Manuscript accepted on :29-10-2024
Published online on: 19-11-2024
Plagiarism Check: Yes
Reviewed by: Dr. Kulvinder Kaur
Second Review by: Dr. Nagham Aljamali
Final Approval by: Dr. Mariia Shanaida
Rina Susilowati1 , Inna Armandari1*
, Inna Armandari1* , Pingky Krisna Arindra2
, Pingky Krisna Arindra2 , David Pakaya1
, David Pakaya1 ,3 and Jens Randel Nyengaard4,5
,3 and Jens Randel Nyengaard4,5
1Department of Histology and Cell Biology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
2Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Universitas Gadjah Mada, Yogyakarta, Indonesia
3Department of Histology, Faculty of Medicine, Tadulako University, Palu, Indonesia
4Core Centre for Molecular Morphology, Section for Stereology and Microscopy, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
5Department of Pathology, Aarhus University Hospital, Denmark
Corresponding Author E-mail:i.armandari@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3020
Abstract
The number of neurons in the sensory ganglion decreases after a peripheral nerve injury (PNI) caused by oral trauma or maxillofacial surgery, resulting in an incomplete nerve regeneration process. Thus, there is an urgent need to reduce the risk for potential complications after PNI, including neuropathic/ectopic pain and allodynia. Citicoline administration reportedly can improve motor function and prevent neuropathic pain in a rat model of PNI. Therefore, the present study aimed to assess the effect of citicoline administration on Brain-derived neurotrophic factor (Bdnf) expression, which is an early indicator of an ongoing nerve regeneration process, and the number of trigeminal neurons at the chronic phase after a PNI in a rat model. The PNI model was established by clamping the mental nerve of Wistar rats with a non-serrated clamp for 30 s. The animals were divided into the following three groups: sham-operated; clamp-injured rats receiving saline as the controls; and clamp-injured rats receiving a daily dose citicoline 50 mg/100g body weight intraperitoneally immediately after surgery for 7 days. They were sacrificed on days 1,3, and 7 for the acute phase analysis to examine the changes in Bdnf expression using quantitative reverse transcriptio polymerase chain reaction. Subsequently, the chronic phase analysis was done by counting the neuron number in the trigeminal ganglion on day 28 post-injury using the stereological method. In the acute phase, citicoline administration increased the Bdnf expression by 2.19 times only on the third-day post-injury, indicating the start of an early regenerative process. However, in the chronic phase, the total number of neurons in the trigeminal ganglion remained similar in all groups, suggesting the possibility of inadequate injury level. In conclusion, although there was no neuronal loss after a mental nerve injury, citicoline administration increased the Bdnf expression at the trigeminal ganglion immediately after the nerve injury, and this may accelerate nerve regeneration.
Keywords
Bdnf; Citicoline; Peripheral Nerve Injury; Trigeminal Ganglion
Download this article as:| Copy the following to cite this article: Susilowati R, Armandari I, Arindra P. K, Pakaya D, Nyengaard J. R. Citicoline Administration Increases the Brain-derived Neurotrophic Factor (Bdnf) Expression in the Trigeminal Ganglion of Rats Post-mental Nerve Injury. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Susilowati R, Armandari I, Arindra P. K, Pakaya D, Nyengaard J. R. Citicoline Administration Increases the Brain-derived Neurotrophic Factor (Bdnf) Expression in the Trigeminal Ganglion of Rats Post-mental Nerve Injury. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3OgSkYY |
Introduction
Peripheral nerve injury (PNI) is a potential complication occurring after oral trauma and maxillofacial surgery caused by surgical procedures, including odontectomy on impacted teeth, dentoalveolar surgery near the mental foramen and mandibular canal, orthognathic surgery, jaw tumor removal, placement of internal fixation screws in fractured mandibular bones, and dental implant placement, which could lead to nerve compression1. The injured neurons in the peripheral nervous system (PNS), unlike those in the central nervous system (CNS), can regenerate spontaneously2,3. Although many cases of PNI are managed through observation over several weeks to months, incomplete nerve regeneration is frequently detected due to the lengthy and insufficient regeneration process4. Interestingly, after PNI, approximately 10%–30% of neurons in the sensory ganglion are likely to die primarily through apoptosis5,6. Thus, the prevention and treatment of PNI are essential to reduce the risk for complications, including neuropathic pain, allodynia, and ectopic pain, and ultimately for complete peripheral nerve regeneration7.
Sensory neurons require neurotrophic factors, including Nerve Growth Factor (NGF), Brain-derived Neurotrophic Factor (BDNF), and Ciliary Neurotrophic factor (CNF), for survival, proliferation, and regeneration6,8. The endogenously secreted neurotrophic factors can enhance the survival of injured neurons and increase their expression during PNI9. However, their responses are slow10–12. BDNF induces an intrinsic neural growth program13, prevents atrophy of neurons after axotomy, stimulates GAP-43 mRNA expression, and promotes axonal regeneration14,15. However, the direct administration of neurotrophic factors is expensive and its preparation is complicated, highlighting the urgent need for alternative treatments. One of the less costly substances, such as mecobalamin, has been shown to induce peripheral nerve regeneration via the upregulation of neurotrophic factors, including BDNF, in the sensory ganglion of a mouse model of PNI16. However, due to the nature of mecobalamin as a supplement that required high-dose administration, its clinical benefits are limited16,17.
Interestingly, citicoline is another potential alternative for promoting peripheral nerve regeneration. Citicoline is an exogenous form of cytidine-5’-diphosphocholine (CDP-choline), an endogenous intermediate in the synthesis of phosphatidylcholine. The local synthesis of phosphatidylcholine in distal axons is critical for normal axon growth18. Citicoline administration induces phospholipid synthesis, maintains membrane integrity, and creates new membrane materials necessary for growing axons18,19. The antioxidant potential of citicoline in preventing free radical damage and fostering phosphatidylcholine synthesis in axons has also been reported20. In clinical practice, citicoline has been used to induce nerve regeneration in various CNS pathologies, including ischemic stroke, cognitive disorders, and glaucoma21–23. In a PNI rat model, citicoline administration can potentially improve motor function24,25 and prevent post-injury neuropathic pain26, in a dose-dependent manner27. As compared with in situ application, the systemic administration of citicoline has a similar effect, in terms of inducing axonal regeneration and motor function recovery28 as well as preventing neuropathic pain26. However, whether citicoline affects peripheral nerve regeneration and prevents neuronal loss in the sensory ganglion is still unknown. Thus, based on the reported effect of citicoline on peripheral nerve regeneration, the present study aimed to assess the effect of systemic citicoline administration on Bdnf gene expression as an early indicator of nerve regeneration in the trigeminal ganglion at the acute phase and to estimate the number of neurons at the chronic phase of the rat PNI model.
Materials and Methods
Animal Model
The animal study was approved by the Medical and Health Research Ethics Committee (MHREC) Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada (number KE/FK/829/EC/2015). Three to four-month-old male Wistar rats were used in this study. The experimental setup is illustrated in Figure 1. Hereto, the animals were divided into the sham-operated, saline-treated, and citicoline-treated groups. In the sham-operated group, an incision was made on the right jaw, which was directly sutured. The saline-treated group was clamp-injured and received saline by intraperitoneal injection, whereas the citicoline-treated group received a daily dose of citicoline of 50mg/100g body weight (BW) (Dexa Medica, Indonesia) intraperitoneally, starting at 5 min post-injury for 7 days.
On the day of surgery, intramuscular anesthesia was administered using a mixture of 0.3-ml ketamine-HCl and 0.05-ml xylazine in 1-ml saline. PNI was established by exposing the right mental nerve to a clamp injury made from a 4-mm non-serrated clamp for 30 s. The clamp strength was 16 kg. Following the surgery, the animals received a daily oral dose of amoxicillin 50mg/100-g BW and 20 mg/100g BW ibuprofen for 3 days. The animals were sacrificed on days 1,3, and 7 post-injury for the acute phase analysis and on day 28 post-injury for the chronic phase analysis.
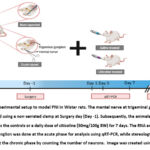 |
Figure 1: Experimental setup to model PNI in Wistar rats. The mental nerve at trigeminal ganglion was clamp-injured using a non-serrated clamp at Surgery day (Day -1).
|
Quantitative Reverse Transcription Polymerase Chain Reaction
Three rats per group were sacrificed on days 1, 3, and 7 post-injury. The rats were decapitated, and the right trigeminal ganglion was dissected for RNA isolation using the RNeasy mini kit (Qiagen, Germany). Altogether, 2 μg RNA was reverse-transcribed using Transcriptor FirstStrand cDNA synthesis (Roche, Switzerland).
Quantitative RT-PCR specific primers were designed using Primer-BLAST from NCBI (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Rat Bdnf expression was analyzed using a LightCycler FS DNA MasterPLUS SYBR Green (Roche, Switzerland) on a LightCycler Carousel (Roche, Switzerland) following manufacturer’s protocol. The Bdnf expression is an early indicator of peripheral nerve regeneration after an injury29. Relative gene expression was calculated based on 2–(Cp Bdnf – Cp Gapdh) equation normalized to the reference gene Gapdh.
Neuron Counting
On day 28, six rats per group were transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate-buffered saline. The right trigeminal ganglions were exposed and excised by cutting the trigeminal roots at their entrance in the brainstem, the mandibular branch 5 mm distally to the ganglion, and the maxillary branch at the level of the orbital fissure. The ganglion was kept in a fixative at 4°C until subsequent processing.
The ganglions were randomly rotated along their longitudinal axis and individually embedded in 20% agar to guarantee isotropy along the longitudinal axis of the ganglion. The ganglion-containing agar underwent dehydration and clearance before being processed for methyl methacrylate plastic embedding Technovit (EMS, USA). Before the final embedding step, each ganglion was rotated once more along its longitudinal axis. Vertical uniform random sections (VURSs) were used instead of the simpler Systematic Uniform Random Sampling (SURS), because the sampling was designed for another study. The VURSs were obtained serially on a rotary microtome at a thickness of 30 µm. Every other section (f1 = ½) was mounted on object glasses and stained with toluidine blue. Every three sections (f2 = ⅓) were observed using an Olympus microscope 60 x objective lens and a numerical aperture of 1.4. Counting frames were laid using the newCAST stereological software (Visiopharm, Denmark) to obtain the area sample fraction (f3 = 1/24.2). The total fraction sampling was 1/2 x 1/3 x 1/24.2 = 6.89 x 10-3. The neurons were counted with the nucleolus as the counting unit. The counted neurons (Q–) were used to calculate the total number of neurons for each ganglion using the following formula: total number of neurons = Q– x 1/f. The coefficient of error was calculated accordingly30.
Data Analysis
Prism 9 (GraphPad, USA) was used for preparing the graphs and statistical analyses. Neuronal counting data were examined by performing a one-way analysis of variance (ANOVA), as indicated in the figure legends. P-values < 0.05 were considered significant.
Results and Discussion
PNI Model in Wistar Rats
Modeling PNI has been performed in in vitro, in vivo to ex vivo studies. Previous in vivo studies have employed different animal types, ranging from Drosophila, zebrafish, and rodents, to non-human primates, depending on the studied nerve types31. Moreover, the proportion of neuronal deaths varied in different injury models and within different time frames31–34. Therefore, in the present study, we used Wistar rats to model PNI with slight modifications from a former study35. The injury was done at the mental nerve, a general somatic afferent sensory nerve of the face. It is a branch of the posterior trunk of the inferior alveolar nerve, itself a branch of the mandibular nerve (CN V3), itself a branch of the trigeminal nerve (CN V). The mental nerve emerges from the mandibular foramen mentalis and branches below the musculus depressor anguli oris into the following three parts: one branch innervates the skin of the chin, and the other two innervates the skin and mucosa of the lower lip. The neuronal cell bodies are located in the ipsilateral trigeminal ganglion36.
Mental nerve injury in rodents has been used as a model of PNI and regeneration models35. The injury was established by clamping the mental nerve using a non-serrated clamp (see Materials and methods). Immediately after surgery, the clamp-injured rats received treatment either with saline as the control group or with a daily dose citicoline for 7 days. In our study, all rats survived until the termination day and no unclosed wound was observed in the operated area. Moreover, daily observation post-surgery and during treatment showed that the eating behaviors of the animals did not change due to the clamping injury at the mental nerve. In our previous study, clamping injury at the mental nerve considerably reduced the diameter of the nerve fiber and axons, as shown by osmium tetroxide (OsO4) staining. Subsequently, the administration of citicoline ameliorated the injury, as evidenced by the larger nerve fiber and axon diameters, suggesting a nerve regeneration process (published data)37. Together, our PNI model is shown to be a representative model for studying the effect of citicoline on peripheral nerve regeneration.
Citicoline Treatment Increases Bdnf Expression in the Trigeminal Ganglion During the Post-Injury Acute Phase
First, to examine the effect of citicoline administration on the PNI model, we assessed the gene expression level of Bdnf, which is among the most important neurotrophic factors for survival, migration, and differentiation in nerve regeneration15.Previously, citicoline was shown to promote nerve regeneration in pathologic conditions, such as multiple sclerosis, (MS) by enhancing early remyelination in the rat model of de- and remyelination even at a low dose38. Moreover, citicoline also improves the functional recovery and regeneration of the sciatic nerve in rats, as shown by the lower density of connective tissue surrounding the nerve, higher axon counts and diameters, thin collagenous scar formation, lesser neuropathic pain intensity, and improved motor function24,26. The mechanisms by which citicoline exerts those effects are well described in the literature as the exogenous source of phosphatidylcholine, a type of phospholipid found in the neuronal membrane, which is critical for neurite growth and neuronal regeneration39.
The effect of citicoline on Bdnf expression after PNI over time is shown in Figure 2. In our study, intraperitoneal citicoline administration for 7 days after mental nerve injury increased the level of Bdnf expression by approximately 1.5 times in the trigeminal ganglion of the saline-treated group, as compared with the sham-operated group, which may suggests the baseline expression of Bdnf after injury (Figure 2). Only on the third day (Day 3), citicoline administration increased the Bdnf expression up to 2.19 times in the trigeminal ganglion of the citicoline-treated group, as compared with the sham-operated group. However, after 7 days, the Bdnf RNA expression level decreased to a level similar to that of the sham-operated group, indicating that the citicoline effect on Bdnf expression only occurs shortly after an injury, as it is likely that BDNF is involved in the early response to nerve injury and accelerates the nerve regeneration process40. To the best of our knowledge, our data showed for the first time the in-time fluctuation of Bdnf expression during the nerve regeneration process after injury. Nevertheless, further studies that include replication samples for qRT-PCR analysis are required to confirm our study findings.
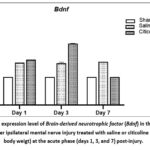 |
Figure 2: The expression level of Brain-derived neurotrophic factor (Bdnf) in the trigeminal ganglion after ipsilateral mental nerve injury treated with saline or citicoline (50mg/100g body weigt) at the acute phase (days 1, 3, and 7) post-injury.
|
Our result is in line with the results of a previous study showing thatciticoline treatment stimulated Bdnf expression in isolated hypothalamic neurons exposed to oxidative stress41. Additionally, assessing the level of Bdnf expression is essential as the serum level of BDNF reportedly can be employed as a predictor for the development of trigeminal neuralgia, a severe chronic neuropathic pain affecting the trigeminal nerve42.
Mental Nerve Injury doesnot Change The Number of Neurons in theTrigeminal Ganglion
Following PNI, most neurons die through apoptosis, which may lead to functional loss of the nerve5,6. To examine the effect of citicoline administration after PNI in rats, neuronal counting was performed in the trigeminal ganglion at the chronic phase (28 days post-injury) in toluidine blue-stained sections using a design-based unbiased stereology. On average, 7—15 VURSs were observed, and 228—448 neurons were counted. The coefficient of errors was between 0.019 and 0.038%. The coefficients of variance of the sham-operated, saline-treated, and citicoline-treated groups were 6.5%, 7.2%, and 7.9%, respectively.
While counting for the total number of neurons, the neuronal types were also determined based on the size of the neurons, namely, the larger neuron A and the smaller neuron B (Figure 3a). Our data showed that the number of neuronal type A and B and the total number of neurons in the trigeminal ganglion after mental nerve injury were not significantly different between the groups (Figure 3b). The mental nerve is a branch of the inferior alveolar nerve, which is a branch of the trigeminal nerve’s mandibular division. Therefore, clamping the mental nerve may be inadequate to produce damage that induces considerable neuronal loss at the trigeminal ganglion.
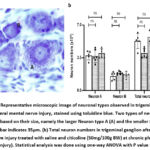 |
Figure 3: (a) Representative microscopic image of neuronal types observed in trigeminal ganglion after ipsilateral mental nerve injury, stained using toluidine blue.
|
Conclusion
Overall, our study data exhibited that citicoline administration after PNI is beneficial in promoting nerve regeneration, as demonstrated by increased Bdnf expression, suggesting that citicoline administration can indeed accelerate nerve regeneration immediately after injury. As the first study that examined the effect of citicoline administration on Bdnf expression after PNI, further studies using BDNF inhibitors are needed to confirm our findings. Whether prolonged BDNF secretion leads to improved functional and structural recovery remains to be investigated. In our study, neuronal loss was absent in the trigeminal ganglion at 28 days after the mental nerve clamping injury. Subsequently, more animals are required in future studies to validate and reproduce this finding, as citicoline has a high potential to be clinically used to prevent the development of complications after PNI and to enhance complete nerve regeneration.
Acknowledgement
We thank the Department of Histology and Cell Biology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Indonesia for the laboratory support and the Department of Clinical Medicine, Section for Stereology and Microscopy, Aarhus University, Denmark for assistance with the stereology analysis.
Conflict of Interest
The author(s) do not have any conflict of interest
Funding Sources
This study was funded by a grant from Dana Masyarakat (UPPM/44/M/05/04/04.15), Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada to RS.
Data Availability Statement
This statement does not apply to this article
Ethics Statement
The animal study was approved by the Medical and Health Research Ethics Committee (MHREC) Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada (number KE/FK/829/EC/2015).
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Authors’ Contribution
Rina Susillowati: Conceptualization, Methodology, Data Collection, Visualization, Funding Acquisition, Supervision, Writing – Original Draft, Review & Editing.
Inna Armandari: Data Collection, Methodology, Analysis, Project Administration, Writing – Review & Editing.
Pingky Krisna Arindra: Methodology, Data Collection, Resources, Writing – Review & Editing.
David Pakaya: Methodology, Data Collection.
Jens Randel Nyengaard: Supervision, Methodology, Resources, Writing – Review & Editing.
References
- Hasstedt KL, Meyer RA, Bagheri SC. Nerve Involvement in Oral Surgery. In: Innovative Perspectives in Oral and Maxillofacial Surgery. Springer International Publishing; 2021:327-342.
CrossRef - Huebner EA, Strittmatter SM. Axon Regeneration in the Peripheral and Central Nervous Systems. In: ; 2009:305-360.
CrossRef - Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, Goldstein LSB, Tuszynski MH. Long-Distance Axonal Growth from Human Induced Pluripotent Stem Cells after Spinal Cord Injury. Neuron. 2014;83(4):789-796.
CrossRef - Antoniadis G, Kretschmer T, Pedro MT, König RW, Heinen C, Richter HP. Iatrogenic Nerve Injuries. Dtsch Arztebl Int. Published online April 18, 2014.
CrossRef - Hart AM, Terenghi G, Wiberg M. Neuronal death after peripheral nerve injury and experimental strategies for neuroprotection. Neurol Res. 2008;30(10):999-1011.
CrossRef - Li R, Li D hui, Zhang H yu, Wang J, Li X kun, Xiao J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol Sin. 2020;41(10):1289-1300.
CrossRef - Goto T, Kuramoto E, Iwai H, Yamanaka A. Cytoarchitecture and intercellular interactions in the trigeminal ganglion: Associations with neuropathic pain in the orofacial region. J Oral Biosci. Published online July 2024.
CrossRef - Xiao J, Wong AW, Willingham MM, Kaasinen SK, Hendry IA, Howitt J, Putz U, Barrett GL, Kilpatrick TJ, Murray SS. BDNF Exerts Contrasting Effects on Peripheral Myelination of NGF-Dependent and BDNF-Dependent DRG Neurons. The Journal of Neuroscience. 2009;29(13):4016-4022.
CrossRef - Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82(4):163-201.
CrossRef - Wan L, Zhang S, Xia R, Ding W. Short‐term low‐frequency electrical stimulation enhanced remyelination of injured peripheral nerves by inducing the promyelination effect of brain‐derived neurotrophic factor on Schwann cell polarization. J Neurosci Res. 2010;88(12):2578-2587.
CrossRef - Saleh A, Roy Chowdhury SK, Smith DR, Balakrishnan S, Tessler L, Martens C, Morrow D, Schartner E, Frizzi KE, Calcutt NA, Fernyhough P. Ciliary neurotrophic factor activates NF-κB to enhance mitochondrial bioenergetics and prevent neuropathy in sensory neurons of streptozotocin-induced diabetic rodents. Neuropharmacology. 2013;65:65-73.
CrossRef - Xu P, Rosen KM, Hedstrom K, Rey O, Guha S, Hart C, Corfas G. Nerve injury induces glial cell line‐derived neurotrophic factor (gdnf) expression in schwann cells through purinergic signaling and the pkc‐pkd pathway. Glia. 2013;61(7):1029-1040.
CrossRef - Geremia NM, Pettersson LME, Hasmatali JC, Hryciw T, Danielsen N, Schreyer DJ, Verge VMK. Endogenous BDNF regulates induction of intrinsic neuronal growth programs in injured sensory neurons. Exp Neurol. 2010;223(1):128-142.
CrossRef - Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W. BDNF and NT-4/5 Prevent Atrophy of Rat Rubrospinal Neurons after Cervical Axotomy, Stimulate GAP-43 and Tα1-Tubulin mRNA Expression, and Promote Axonal Regeneration. The Journal of Neuroscience. 1997;17(24):9583-9595.
CrossRef - McGregor CE, English AW. The Role of BDNF in Peripheral Nerve Regeneration: Activity-Dependent Treatments and Val66Met. Front Cell Neurosci. 2019;12.
CrossRef - Gan L, Qian M, Shi K, Chen G, Gu Y, Du W, Zhu G. Restorative effect and mechanism of mecobalamin on sciatic nerve crush injury in mice. Neural Regen Res. 2014;9(22):1979.
CrossRef - Tamaddonfard E, Farshid A, Samadi F, Eghdami K. Effect of Vitamin B12 on Functional Recovery and Histopathologic Changes of Tibial Nerve-Crushed Rats. Drug Res. 2014;64(09):470-475.
CrossRef - Qureshi IA, Endres JR. Citicoline: A Novel Therapeutic Agent with Neuroprotective, Neuromodulatory, and Neuroregenerative Properties. In: ; 2010. https://api.semanticscholar.org/ CorpusID: 16494787
- Parisi V, Coppola G, Centofanti M, Oddone F, Maria Angrisani A, Ziccardi L, Ricci B, Quaranta L, Manni G. Evidence of the neuroprotective role of citicoline in glaucoma patients. In: ; 2008:541-554.
CrossRef - González-Pacheco H, Méndez-Domínguez A, Hernández S, López-Marure R, Vazquez-Mellado MJ, Aguilar C, Rocha-Zavaleta L. Pre-Conditioning with CDP-Choline Attenuates Oxidative Stress-Induced Cardiac Myocyte Death in a Hypoxia/Reperfusion Model. The Scientific World Journal. 2014;2014:1-8.
CrossRef - Secades JJ, Alvarez-Sabín J, Castillo J, Díez-Tejedor E, Martínez-Vila E, Ríos J, Oudovenko N. Citicoline for Acute Ischemic Stroke: A Systematic Review and Formal Meta-analysis of Randomized, Double-Blind, and Placebo-Controlled Trials. Journal of Stroke and Cerebrovascular Diseases. 2016;25(8):1984-1996.
CrossRef - Bonvicini M, Travaglini S, Lelli D, Antonelli Incalzi R, Pedone C. Is Citicoline Effective in Preventing and Slowing Down Dementia?—A Systematic Review and a Meta-Analysis. Nutrients. 2023;15(2):386.
CrossRef - Levin LA, Peeples P. History of neuroprotection and rationale as a therapy for glaucoma. Am J Manag Care. 2008;14(1 Suppl):S11-4.
- Özay R, Bekar A, Kocaeli H, Karlı N, Filiz G, Ulus İH. Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scarring after peripheral nerve surgery in rats. Surg Neurol. 2007;68(6):615-622.
CrossRef - Aslan E, Kocaeli H, Bekar A, Tolunay Ş, Ulus IH. CDP-choline and its endogenous metabolites, cytidine and choline, promote the nerve regeneration and improve the functional recovery of injured rat sciatic nerves. Neurol Res. 2011;33(7):766-773.
CrossRef - Emril D, Meliala L, Susilowati R, Wibowo S. Cytidine 5’-diphosphocholine administration prevents peripheral neuropathic pain after sciatic nerve crush injury in rats. J Pain Res. 2016;9:287-291.
CrossRef - Kaplan T, Kafa IM, Cansev M, Bekar A, Karli N, Taskapilioglu MO, Kanar F. Investigation of the dose-dependency of citicoline effects on nerve regeneration and functional recovery in a rat model of sciatic nerve injury. Turk Neurosurg. 2014;24(1):54-62
CrossRef - Caner B, Kafa MI, Bekar A, Kurt MA, Karli N, Cansev M, Ulus IH. Intraperitoneal administration of CDP-choline or a combination of cytidine plus choline improves nerve regeneration and functional recovery in a rat model of sciatic nerve injury. Neurol Res. 2012;34(3):238-245.
CrossRef - Shi Z liang, Fan Z yong, Zhang H, Li S tai, Yuan H, Tong J hui. Localized delivery of brain-derived neurotrophic factor from PLGA microspheres promotes peripheral nerve regeneration in rats. J Orthop Surg Res. 2022;17(1):172.
CrossRef - Foldager CB, Nyengaard JR, Lind M, Spector M. A Stereological Method for the Quantitative Evaluation of Cartilage Repair Tissue. Cartilage. 2015;6(2):123-132.
CrossRef - Li A, Pereira C, Hill EE, Vukcevich O, Wang A. In Vitro, In Vivo and Ex Vivo Models for Peripheral Nerve Injury and Regeneration. Curr Neuropharmacol. 2022;20(2):344-361.
CrossRef - McKay Hart A, Brannstrom T, Wiberg M, Terenghi G. Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat. Exp Brain Res. 2002;142(3):308-318.
CrossRef - Burland M, Paris L, Quintana P, Bec JM, Diouloufet L, Sar C, Boukhaddaoui H, Charlot B, Braga Silva J, Chammas M, Sieso V, Valmier J, Bardin F. Neurite growth acceleration of adult Dorsal Root Ganglion neurons illuminated by low-level Light Emitting Diode light at 645 nm. J Biophotonics. 2015;8(6):480-488.
CrossRef - Gong WL, Zhang YN, Zhang J, Jiang X, Xian WJ. Biological differences and molecular mechanism of different peripheral nerves after injury. Eur Rev Med Pharmacol Sci. 2024;28(2):778-788.
- Li B, Jung HJ, Kim SM, Kim MJ, Jahng JW, Lee JH. Human periodontal ligament stem cells repair mental nerve injury. Neural Regen Res. 2013;8(30):2827-2837.
- Iwanaga J, Saga T, Tabira Y, Nakamura M, Kitashima S, Watanabe K, Kusukawa J, Yamaki K. The clinical anatomy of accessory mental nerves and foramina. Clinical Anatomy. 2015;28(7):848-856.
CrossRef - Pingky Krisna Arindra, Masykur Rahmat, Rahardjo. Regenerasi nervus mentalis akibat cedera penjepitan setelah kombinasi platelet rich plasma yang diaktivasi spons kolagen dan cytidine 5’-diphosphocholine. Jurnal Kedokteran Gigi. 2016;7(2):138-144.
- Gudi V, Schäfer N, Gingele S, Stangel M, Skripuletz T. Regenerative Effects of CDP-Choline: A Dose-Dependent Study in the Toxic Cuprizone Model of De- and Remyelination. Pharmaceuticals. 2021;14(11):1156.
CrossRef - Secades Ruiz JJ, Gareri P. Citicolina: revisión farmacológica y clínica, actualización 2022. Rev Neurol. 2022;75(S05):S1.
CrossRef - Boyd JG, Gordon T. Neurotrophic Factors and Their Receptors in Axonal Regeneration and Functional Recovery After Peripheral Nerve Injury. Mol Neurobiol. 2003;27(3):277-324.
CrossRef - Mastropasqua L, Agnifili L, Ferrante C, Sacchi M, Figus M, Rossi GCM, Brescia L, Aloia R, Orlando G. Citicoline/Coenzyme Q10/Vitamin B3 Fixed Combination Exerts Synergistic Protective Effects on Neuronal Cells Exposed to Oxidative Stress. Nutrients. 2022;14(14):2963.
CrossRef - Song Y, Zhou M, Xiong J, Huang R, Shen W, Zhan T, Xie Y, Gao Y, Xiong W. Effects of carbamazepine on BDNF expression in trigeminal ganglia and serum in rats with trigeminal neuralgia. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024;49(1):11-20.







