-z.Tools
CrossRef - Orsso CE, Tibaes JRB, Oliveira CLP, et al. Low muscle mass and strength in pediatrics patients: Why should we care? Clin Nutr. 2019;38(5):2002-2015. doi:10.1016/j.clnu.2019.04.012
CrossRef - Sottier D, Petit J-M, Guiu S, et al. Quantification of the visceral and subcutaneous fat by computed tomography: interobserver correlation of a single slice technique. Diagn Interv Imaging. 2013;94(9):879-884. doi:10.1016/j.diii.2013.04.006
CrossRef - Triarico S, Rinninella E, Mele MC, Cintoni M, Attinà G, Ruggiero A. Prognostic impact of sarcopenia in children with cancer: a focus on the psoas muscle area (PMA) imaging in the clinical practice. Eur J Clin Nutr. 2022;76(6):783-788. doi: 10.1038/s41430-021-01016-y.
CrossRef - Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16(1):10-15. doi:10.1097/med.0b013e328320d54c
CrossRef - Griffiths A, Toovey R, Morgan PE, Spittle AJ. Psychometric properties of gross motor assessment tools for children: a systematic review. BMJ Open. 2018;8(10):e021734. doi:10.1136/bmjopen-2018-021734
CrossRef - Bianco A, Jemni M, Thomas E, et al. A systematic review to determine reliability and usefulness of the field-based test batteries for the assessment of physical fitness in adolescents -The ASSO Project. Int J Occup Med Environ Health. 2015;28(3):445-478. doi:10.13075/ijomeh.1896.00393
CrossRef - Geiger R, Strasak A, Treml B, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150(4):395-399, 399.e1-2. doi:10.1016/j.jpeds.2006.12.052
CrossRef - Fernandez-Santos JR, Ruiz JR, Cohen DD, Gonzalez-Montesinos JL, Castro-Piñero J. Reliability and Validity of Tests to Assess Lower-Body Muscular Power in Children. J strength Cond Res. 2015;29(8):2277-2285. doi:10.1519/JSC.0000000000000864
CrossRef - Nunez-Gaunaurd A, Moore JG, Roach KE, Miller TL, Kirk-Sanchez NJ. Motor proficiency, strength, endurance, and physical activity among middle school children who are healthy, overweight, and obese. Pediatr Phys Ther Off Publ Sect Pediatr Am Phys Ther Assoc. 2013;25(2):130-138; discussion 139. doi:10.1097/PEP.0b013e318287caa3
CrossRef - Silva PFS, Quintino LF, Franco J, Faria CDCM. Measurement properties and feasibility of clinical tests to assess sit-to-stand/stand-to-sit tasks in subjects with neurological disease: a systematic review. Brazilian J Phys Ther. 2014;18(2):99-110. doi:10.1590/s1413-35552012005000155
CrossRef - Zaino CA, Marchese VG, Westcott SL. Timed up and down stairs test: preliminary reliability and validity of a new measure of functional mobility. Pediatr Phys Ther Off Publ Sect Pediatr Am Phys Ther Assoc. 2004;16(2):90-98. doi:10.1097/01.PEP.0000127564.08922.6A
CrossRef - Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to assessment of muscle mass and myosteatosis on computed tomography: A systematic review. Journals Gerontol – Ser A Biol Sci Med Sci. 2019;74(10):1671-1678. doi:10.1093/gerona/glz034
CrossRef - Anjanappa M, Corden M, Green A, et al. Sarcopenia in cancer: Risking more than muscle loss. Tech Innov Patient Support Radiat Oncol. 2020;16:50-57. doi:10.1016/j.tipsro.2020.10.001
CrossRef - van Vugt JLA, Levolger S, de Bruin RWF, van Rosmalen J, Metselaar HJ, IJzermans JNM. Systematic Review and Meta-Analysis of the Impact of Computed Tomography–Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am J Transplant. 2016;16(8):2277-2292. doi:10.1111/ajt.13732
CrossRef - Giusto M, Lattanzi B, Albanese C, et al. Sarcopenia in liver cirrhosis: The role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol. 2015;27(3):328-334. doi:10.1097/MEG.0000000000000274
CrossRef - Ferrara P, Marrone G, Emmanuele V, et al. Homotoxicological remedies versus desmopressin versus placebo in the treatment of enuresis: a randomised, double-blind, controlled trial. Pediatr Nephrol. 2008;23(2):269-274.
CrossRef - Yeh DD, Ortiz-Reyes LA, Quraishi SA, et al. Early nutritional inadequacy is associated with psoas muscle deterioration and worse clinical outcomes in critically ill surgical patients. J Crit Care. 2018;45:7-13. doi:10.1016/j.jcrc.2017.12.027
CrossRef - Falsini B, Iarossi G, Chiaretti Aet al. NGF eye-drops topical administration in patients with retinitis pigmentosa, a pilot study. J Transl Med. 2016;14:8. doi: 10.1186/s12967-015-0750-3.
CrossRef - Akahoshi T, Yasuda M, Momii K, et al. Sarcopenia is a predictive factor for prolonged intensive care unit stays in high‐energy blunt trauma patients. Acute Med Surg. 2016;3(4):326-331. doi:10.1002/ams2.195
CrossRef - Moisey LL, Mourtzakis M, Cotton BA, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17(5):1. doi:10.1186/cc12901
CrossRef - Mangus RS, Bush WJ, Miller C, Kubal CA. Severe Sarcopenia and Increased Fat Stores in Pediatric Patients with Liver, Kidney, or Intestine Failure. J Pediatr Gastroenterol Nutr. 2017;65(5):579-583. doi:10.1097/MPG.0000000000001651
CrossRef - Lurz E, Patel H, Frimpong RG, et al. Sarcopenia in Children With End-Stage Liver Disease. J Pediatr Gastroenterol Nutr. 2018;66(2):222-226. doi:10.1097/MPG.0000000000001792
CrossRef - Chiaretti A, Ruggiero A, Barone G, et al. Propofol/alfentanil and propofol/ketamine procedural sedation in children with acute lymphoblastic leukaemia: safety, efficacy and their correlation with pain neuromediator expression. Eur J Cancer Care. (Engl) 2010;19(2):212-220
CrossRef - Suzuki D, Kobayashi R, Sano H, Hori D, Kobayashi K. Sarcopenia after induction therapy in childhood acute lymphoblastic leukemia: its clinical significance. Int J Hematol. 2018;107(4):486-489. doi:10.1007/s12185-017-2388-9
CrossRef - López JJ, Cooper JN, Albert B, Adler B, King D, Minneci PC. Sarcopenia in children with perforated appendicitis. J Surg Res. 2017;220:1-5. doi:10.1016/j.jss.2017.05.059
CrossRef - Zhang H, Tao Y, Wang Z, Lu J, Bhatt GC. Evaluation of nutritional status and prognostic impact assessed by the prognostic nutritional index in children with chronic kidney disease. Med (United States). 2019;98(34). doi:10.1097/MD.0000000000016713
CrossRef - Kawakubo N, Kinoshita Y, Souzaki R, et al. The Influence of Sarcopenia on High-Risk Neuroblastoma. J Surg Res. 2019;236:101-105. doi:10.1016/j.jss.2018.10.048
CrossRef - Lurz E, Patel H, Lebovic G, et al. Paediatric reference values for total psoas muscle area. J Cachexia Sarcopenia Muscle. 2020;11(2):405-414. doi:10.1002/jcsm.12514
CrossRef - Morrell GR, Ikizler TA, Chen X, et al. Psoas Muscle Cross-sectional Area as a Measure of Whole-body Lean Muscle Mass in Maintenance Hemodialysis Patients. J Ren Nutr. 2016;26(4):258-264. doi:10.1053/j.jrn.2016.02.002
CrossRef - Anita. Assessment of malnutrition using Z-scores and Composite Index of Anthropometric Failure among street children in Delhi. Nutrition. 2024;125:112487. doi: 10.1016/j.nut.2024.112487
CrossRef - Mengqin Z, Yalin H, Xing L, Ya L, Yalin T, Xin D, Jianhua R. Trends in nutritional status and factors affecting prognostic nutritional index in ovarian cancer patients during chemotherapy: a prospective longitudinal study based on generalized estimating equations. Support Care Cancer. 2024;32(3):191. doi: 10.1007/s00520-024-08384-8
CrossRef - Posteraro B, Bruno S, Boccia S, et al. Candida parapsilosis bloodstream infection in pediatric oncology patients: results of an epidemiologic investigation. Infect Control Hosp Epidemiol. 2004;25(8):641-5. doi: 10.1086/502454.
CrossRef - Jin Y, Ma X, Yang Z, Zhang N. Low L3 skeletal muscle index associated with the clinicopathological characteristics and prognosis of ovarian cancer: a meta-analysis. J Cachexia Sarcopenia Muscle. 2023 Apr;14(2):697-705. doi: 10.1002/jcsm.13175.
CrossRef - Ruggiero A, Rizzo D, Attinà G, Lazzareschi I, Mastrangelo S, Maurizi P, Migliorati R, Bertolini P, Pastore M, Colosimo C, Riccardi R. Phase I study of temozolomide combined with oral etoposide in children with recurrent or progressive medulloblastoma. Eur J Cancer. 2010 Nov;46(16):2943-9.
CrossRef - Su Y, Wu Y, Li C, Sun T, Li Y, Wang Z. Sarcopenia among treated cancer patients before and after neoadjuvant chemotherapy: a systematic review and meta-analysis of high-quality studies. Clin Transl Oncol. 2024 Mar 12. doi: 10.1007/s12094-024-03421-8.
CrossRef - Vogele D, Mueller T, Wolf D, Otto S, Manoj S, Goetz M, Ettrich TJ, Beer M. Applicability of the CT Radiomics of Skeletal Muscle and Machine Learning for the Detection of Sarcopenia and Prognostic Assessment of Disease Progression in Patients with Gastric and Esophageal Tumors. Diagnostics (Basel). 2024;14(2):198. doi: 10.3390/diagnostics14020198.
CrossRef - Giordano P, Lassandro G, Barone A, et al. Use of Eltrombopag in Children With Chronic Immune Thrombocytopenia (ITP): A Real Life Retrospective Multicenter Experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Front Med (Lausanne). 2020;7:66. doi: 10.3389/fmed.2020.00066.
CrossRef - Lien Y-C, Hsieh C-C, Wu Y-C, et al. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8(8):1041-1048. doi:10.1016/j.gassur.2004.09.033
CrossRef - Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14(2):381-389. doi:10.1245/s10434-006-9093-x
CrossRef - Nozoe T, Matsumata T, Sugimachi K. Preoperative elevation of serum C-reactive protein is related to impaired immunity in patients with colorectal cancer. Am J Clin Oncol. 2000;23(3):263-266. doi:10.1097/00000421-200006000-00011
CrossRef - Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140(9):1537-49. doi: 10.1007/s00432-014-1714-3
CrossRef - Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: A tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443. doi:10.1007/s00595-009-4065-y
CrossRef - Triarico S, Rinninella E, Cintoni M, et al. Impact of malnutrition on survival and infections among pediatric patients with cancer: a retrospective study. Eur Rev Med Pharmacol Sci. 2019;23(3):1165-1175. doi:10.26355/eurrev_201901_17009
- Lurz E, Patel H, Lebovic G, et al. Paediatric reference values for total psoas muscle area. J Cachexia Sarcopenia Muscle. 2020;11(2):405-414. doi:10.1002/jcsm.12514
CrossRef - Han JM, Kim DH, Lee BC. Association between the thickness or area of the temporal muscle and skeletal muscle mass in bioimpedance analysis. Gerontology. 2024 Apr 24. doi: 10.1159/000539063. Epub ahead of print.
CrossRef - Analay P, Kara M, Özçakar L. Temporal Muscle Thickness: A Far-Fetched Measurement in Sarcopenia. J Am Med Dir Assoc. 2024;25(4):671. doi: 10.1016/j.jamda.2024.02.008.
CrossRef - Borda MG, Baldera JP, Samuelsson J, et al. Temporal Muscle Thickness: A Practical Approximation for Assessing Muscle Mass in Older Adults. J Am Med Dir Assoc. 2024;25(4):664-670.e3. doi: 10.1016/j.jamda.2023.12.009.
CrossRef - Di Bella A, Croisier E, Blake C, Pelecanos A, Bauer J, Brown T. Assessing the Concurrent Validity and Interrater Reliability of Patient-Led Screening Using the Malnutrition Screening Tool in the Ambulatory Cancer Care Outpatient Setting. J Acad Nutr Diet. 2020;120(7):1210-1215. doi: 10.1016/j.jand.2019.10.015
CrossRef - Elia M. Screening for Malnutrition: A Multidisciplinary Responsibility. Development and Use of the ‘Malnutrition Universal Screening Tool’ (‘MUST’) for Adults. Worcestershire, UK: BAPEN; 2003.
- Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition the mini nutritional assessment. Clinics in Geriatric Medicine. 2002;18(4):737–757.
CrossRef - Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321-36. doi: 10.1016/s0261-5614(02)00214-5
CrossRef - Sakuma K, Hamada K, Yamaguchi A, Aoi W. Current Nutritional and Pharmacological Approaches for Attenuating Sarcopenia. Cells. 2023;12(19):2422. doi: 10.3390/cells12192422
CrossRef - Zhao X, Yuan F, Wan H, Qin H, Jiang N, Yu B. Mechanisms of magnoliae cortex on treating sarcopenia explored by GEO gene sequencing data combined with network pharmacology and molecular docking. BMC Genom Data. 2022;23(1):15. doi: 10.1186/s12863-022-01029-x
CrossRef - Zhang G, Jin C, Zhu Y, Fu F, Wang G, Li S. Sulforaphene inhibits the progression of osteosarcoma via regulating FSTL1/NF-κB pathway. Life Sci. 2020;263:118485. doi:10.1016/j.lfs.2020.118485
CrossRef - Gasmi A, Gasmi Benahmed A, Shanaida M, et al. Anticancer activity of broccoli, its organosulfur and polyphenolic compounds. Crit Rev Food Sci Nutr. 2023:1-19. doi: 10.1080/10408398.2023.2195493
CrossRef - Shalaby N, Zaki HF, Badary OA, et al. Efficacy and Toxicity of Vincristine and CYP3A5 Genetic Polymorphism in Rhabdomyosarcoma Pediatric Egyptian Patients. Asian Pac J Cancer Prev. 2024;25(4):1391-1409. doi: 10.31557/APJCP.2024.25.4.1391
CrossRef - Özdemir B, Gerçeker GÖ, Özdemir EZ, Yildirim BG, Ören H, Yiş U, Günay Ç, Thomas GÖ. Evaluation of vincristine-induced peripheral neuropathy in children with cancer: Turkish validity and reliability study. J Pediatr Nurs. 2023;72:185-190. doi: 10.1016/j.pedn.2023.04.006
CrossRef - Yadav A, Yadav SS, Singh S, Dabur R. Natural products: Potential therapeutic agents to prevent skeletal muscle atrophy. Eur J Pharmacol. 2022;925:174995. doi: 10.1016/j.ejphar.2022.174995
CrossRef - Vargas-Mendoza N, Madrigal-Santillán E, Álvarez-González I, et al. Phytochemicals in Skeletal Muscle Health: Effects of Curcumin (from Curcuma longa Linn) and Sulforaphane (from Brassicaceae) on Muscle Function, Recovery and Therapy of Muscle Atrophy. Plants. 2022; 11(19):2517. https://doi.org/10.3390/plants11192517)
CrossRef







