Phytochemicals, Anti-microbial and Anti-oxidant Investigations on Cassia fistula Fruit Pulp Extracts
Nirmala Arunachalam1* , Shivashankar Jayasankar1, Jayaprakash Rajendran2
, Shivashankar Jayasankar1, Jayaprakash Rajendran2 and Radhamani Thamilarasan1
and Radhamani Thamilarasan1
1Department of Biotechnology, Aarupadai Veedu Institute of Technology, Vinayaka Mission’s Research Foundation (Deemed to be University), Paiyanoor, Chennai, Tamil Nadu, India.
2Department of Chemistry, School of Arts and Science, Aarupadai Veedu Institute of Technology, Vinayaka Mission’s Research Foundation (Deemed to be University), Paiyanoor, Chennai, Tamil Nadu, India.
Corresponding Author E-mail: drnirmala81@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3044
Abstract
Plants are used as medicinal substances in many cultures and operate as a valuable reservoir for several powerful drugs owing to the existence of specific bioactive components. The process of discovering novel medications starts with the discovery of bioactive compounds derived from natural sources. Cassia fistula (Cf), also known as Konrai, is a natural plant belonging to the Caesalpiniaceae family. It is a significant tropical fruit and is renowned for its therapeutic characteristics. The current research aims to assess the phytochemical composition of the Cf fruit pulp by analysis of its aqueous and chloroform extracts. Additionally, the study will investigate the antioxidant and antimicrobial properties of these extracts. The analysis indicates the presence of beneficial phytochemicals except Cardiac glycosides, Polyphenol, and Anthroquinone. In FT-IR analysis, the phytochemical profile of the chloroform extracts demonstrated the existence of important phytochemical functional groups. Around 30 substances were found in the GCMS of the extract. Likely, the DPPH assay of the fruit pulp extracts exhibits the highest concentration (750μg/ml) and good antioxidant activity. Further, the antimicrobial activity was evaluated against disease-causing stains Staphylococcus aureus, E.coli, A.niger and C. albicans. The extract exhibited strong antibacterial activity in the chloroform extract compared to the aqueous extract. The findings indicated that chloroform extracts exhibited significant antifungal activity against A.niger in comparison to C.albicans. The aforementioned investigation validates that the chloroform extracts of Cf fruit pulp contains a diverse range of phytochemicals and other active functional groups, which are used for different therapeutic applications.
Keywords
Antioxidants activity; Cassia fistula; Medicinal properties; phytochemical analysis; Pulp extract
Download this article as:| Copy the following to cite this article: Arunachalam N, Jayasankar S, Rajendran J, Thamilarasan R. Phytochemicals, Anti-microbial and Anti-oxidant Investigations on Cassia Fistula Fruit Pulp Extracts. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Arunachalam N, Jayasankar S, Rajendran J, Thamilarasan R. Phytochemicals, Anti-microbial and Anti-oxidant Investigations on Cassia Fistula Fruit Pulp Extracts. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3ZfUzSz |
Introduction
Plants and the components they contain have been employed in traditional medicine for as long as humans have lived. Various medicinal plants with biological features such as antioxidant, anti-inflammatory, and anti-diabetic have shown therapeutic results in the management of health 1,2. Different phytochemicals, sometimes referred to as secondary metabolites, are found in plants. Because they can work alone, in combination, or synergistically to promote health, phytochemicals can be helpful in the treatment of many diseases 2. Phytochemicals are essential for the pharmaceutical industries for formulation of novel medications and medicinal substance 3. Various active phytochemicals of plant extracts are showing significant biological activities, such as anti-inflammatory, anti-diarrheal, anti-ulcer, and anticancer properties4. The golden shower tree, Cassia fistula Linn, is one such therapeutic plant found in nature. In tropical Africa, Bangladesh, Pakistan, and India, Cassia fistula (Cf) is a widely planted plant. The bulk of the naturally occurring bioactive compounds from Cf plant are secondary metabolites, and they are being used in medicines, dietary supplements, antibacterial properties and other useful industrial products 5,6. Reports of medicinal plants’ antimicrobial qualities are approaching from all over the world. By looking at Cf, this study seeks to increase the variety of antibacterial agents made from natural resources, a member of the Caesalpiniaceae subfamily of Leguminosae 7, 8. It has been suggested that this plant can aid with skin diseases, liver issues, tuberculosis, haematemesis, pruritus, leucoderma, and diabetes9.
Cf extract is used as an antiperiodic and to treat rheumatism. It has been established that plant components may be used as a medication to treat hyper cholesterolemia, in part due to their high fiber and mucilage content10. Hepato protective, anti-implantation, laxative, anti-inflammatory, smooth muscle stimulant, antiarthritic, antibacterial, antipyretic, antitussive, analgesic, antifungal, antiviral, and hypoglycemia are among the pharmacological effects of Cassia fistula 11, 12. Fruits can help to treat diabetes, act as an antipyretic, abortifacient, demulcent, and reduce body heat. They can also help with chest pain, throat issues, liver problems, eye disorders, and grasping problems 13,14. According to the previous literature, a lot of scientific studies were conducted on different parts of Cf plant but very limited study was witnessed in Cassia fistula fruit pulp. The current study examined the “Phytochemical analysis of Cf fruit pulp and its anti-oxidant and anti-microbial property” based on the previously mentioned information.
Materials and Methods
Collection and Preparation of Sample
For the study purpose, CfFruit (Fig-1(a) was collected from the forest area in and around Chennai, Tamil Nadu, India, where it was found naturally (Fig-1(b). The plant specimen authentication was confirmed in Plant Anatomy Research Centre (PARC) Chennai, by denoting the placed specimen. The voucher number of the specimen is PARC/ 2022/ 4819. Segregation of fruit pulp from fruit pod (Fig -2a) has been done. They were cut into small pieces. It was shade-dried for 4 -5 days and ground to a fine powder using a Mixer or Blender. To facilitate additional analysis, the sample was kept in a sterile container.
 |
Figure 1: (a). Collection of C. fistula Fruit, (b) C. fistula plant fruit with flower
|
The shade-dried powdered fruit pulp materials were extracted using two solvents -water and Chloroform at room temperature. The extract of the Cffruit pulp was prepared by soaking 10 g of fine powder in 100 ml of water and chloroform respectively and kept in Rotary Shaker apparatus for 24 hours as shown in Fig-2(b).
Phytochemical Analysis
To identify the different preliminary phytochemical constituents (Hexane, petroleum ether and water extracts) such as Terpeniods, Carbohydrates, Phytosterol, Alkaloids, Quinone, Saponin, Steriods, Flavonoids, Cardiac Glycosides, Glycosides, Anthroquinones, Polyphenols.Chemical tests were carried out on the plant extracts using standard procedure 15.
 |
Figure 2: a). Segregation of fruit pulp from fruit pod b). Extraction of C. fistula fruit pulp using chloroform and water
|
Characterization of CF pulp Extract
Fourier Transform Infrared (FTIR) Procedure
It is possible that Fourier Transform Infrared Spectrophotometer (FTIR) most efficient technique for identifying the different kinds of chemical bonds, commonly referred to as functional groups, present in various compounds. In this analysis, solvent extracts from dried powdered fruit pulp were employed. To prepare the samples, 10 ml of the extract was enclose within a 100 mg KBr pellet, resulting in translucent discs. Each pulverized samples from the plant specimens was then analyzed using an FTIR spectroscope (Shimadzu, IR Affinity 1, Japan), which operates with a resolution of cm-1 and a scanning range from 600 to 3600 cm-1.
Gas Chromatography-Mass Spectrometer (GC-MS) Analysis:
In the realm of phytochemical analysis and chemotaxonomic studies of medicinal plants containing biologically active compounds, the use of GCMS is essential. Before the Gas Chromatography-Mass Spectrometer (GC-MS) examination, roughly 1-gram dry extract was suspended in ethyl acetate, filtered through Whatman filter paper, and nitrogen-purged to eliminate any remaining ethanol. 1.0μL sample was introduced into a Shimadzu GCMS-QP2010 Ultra fitted with a Restek fused silica capillary column (30 m in length, 0.25 mm in diameter, and 0.25 film thickness made entirely of diphenyl dimethyl polysiloxane). The oven temperature was adjusted at a rate of 5 °C per minute, ranging from 70 °C (2 minutes) to 280 °C (30 minutes). The carrier gas, helium (99.999%), was employed at a steady flow rate of 1.84 mL min-1. 200°C was the ion source temperature, 119.2 kPa pressure, split injection mode (1:10), and injector temperature. Mass spectra were obtained between 40 and 600 m/z at a scan period of 0.5 s at 70 eV. For the extract, two duplicate injections total 35 minutes of GC running time were made. The relative percentage quantity of each component was ascertained by comparing its average peak area to the total area. The identification process involved in comparing the unknown component’s spectrum to the recognized compound’s spectra found in the National Institute Standard and Technique database.
DPPH assay
The total capacity of the extracts from various plant samples to scavenge free radicals was assessed based on a previously established method 16, with minor modifications, utilizing the stable DPPH radical. Prepare the 0.1 mM of DPPH solution in methanol and add 100 μl of this solution to 300 μl of the solution of samples respectively at different concentration (5, 10,15,20 μg/ml). The reaction mixture was allowed to incubate for 30 minutes in a dark environment at room temperature. Then the absorbance has to be measured at 517 nm using a UV-VIS spectrophotometer (Ascorbic acid can be used as the reference). Lower absorbance values of reaction mixture indicate higher free radical scavenging activity. The free radical scavenging activity of the extracts was indicated by the reduction of the initial purple hue. The capability of scavenging the DPPH radical can be calculated by using the following formula.
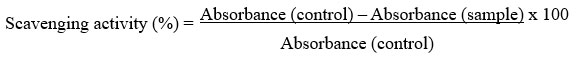
Antibacterial Activity
Using the agar well-diffusion method, the antibacterial activity of the extracts was evaluated against Gram-positive bacteria Staphylococcus aureus, Gram-negative bacteria Escherichia coli, fungus sps of Candida albicans and Aspergillus niger . Staphylococcus aureus and Escherichia coli were grown on nutrient agar (Himedia, India); whereas, Candida albicans and Aspergillus Niger were grown on Sabouraud Dextrose Agar. Antibacterial activity of extracts against bacterial strains was performed on Mueller Hinton agar. After that, the culture plates were incubated for 24 hours at 37 °C. Aspergillus niger cultures plates were kept at room temperature for three days. After incubation, the zone of inhibition(mm) was measured and recorded in each plate. The mean value of the three replicate trials was used to express the results.
Determination of minimum inhibitory concentration
Based on the ZOI findings, the minimum inhibitory concentration (MIC) of the extracts against Staphylococcus aureus was determined using the broth dilution technique. In summary, a 0.5 McFarland: 1.5 × 108 CFU/mL Staphylococcus aureus culture was introduced to the nutritional broth, which contained extracts diluted at different concentrations (10-1 to 10-7). The lowest concentration that prevents the organisms from growing visibly is known as the sample minimum inhibitory concentration or MIC. Using a UV-visible spectrophotometer, the growth of Staphylococcus aureus in the tubes was observed at 600 nm following a 24-hours incubation period. The minimum inhibitory concentration (MIC) was identified as the lowest concentration that demonstrated greater than 75% suppression of organism development.
Determination of minimum bactericidal concentration
For the purpose of measuring the minimum bactericidal concentration (MBC) of the extracts against Staphylococcus aureus, fresh nutrient agar plates were cultured with the broth used for the MIC test. MBC is the lowest medication concentration at which 99.9% of the tested microorganisms are killed. Every experiment was run three times, and the mean values were used to express the results.
Results and Discussion
Phyto constituents Screening of C. fistula fruit pulp extracts
To identify the presence of various chemical groups, the aqueous (A-CFFP) and chloroform (C-CFFP) extracts of the Cffruit pulp were subjected to preliminary phytochemical testing. Terpenoids, carbohydrates, phytosterol, alkaloids, quinone, saponin, steroids, flavonoids, cardiac glycosides, glycosides, anthraquinones, and polyphenols were all phyto chemicals analyzed in both extracts.
Aqueous and Chloroform extracts of Cf fruit pulp were subjected to phytochemical analysis (Table -1). The finding shows the presence of bioactive phytochemicals like terpenoids, carbohydrates, phytosterol, quinone, saponin, and cardiac glycosides but lacks anthroquinone, polyphenols, and cardiac glycosides. Alkaloids, flavonoids, and terpenoids are thought to have significant antioxidant properties. According to previousreport Cf fruit pulp contains more or less the same components of phytochemicals such as saponin, triterpenoids, steroids, glycosides, anthraquinone, flavonoids, gum, mucilage, proteins and amino acids17.Another study reported that Cf plant is rich in phenolic compnd, antioxidants such as anthraquinones, flavonoids and flavan 3-ol derivatives. The results shows positive for alkaloids, terpenoids, reducing sugars, saponins, tannins, carbonyl, phlobatanin, and steroids 18 . In the present study also, we observed the presence of many phytochemicals.
Table 1: Phytochemicals analysis of Cassia fistula fruit pulp extracts
|
Active Functional Group |
Aqueous fruit pulp extract of Cassia fistula |
Chloroform fruit pulp extract of Cassia fistula |
|
Terpenoids Test |
++ |
+++ |
|
Carbohydrate Test |
++ |
++ |
|
Phytosterol Test |
+ |
+++ |
|
Alkaloid Test |
– |
++ |
|
Quinone Test |
++ |
++ |
|
Saponin Test |
+ |
++ |
|
Steroids Test |
++ |
++ |
|
Flavonoids Test |
+ |
++ |
|
Cardiac Glycosides Test |
+ |
– |
|
Glycosides Test |
– |
+ |
|
Anthroquinone Test |
– |
– |
|
Polyphenol Test |
– |
– |
(-) Absent, (+) Present, (++) Moderate present , (+++) Highly present
FTIR spectrum of Cassia fistula fruit pulp extracts:
The functional molecules of the phytochemicals present in the plant extracts or other materials are identified using FTIR characterization studies. The peak values and most likely functional groups present in the fruit pulp of Cf are displayed in the FTIR spectrum of the chloroform extract. A peak was visible in the FT-IR spectra of chloroform extract of the Cf fruit pulp at 3286.70cm -1 which indicates the O-H alcohol group stretching; the peak at 2314.58cm -1 which indicates the Alkyne group; the peak at 1635.64cm -1 which indicates the Alkene group C=C; the peak at 678.94 cm -1 which indicates the halogen compound [iodo- compound CC-11]; the peak at 555.50 cm -1 which indicates the halogen compound [chloro-compound CC-1]; The bending at 408.91 cm-1 which indicates the presence of NH functional group in the Chloroform extracts of Cffruit pulp(Fig-3).
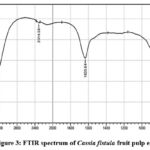 |
Figure 3: FTIR spectrum of Cassia fistula fruit pulp extracts
|
Functional molecules of the chloroform extract of Cf leaves were reported by previous study 18. Phenols are O-H stretch alcohols with an H bond, and this is related to the strong and wide band observed at 3258 cm-1. At 25 absorption peaks of 1042 and 878cm-1, there are narrow bands that have been recognized as C-N stretched aliphatic amines and N-H primary and secondary amines, respectively. This work concludes that functional groups including carboxylic acids, amines, and alcohol are present in the chloroform extract of Cf. These functional groups are connected to the bioactive phytochemicals present in it. In the current study, we found that the chloroform extracts of Cf fruit pulp had a broadband, which indicates the stretching of the O-H alcohol group, and the least narrow band, which shows the presence of NH functional group. In previous study report says that the active functional groups found in C. fistula hexane extract include phenols, alcohols, carboxylic acids, ethers, and ester. The leaf extract’s bioactive phytochemicals are linked to these functional groups19.
Gas Chromatography & Mass Spectrometer (GC-MS) Analysis of Chloroform extracts of Cassia Fistula Fruit Pulp:
A gas chromatogram derived from the chloroform extracts of Cf fruit pulp revealed many peaks, each of which represented a distinct chemical or functional component (Fig.4). Mass spectroscopy revealed the presence of 30 compounds in chloroform extracts of Cffruit pulp(Table -2). The identification of compounds was based on their Area, Retention time, Height and Compound name and Molecular formula. Gas Chromatography and Mass Spectrometer (GC-MS) screening of the Chloroform extract of Cffruit pulp revealed that the presence of compounds such 2-Ethoxyacetaldehyde, Cyclopent-one, tricosane, butanoic acid, (2E)-3,7-dimethylocta-2,6-dien-1-ol,Undecanoic acid,Cyclonoxiloxane Octadecane,6-methoxycyclohexane-1,2,3,4,5-pentol,1-Dodecanol,Tetracontane,n-Decanoicacid,1,4-ditert-butylbenzene,and Octadecanoic acid, remaining more components are present in it. The identification of compounds was based on their peak area (which represents the percentage of that compound), Retention time, Height, Compound name and molecular formula suggesting that Cf fruit pulp extracts showed the presence of many functional compounds and phytochemicals. It could be responsible for the therapeutic effects of the plant.
Table 2: Phytochemical constituents identified in Chloroform extracts of Cassia fistula fruit pulp by using GC-MS analysis
|
Retention |
Area (mv.s) |
Height (mv) |
Compound Name |
Molecular Formula |
|
3.027 |
179.112 |
2.168 |
2-Ethoxyacetaldehyde |
C4H8O2 |
|
4.000 |
2.399 |
0.374 |
Cyclopent-one |
C5H10 |
|
5.487 |
9418.607 |
1902.217 |
Not identified |
Not identified |
|
6.670 |
10.687 |
0.831 |
Tricosane |
C23H48 |
|
7.247 |
22.024 |
1.073 |
Butanoic acid |
C4H8O2 |
|
7.587 |
27.746 |
1.578 |
Not identified |
Not identified |
|
8.373 |
10.625 |
0.378 |
(2E)-3,7-dimethylocta-2,6-dien-1-ol |
C10H18O |
|
9.040 |
22.233 |
1.400 |
Not identified |
Not identified |
|
9.913 |
32.067 |
1.844 |
3,7,11-trimethyldodeca-2,6,10-trien-1-ol |
C15H26O |
|
10.837 |
143.230 |
3.782 |
Undecanoic acid |
C11H22O2 |
|
11.697 |
9.358 |
0.572 |
Cyclonoxiloxane Octadecane |
C18H38 |
|
12.823 |
2.176 |
0.183 |
6-methoxycyclohexane-1,2,3,4,5-pentol |
C7H14O6 |
|
13.637 |
105.977 |
3.925 |
1-Dodecanol |
C12H26O |
|
14.743 |
88.602 |
3.131 |
3,7,11,15-tetramethylhexadec-2-en-1-ol |
C20H40O |
|
15.187 |
102.252 |
3.183 |
Tetracontane |
C40H82 |
|
16.247 |
45.955 |
1.215 |
n-Decanoic acid |
C10H20O2 |
|
17.570 |
26.613 |
1.116 |
1,4-ditert-butylbenzene |
C14H22 |
|
18.613 |
375.956 |
20.481 |
Phytol-3,7,11,15-tetramethylhexadec-2-en-1-ol |
C20H40O |
|
19.527 |
76.354 |
2.602 |
Octadecanoic acid |
C18H36O2 |
|
20.027 |
44.018 |
1.993 |
3-hydroxy-3-methylpentanedioic acid |
C6H10O5 |
|
20.443 |
8.106 |
5.429 |
1,3-diaminothiourea |
CH6N4S |
|
22.040 |
160.128 |
5.266 |
4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene |
C15H24 |
|
26.510 |
5964.756 |
47.243 |
5-isothiocyanatopentylbenzene |
C12H15NS |
|
28.067 |
4010.379 |
44.131 |
7,11,15-trimethyl-3-methylidenehexadec-1-ene |
C20H38 |
|
28.710 |
8367.747 |
192.802 |
2,3-dihydroxypropyl octanoate |
C11H22O4 |
|
0.687 |
5.841 |
0.503 |
Not identified |
Not identified |
|
30.917 |
3062.129 |
143.482 |
Cholest-7-en-3-ol C27H46O |
C27H46O |
|
33.180 |
9864.300 |
136.368 |
Not identified |
Not identified |
|
34.460 |
2669.126 |
50.992 |
Phytol |
C20H40O |
|
36.040 |
117.332 |
5.059 |
n-propyl-9,12-octadecadienoate |
C21H38O2 |
According to previous research GC-MS analysis of chloroform extracts of Cf leaf revealed the presence of 13 components at various retention times20. The another research reported that nearly thirty one chemicals were found in the n-hexane fraction of the methanolic flower extract of C. fistula, according to the GC-MS chromatogram. With a peak area of 17.81%, butanoic acid, methyl ester, was the main component in this fraction. This substance is part of the methyl ester group of fatty acids. The majority of this group’s members have insecticidal21 antifungal22 and antibacterial properties23
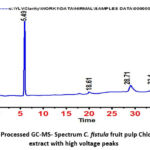 |
Figure 4: Processed GC-MS- Spectrum C. fistula fruit pulp Chloroform extract with high voltage peaks
|
Antioxidant activity of Chloroform extract of Cassia fistula fruit pulp:
The percentage of the antioxidant activity of the fruit pulp of chloroform extracts shows increased (150, 300, 450, 600, and 750μg/ml) antioxidant activity in a dose-dependent manner (Figure -5). At 150μg/ml, the fruit pulp extract exhibited a percentage inhibition of 2.5, while at 450μg/ml, it was 55. The fruit pulp extract’s 50% inhibition concentration (IC50) values were determined to be 2.5μg/ml and 55μg/ml, respectively.
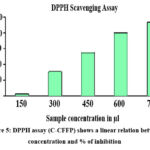 |
Figure 5: DPPH assay (C-CFFP) shows a linear relation between concentration and % of inhibition
|
The plant extract has a good linear relationship between concentration and inhibitory activity, according to analysis. Based on the stable DPPH free radical absorbing an electron from molecules, antioxidant activity is calculated. It can be distinguished visually by turning from brown to yellow. The antioxidant activity in this study was attributed to flavonoids, tannins, alkaloids, and phenols; these compounds destroy free radicals by donating an electron to DPPH. As a result, it is evident from the graph that the maximum concentration of the Cfchloroform extract has a greater ability to scavenge free radicals, specifically DPPH. The antioxidant activity of the Cf plant’s flower extract was mentioned in the early study17 .In past study report says that, flavonoids, phenols, and tannins function as free radical scavengers and may be the cause of the effects24. Other studies have shown that tannins, flavonoids25 phenol 23, 26, 27, saponins, and tannins have strong anti-inflammatory, antibacterial, and antioxidant properties. To assess the antioxidant qualities ofCf bark, stem, leaf, and root, a separate study was carried out. The findings indicated that extracts from the bark of various age groups exhibited greater antioxidant activity compared to the other plant parts 28.
A recent study assessed Cf Linn.’s antioxidant potential and ability to shield erythrocytes from hydrogen peroxide-induced oxidative damage. According to the results, Cf aqueous extract had a 75% antioxidant and protective efficacy, while its ethanolic extract had a strong antioxidant activity and shielded erythrocytes from damage by over 90% 24. We found that the A- CFFP and C-CFFP extracts from Cf exhibit strong antioxidant properties as concentrations rise in this investigation as well.
Zone of inhibition (ZOI) against selected bacteria
At varying dosages, such as 5 μg/ml, 25 μg/ml, 50 μg/ml, and 100 μg/ml, the antibacterial activity of the Cf fruit pulp extract (A-CFFP and C-CFFP) was evaluated against Gram-positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli). The extracts’ antibacterial activity dose in a linear fashion as their concentration (μg/ml) increased (Table 3). When the obtained results were contrasted with those of prescription medications, it became clear that both extracts showed strong antibacterial activity (Table -4).
Table 3: Antibacterial activity of the Cassia fistula fruit pulp extracts (A- CFFP and C- CFFP)
|
S.no |
Microorganism |
Zone of Inhibition (mm) |
|||||||
|
Aqueous extract (μg/ml) |
Chloroform extracts (μg/ml) |
||||||||
|
5 |
25 |
50 |
100 |
5 |
25 |
50 |
100 |
||
|
1. |
S. aureus |
– |
13 |
14 |
16 |
– |
15 |
16 |
20 |
|
2 |
E. coli |
– |
12 |
13 |
15 |
– |
12 |
14 |
17 |
Table 4: Antibacterial activity of standard drugs
|
S.no |
Drug (μg/mL) |
Zone of Inhibition (mm) |
|||||||
|
S. aureus |
E. coli |
||||||||
|
5 |
25 |
50 |
100 |
5 |
25 |
50 |
100 |
||
|
1. |
Ampicillin |
14 |
16 |
19 |
23 |
12 |
16 |
18 |
21 |
|
2 |
Chloramphenicol |
13 |
15 |
17 |
20 |
13 |
16 |
18 |
19 |
Zone of inhibition (ZOI) against selected Fungus
The fruit of Cf has antibacterial action, according to earlier study report 29. Cf at a 500 μg/ml concentration using the agar dilution streak method. Just E. coli was only slightly inhibited, although B. subtilis as well as S. epidermidis no inhibition. Nonetheless, our study demonstrated strong antibacterial efficacy against the two selected bacterial species. Earlier scientific study reported that use of disc diffusion and MIC techniques to examine the antibacterial qualities of Cf 30. The inhibition against S. aureus was stronger in alcoholic extracts as compared to aqueous extract. Furthermore, we found that, in comparison to aqueous extract, the fruit pulp of Cfexhibited higher inhibition in chloroform extract.
Table 5: Antifungal activity of Cassia fistula fruit pulp extracts (A- CFFP and C- CFFP)
|
S.no |
Microorganism |
Zone of Inhibition (mm) |
|||||||
|
Aqueous extract (μg/ml) ( A-CCFP) |
Chloroform extracts (μg/ml) (C- CFFP) |
||||||||
|
5 |
25 |
50 |
100 |
5 |
25 |
50 |
100 |
||
|
1. |
A. niger |
– |
13 |
14 |
17 |
– |
16 |
18 |
19 |
|
2 |
C. albicans |
– |
15 |
16 |
18 |
– |
12 |
15 |
17 |
Table 6: Antifungal properties of common medications
|
S.no |
Drug (μg/mL) |
Zone of Inhibition (mm) against selected micro-organisms (Fungal sps) |
|||||||
|
A. niger |
C. albicans |
||||||||
|
5 |
25 |
50 |
100 |
5 |
25 |
50 |
100 |
||
|
1. |
Greseofulvin |
12 |
15 |
18 |
20 |
13 |
15 |
19 |
22 |
|
2 |
Nystatin |
13 |
18 |
20 |
23 |
12 |
18 |
19 |
22 |
The efficacy of the Cf extracts (aqueous and chloroform) against certain fungus species was evaluated by measuring their zone of inhibition. As the quantities of the extracts (5 μg/ml, 25 μg/ml, 50 μg/ml, and 100 μg/ml) increased, their antifungal activity increased in a linear fashion. (Table -5). When the results were contrasted with those of common antifungal medications, it became clear that both extracts showed strong antifungal action (Table -6).
Finding of minimum inhibitory concentration (MIC):
From the ZOI results, MIC of both Cffruit pulp extract (A- CFFP and C- CFFP) against Staphylococcus aureus were determined by microdilution method. The MIC result represents that the growth of Staphylococcus aureus was inhibited (above 75%) by A- CFFP and C-CFFP at a dilution factor of 10 -3 to 10 -4 and 10 -4 to 10 -5, respectively. It confirms that C- CFFP exhibits high efficiency (high inhibition at low concentration) compared to A-CFFP.
Conclusion
From the current investigations concluded that the fruit pulp of Cf includes a variety of active phytochemicals. The results of this study demonstrate the existence of certain phytochemicals with biological activity that may have a significant therapeutic index. When creating novel medication formulations, the plant may be a safer substitute. Except for cardiac glycosides, polyphenol, and anthraquinone, a qualitative phytochemical examination disclosed the existence of alkaloids, saponins, flavonoids, carbohydrates, steroids, quinone, phytosterol, and terpenoids. FT-IR and GC-MS analyses were used to observe and confirm their active functional groups and phytoconstituents. The DPPH assay of the fruit pulp extracts exhibits the highest concentration (750μg/ml) and good antioxidant activity. The extract exhibited strong antibacterial activity in the chloroform extract compared to the aqueous extract. However, toxicological research and pharmacological activity testing should be done after isolating the pure chemical before use in humans. However, more research is required to more accurately assess the potential efficacy of the crude extracts.
Acknowledgment
The authors are grateful to Aarupadai Veedu Institute of Technology, Tamil Nadu, India for providing lab facilities and technical administrative support to complete the work successfully.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Authors contribution
Nirmala Arunachalam- work design , guiding the entire work , Manuscript preparation
Shivashankar Jayasankar Research Work done by the candidate
Jayaprakash Rajendran- Manuscript preparation and technical support
Radhamani Thamilarasan- Manuscript preparation and support
References
- Rahmani A.H and Aly S.M. Nigella sativa and its active constituents thymoquinon shows pivotal role in the diseases prevention and treatment. Asian. J. Pharm. Clin Res., 2015; 8 : 48–53.
- Rahmani A.H, Aly S.M, Ali H, Babiker A.Y, Srikar S and Khan A.A. Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, anti-oxidant and anti-tumour activity. Int. J. Clin. Exp. Med.,2014;7 : 83–91.
- Al-Bukhari M.I. Division 71 on medicine. In: Al-Bukhari S, editor. The Collection of Authentic Sayings of Prophet Mohammad (Peace be Upon Him) 2nd ed. Ankara, Turkey: Hilal Yayinlari .1976;81: 435-73.
- Satyavati G.Y and Sharma M. In Medicinal plant in India ICMR. NEW DELHI .1989;12: 5-32.
- Vashista P.C. Taxonomy of Agisosperme. P.B.M Press, New DELHI, India balunas M.J.and A.P. Kingholn .2005; 193.
- Dahanukar S.A, Kulkarani R.A and Rege N.N. Pharamacology of medicine plants and natural produce. Indian.J. Pharmacol.2000; 32: S81.
- Collett Henry. col. Sir. Flora simlensis (Flowering plants of simala) Bengal army New Connaught place , deharadun: Bishen singh mahandrapal singh . 1971; 108.
- Duuta A.C. Botany for degree students 6 th ed. Calcutta Oxford university press. 2002; 558.
- Asolkar L.V, Kakkar K.K and Chakre O.J. Second supplement to glossary of Indian medicinal plants with active principles. New Delhi publication and information directorate, CSIR. 1992; 177.
- EL-Saadany S.S, BL –massry R.A, Labib S.M and Sitohy M.Z. The biochemical role and hypo cholesterolaemic potential of the legume cassia fistula in hypercholesterolaemia rats. Die. Nahrung. 1991; 35: 807-15.
CrossRef - Pandey G, Dravyaguna vijiana 3 rd ed. Reprint part 3. Varanasi: chowkhamba krishnadas Academy. 2005; 167.
- Lavekar G.S. Database on medicinal plants in Ayurveda and siddha . vo; 6. Central for research in Ayurveda and siddha department of AYUSH, Ministry of health and family welfare , government of India .2009; 183.
- Kirtikar K.R and Basu B.D. Indian medicinal plants. International book distributors, 2006.
- Padma S. Indian .J .of Microbiol. 2006; 46(26): 169-170.
CrossRef - Harborne J.B. Textbook of Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. 5th Edition. Chapman and Hall Ltd, London,1998; 21-72.
- Dahanukar S.A, Kulkarni R.A and Rege N.N. Pharmacology of Medicinal Plants and Natural Products. Indian J Pharmacol. 2000; 32: S81-118.
- Bhalodia N.R, Nariya P.B, Acharya R.N and Shukla V.J. Evaluation of in vitro Antioxidant activity of Flowers of Cassia fistula Linn . Inter. Journ. of Pharm Tech Research., 2011; 3(1): 589-599.
CrossRef - Ashraf Ali, Cassia fistula linn: A review of phytochemical and pharmacological studies,Inter. Jour. of Pharm. Science and Research ., 2014; Vol. 5(6): 2125-2130.
- Sujatha J and Asokan S.Phytochemical analysis and Antioxidant effect of hexane extract of Cassia fistula using FT-IR and GC-MS analysis, Int. J. pharmaceutics & drug analysis, Vol.6 ISSUE 2, 2018; 173 – 179 ;
- Sujatha. Antioxidant effect and Phytochemical Analysis of Chloroform Extract of Cassis fistula using FT-IR, HPLC and GC-MS analysis. Int. J. Pharm. Sci. Rev. Res. 2017 ; 46(1): 129-133.
- De Meloa A.R, Garciaa I.J.P, Serrãob J.E, Santosa H.L, Limaa L.A.R.S and Alves S.N. Toxicity of different fatty acids and methyl esters on Culex quinquefasciatus larvae. Ecotoxicol. Environ. Saf. 2018;154: 1-5.
CrossRef - Suresh A, Praveenkumar R, Thangaraj R, Oscar FL, Baldev E, Dhanasekaran D and Thajuddin N. Microalgal fatty acid methyl ester a new source of bioactive compounds with antimicrobial activity. Asian Pac. J. Trop. Dis., 2014,4: S979-S984.
CrossRef - Ali A, Javaid A and Shoaib A . GC-MS analysis and antifungal activity of methanolic root extract of Chenopodium album against Sclerotium rolfsii. Planta Daninha 35: Article ID e017164713, 2017.
CrossRef - Kaur G.J and Arora D.S. Antibacterial and phytochemical screening of Anethumgraveolens, Foeniculumvulgare and Trachyspermumammi. BMC Compl Altern Med. 2009; 9: 30.
CrossRef - Lai H.Y, Yau Y.Y and Kim K.H. Blechnumorientale Linn – a fern with potential as antioxidant, anticancer and antibacterial agent. BMC. Complem. Altern. Med. 2010;10: 15.
CrossRef - Garg V.K.R, Jain M, Sharma P.K.R and Garg G. Anti-inflammatory activity of Spinaciaoleracea. Int .J Pharma. Prof Res. 2010; 1(1):1-4.
- Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini .Rev .Med Chem. 2009; 9: 31-59.
CrossRef - Mandal P, Babu S.S.P and Mandal N.C. Antimicrobial activity of saponins from Acacia auriculiformis. Fitoterapia., 2005;76: 462-465.
CrossRef - Kumar A, Pande C.S and Kaul R.K. Chemical examination of Cassia fistula flowers. Indian. J. Chem. 1966; 4: 460.
- Vimalraj T.R, Saravanakumar S, Vadivel S, Ramesh S and Thejomoorthy P. Antibcaterial effects of Cassia fistula extracts on pathogenic bacteria of veterinary importance . Tamilnadu J Veter Anim Sci. 2009; 5: 109–1








