Ratnali Bania* , Sofiqul Islam
, Sofiqul Islam , Nekibur Rahman
, Nekibur Rahman , Satyendra Deka
, Satyendra Deka , Debabrata Nath
, Debabrata Nath , Abhishek Parasar
, Abhishek Parasar and Madhusmita Kumari
and Madhusmita Kumari
Pratiksha Institute of Pharmaceutical Sciences, Panikhaiti, Guwahati, Kamrup (M), Assam, India.
Corresponding Author E-mail:baniaratnali30@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3036
Abstract
Herbal medicine therapy is an ancient practice to treat various health issues, including viral and non-infectious skin diseases. At present time, people are running behind natural or herbal sources because of their fewer side effects and add-on benefits as compared to allopathy medicines. In the Indian traditional system of medicine (Ayurveda), the Amaranthus spinosus (Amaranthaceae) plant is used for analgesic, antipyretic, laxative, diuretic, digestible, antidiabetic, anti-snake venom, antileprotic, blood diseases, bronchitis, piles, and anti-gonorrheal. The study's objective was to perform extraction and phytochemical studies of A. spinosus leaves, perform the in-vitro antibacterial and antioxidant activity of the extract, and formulate a topical cream. The ethanol water leaf extract exhibited higher antibacterial efficacy than ciprofloxacin against Staphylococcus aureus at 15 and 30 µg, while ciprofloxacin was more effective at 60 µg. However, the extract showed more effectiveness than ciprofloxacin against Escherichia coli at all tested concentrations. The study revealed that the leaf extract showed good antibacterial and antioxidant activity. The physical evaluation of the formulated cream showed that the cream was stable with potential application to reduce skin infection with consequent health benefits.
Keywords
Antibacterial agent; Antioxidant; A. spinosus; Cream; Herbal medicine; Leaf extract
Download this article as:| Copy the following to cite this article: Bania R, Islam S, Rahman N, Deka S, Nath D, Parasar A, Kumari M. Formulation of Antioxidant and Antibacterial Cream Containing Amaranthus spinosus Leaf Extract. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Bania R, Islam S, Rahman N, Deka S, Nath D, Parasar A, Kumari M. Formulation of Antioxidant and Antibacterial Cream Containing Amaranthus spinosus Leaf Extract. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3YPK5rw |
Introduction
Exploring new bioactive substances derived from natural sources has recently attracted much attention because of their possible therapeutic uses in various fields, including pharmaceuticals and cosmetics. Plants are an eminent source of bioactive substances with a wide range of pharmacological and phytochemical characteristics. Developing topical formulations like cream using plant extracts offers various pharmacological properties, including antibacterial, antioxidant, and anti-inflammatory1,2. Topical creams are a perfect vehicle for maximizing the medicinal potential of plant extracts because of their safe, non-greasy texture, ease of application, and a good vehicle to deliver the substances to the skin2,3. It has long been known that plants are an excellent source of bioactive chemicals with antioxidant and antibacterial properties, which makes them a good choice for creating topical treatments to treat skin diseases2,4.
Since ancient times, herbal sources like plants have been used to prevent and treat many diseases. In India and other countries, medicinal plants have been used without any checking parameters as traditional healers5. These plants have different medicinal properties that help the body by boosting protective enzymes and strengthening its antioxidant system. When the body produces too many reactive oxidative species (ROS), it can damage cells and tissues and start inflammation. By controlling ROS levels, these plants can potentially treat various diseases and show biological activities including antimicrobial, antioxidant, anti-cancer, anti-inflammatory, etc.6
Antibacterial activity or agents describe compounds that either eradicate or inhibit the development of bacteria. Although scientists have found many antibiotics that can fight different infections well, some bacteria are becoming resistant to other antibiotics, making it hard to treat infections. This is becoming a challenge for human health. Also, antibiotic resistance increases mortality rates and the costs of treating diseases. To minimize or overcome the problem, researchers focus on plant-based or natural antibacterial agents. Additionally, phytoconstituents have diverse chemical structures, which reduce the probability of bacteria developing resistance compared to conventional antibiotics. Furthermore, using antibiotics produced from plants has several benefits, such as their natural abundance, comparatively cheap extraction costs, and the possibility of using environmentally friendly manufacturing techniques. Also, many of these compounds have been conventionally used in herbal medicine for their antimicrobial properties, indicating their safety for human consumption 7. A. spinosus Linn. (Amaranthaceae) is commonly known as Katakhutura (figure 1) in Assamese culture. About 20 species are found or cultivated in India and worldwide four hundred Amaranthus species occur in tropical, subtropical, and temperate climate zones8–10.
 |
Figure 1: Amaranthus spinosus Plant |
A. spinosus is used as a vegetable in different regions of India. According to Indian tradition, this plant treats leprosy, blood disease, leucorrhoea, bronchitis, rheumatic pain, eczema, gastrointestinal diseases, boils, burns, abscesses, colic menorrhagia, etc. The plant is also an expectorant, digestive, diuretic, antipyretic, antileprotic, anti-gonorrhoeal, antidiabetic, antimalarial, antiviral, anti-inflammatory, etc. The boiled roots and leaves of A. spinosus are given to children as emollients, laxatives, and for burns and boils4,11 . The juice of A. spinosus was used in a village area of Assam, India, to prevent swelling around the stomach, while the leaves were boiled without salt and consumed for 2-3 days to cure jaundice. A. spinosus is also used, as reported, for its anti-inflammatory, antimalarial, immunomodulatory, anti-diabetic, anti-hyperlipidemic, antioxidant, and spermatogenic activities1,3,12,13. Phytoconstituents like alkaloids, glycosides, flavonoids, steroids, phenolic acids, amino acids, saponins, glycosides, lipids, terpenoids, tannins, betanin, b-sitosterol, stigmasterol, linoleic acid, rutin, and beta-carotene8,9,14.
Studies showed that A. spinosus extract has antimicrobial activity in different microbial strains like Staphylococcus aureus, Aspergillus flavus, and Escherichia coli. The plant extract has restored the level of oxidative free radicals in rats, which has helped in the fast recovery of wounds. The phytochemical studies confirmed the presence of terpenoids, alkaloids, and saponins. Different studies confirmed that A. spinosus can be used as a substitute treatment for infectious or non-infectious wounds15–17.
The major objective of the present study is to formulate an antioxidant and antibacterial cream with the extract of A. spinosus leaves. Also, evaluating the physical properties, such as color, odour, pH, viscosity and spreadability of the formulated cream is another objective. The ultimate goal is to create a cream with extract from A. spinosus that offers a safe, natural option for treating bacterial skin infections.
Materials and Methods
Collection, Authentication, and Extraction of Plant
The flowering plants were collected in the winter season from the village area of Dhubri district, Assam, India. The plant was authenticated at the Botanical Survey of India, Shillong, Meghalaya, India. For the extraction, the leaves were first separated, dried under a shed, and powdered using a grinder. The powdered leaves were sieved through a #40 sieve to get the uniform size of the powdered leaves. The extraction was performed using digestion with different solvents like petroleum ether, ethyl acetate, acetone, ethanol, and hydroalcoholic (ethanol: water). About 25 grams of powdered leaves were mixed with 100 ml of solvent and kept in a water bath for 30 minutes. The extract was separated and dried using filtration and evaporation methods.
Preliminary Phytochemical Screening of Leaf Extract
Using standard methods, the phytochemical screening of the ethanolic extract of A. spinosus was conducted to determine the presence of phytochemical constituents18.
Antibacterial Activity
The antibacterial activity of leaf extract was performed using the agar disk diffusion method. Solid agar media plates are used for the growth of test organisms in this method. The extract is loaded into the paper discs and placed on the solid agar plate after the development of the test organism. Once the extract diffuses into the agar plate, it will stop the growth of bacteria up to a particular area, known as the zone of inhibition, and it will be measured using an antibiotic zone reader. Staphylococcus aureus and Escherichia coli are the two most commonly used bacterial strains used for this antibacterial study, for the solid agar plates were prepared by dissolving the required quantities mentioned in table 1 of peptone, beef extract, sodium chloride, and agar in water with heat. Sterilization was performed using autoclaving for the liquid agar media and after that, it was allowed to solidify in Petri dish.
Table 1: Composition of nutrient agar
|
Nutrient Agar |
Quantity (g) |
|
Peptone |
5 |
|
Beef Extract |
3 |
|
Sodium chloride |
5 |
|
Agar |
15 |
|
Distilled water |
1000ml |
A stock solution was prepared in 1ml of dimethyl sulfoxide (DMSO) by dissolving 0.06 gm of extract. The sterilized paper discs were dipped into the solution to make 60 μg/disc concentrations. Further test samples of 30μg/disc and 15μg/disc concentrations were prepared by diluting the stock solution. The paper discs loaded with extract, Ciprofloxacin as a standard drug, and control DMSO were placed in the agar plates and incubated for up to 24 hours. The zone of inhibitions was measured and recorded 6,7.
Antioxidant Activity
The activity was performed using radical scavenging of the unchanged 2,2’ diphenyl-1-picrylhydrazyl (DPPH) assay for the plant extract. The standard solution was an ascorbic acid with 1 to 10 µg/ml concentrations. The assay was carried out using a UV visible spectrophotometer, with an ascorbic acid solution ranging from 1 to10 µg/ml used as the standard solution.
The sample solution was prepared using a 0.005% DPPH solution in ethanol, where 2 ml of extract solution was mixed. The samples were incubated at room temperature for 30 mins. A UV spectrophotometer measured the optical density at 517 nm for every sample in triplicate 16,17. The antioxidant activity was determined by using the following equation:
% inhibition: [(Acontrol – Asample)/ Acontrol) X100]
Where A control is the absorbance of control at the moment of solution preparation and A sample is the absorbance of the sample after 45 min.
Formulation of cream-containing extract
The oil phase and water phase were prepared separately by melting the required ingredients mentioned in table 2. Both phases were mixed slowly with continuous stirring at 70 °C. After getting the required consistency, the leaf extract was added to the cream. For a smooth and stable cream preparation, the ingredients are added slowly and step-by-step 3,4,19.
Table 2: Composition of cream with A.spinosus extract
|
Ingredients |
Amount(g) |
|
Stearic acid |
1 |
|
Spermaceti |
0.5 |
|
Cetyl alcohol |
0.5 |
|
Glycerine |
0.5 |
|
Triethanolamine |
0.2 |
|
Benzyl alcohol |
0.2 |
|
Water |
7 |
|
Leaf Extract of A. spinosus |
0.1 |
Evaluation of Prepared Cream
The following evaluation tests are performed to measure the quality of formulated cream. These evaluation parameters help in ensuring the suitable compatibility of cream with skin 19,20.
Homogeneity: the prepared cream was observed visually to check the homogeneity.
Determination of pH: A digital pH meter was used to check the pH of the prepared cream.
Organoleptic test: properties like appearance, odour and texture were evaluated by applying a small portion of cream to the skin.
Consistency: to check the consistency of the prepared cream, a cone was dropped from a 10 cm fixed height into a measuring cylinder. The measuring cylinder was filled with prepared cream.
Spreadability: The test was performed by observing how easily and quickly the cream spreads over the area after application.
Emolliency: The emollient and greasiness effects of cream were observed by applying it to the skin.
Skin irritation test: The test was performed by applying a small portion of cream to an area of skin and being observed for 15-20 minutes to see if any irritation occurred.
Stability Study: Prepared cream was kept for three months at three different temperatures, such as at 40C, 20-300C and 40-500C. Other parameters were noted.
Results and Discussion
Percentage Yield of Extract
After extracting powdered leaves, extracts were dried using the evaporation method, and the yield values as a percentage were determined. The table 3 displays the results. The highest percentage yield was found when ethanol and water were used for extraction in a 1:1 ratio.
Table 3: Percentage yield of extract
|
The solvent used for extraction leaves |
Weight of Extract (gm) |
% of Yield |
|
Pet. Ether |
0.87 |
3.48 |
|
Ethyl acetate |
1.2 |
4.8 |
|
Acetone |
2.1 |
8.4 |
|
Ethanol |
2.8 |
11.2 |
|
Ethanol: water (1:1) |
3.9 |
15.6 |
Preliminary Phytochemical Analysis
After performing the preliminary phytochemical study, it was found that alkaloids, flavonoids, tannins, and phenolic compounds were present in the extract of acetone, ethanol, and ethanol: water. Details of the results are shown in table 4.
Table 4: Phytochemical constituent present in the extract of A. spinosus leaf
|
Name of the constituents |
Pet. ether |
Ethyl acetate |
Acetone |
Ethanol |
Ethanol: water (1:1) |
|
Alkaloids |
– |
– |
+ |
+ |
+ |
|
Glycoside |
– |
– |
– |
– |
– |
|
Flavonoids |
– |
– |
+ |
+ |
+ |
|
Steroids |
– |
– |
– |
– |
– |
|
Tannins and phenolic Compound |
– |
– |
+ |
+ |
+ |
**+ = Present the phytochemical constituent and – = Absent the phytochemical
Antibacterial Activity
After performing the antibacterial test of ethanol water leaf extract by disk diffusion method, it was shown that at concentrations of 15 and 30 microgram, the extract showed somewhat higher efficiency against S. aureus bacteria than Ciprofloxacin. On the other hand, ciprofloxacin showed better effectiveness at a concentration of 60 microgram than extract. The extract showed more effectiveness in all three concentrations than ciprofloxacin against E.coli bacteria.
The results of the disc diffusion experiment, which are presented in the accompanying table 5 and figure 2, highlight the significant antibacterial qualities of the leaf extract of A. spinosus.
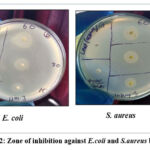 |
Figure 2: Zone of inhibition against E.coli and S.aureus bacteria |
Table 5: Antibacterial activity test of A. spinosus leaf against bacterial strains
|
Compounds |
Concentration |
Zone Of Inhibition (mm) |
|
|
S.aureus |
E.coli |
||
|
Leave extract of A.spinosus in ethanol:water (1:1 ) |
60 𝜇g |
13 |
16 |
|
30 𝜇g |
12 |
12 |
|
|
15 𝜇g |
10 |
9.5 |
|
|
Ciprofloxacin |
60 𝜇g |
15 |
13 |
|
30 𝜇g |
11 |
11 |
|
|
15 𝜇g |
—- |
— |
|
Antioxidant Activity
The IC50 value represents the concentration of a substance required to inhibit a particular biological or biochemical function by 50%. In this study, the IC50 values indicate the efficiency of the test substance and the standard in scavenging free radicals. The IC50 value for the test substance was 69.28 µg/ml. The IC50 value for the standard substance was 49.55 µg/ml. A lower IC50 value signifies a higher potency of the substance in scavenging free radicals. Therefore, the standard substance is more effective in free radical scavenging compared to the test substance (shown in the figure 3). However, the test substance also shows significant scavenging activity, making it a potential candidate for further investigation and development as an antioxidant agent. It also opens up the possibility of exploring the plant further and isolating the compound responsible for the antioxidant activity shown by the plant.
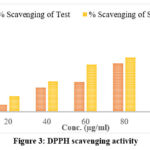 |
Figure 3: DPPH scavenging activity |
Formulation and Evaluation of Antibacterial Cream
The cream containing A.spinosus extract was formulated (shown in the figure 4) with the specified ingredients in the table 2. After the formulation of antibacterial cream, a comprehensive evaluation was conducted to assess its quality and performance. The evaluation test included observations about colour, viscosity, and spreadability and several tests, such as a skin irritation test, homogeneity analysis, and pH measurement. The results are shown in the table 6. The result of the evaluation parameters ensured the suitability of the cream for topical application.
 |
Figure 4: Prepared cream containing A. spinosus extract |
Table 6: Results of evaluation of prepared cream
|
Parameter |
Leaf extract cream |
|
Colour |
Light green |
|
PH |
5.6 |
|
Homogeneity |
Excellent |
|
Consistency (60 sec) |
5mm |
|
Skin Irritation |
Nil |
|
Viscosity |
1375 Cp |
|
Spreadability (g.cm/sec) |
36 |
Stability Study of Cream
The stability test of the cream was carried out at room temperature and accelerated temperature for at least three months. The results of evaluation parameters were listed in the table 7 and it was observed that the cream was stable.
Table 7: Evaluation of stability study
|
Parameter |
Room temperature (at 250C ± 2) |
Accelerated temperature (at 400C ± 2) |
||||
|
1 month |
2 months |
3 months |
1 month |
2 months |
3 months |
|
|
Colour |
Light green |
Light green |
Light green |
Light green |
Light green |
Light green |
|
pH |
5.6 |
5.7 |
5.7 |
5.5 |
5.6 |
5.7 |
|
Homogeneity |
Excellent |
Excellent |
Excellent |
Excellent |
Excellent |
Excellent |
|
Consistency (60 sec) |
5mm |
5mm |
6mm |
6mm |
5mm |
6mm |
|
Skin Irritation |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
|
Viscosity |
1375 Cp |
1410 Cp |
1457 Cp |
1390 Cp |
1426 Cp |
1498 Cp |
|
Spreadability (g.cm/sec) |
36 |
37 |
36 |
36 |
38 |
37 |
Conclusion
The study concludes by indicating A. spinosus potential as a useful source of antioxidant antibacterial compounds for topical therapy formulation. The study started with collecting and authenticating A. spinosus plants from the Dhubri area in Assam, India, and then extracting the leaves using different solvents. The phytochemical analysis revealed the presence of alkaloids, flavonoids, tannins, and phenolic compounds in the leaf extracts, which are known for their antimicrobial properties. Using the disk diffusion technique, the antibacterial activity of the A. spinosus leaf extract was evaluated against two common bacterial strains: E. coli and S. aureus. The antibacterial study revealed that the leaf extracts showed better efficacy when compared to the Ciprofloxacin. The DPPH antioxidant confirmed the leaf extract has good antioxidant property. The antioxidant and antibacterial activity results revealed that the plant A. spinosus can be a natural substitute for treating bacterial and other diseases like wound healers, aging, etc. A topical cream formulated to administer the drug easily and efficiently on a required body site. The prepared topical cream was evaluated for physical parameters. The evaluation study confirmed that the cream was stable and suitable for topical application.
However, it is important to acknowledge certain limitations and areas for further exploration. Although A. spinosus extract’s antibacterial activity was shown in vitro, more research is necessary to confirm its effectiveness in vivo and evaluate its safety profile over an extended period. More investigation is necessary to clarify the mechanisms of action of phytoconstituents and maximize their effectiveness for therapeutic use. Translating these discoveries into real-world applications also requires addressing issues including formulation development, regulatory approval, and standardization of extraction procedures. Furthermore, optimizing the cream formulation, including concentration adjustments and incorporating other synergistic ingredients, could enhance its efficacy and stability.
Acknowledgement
We want to thank the Institutional research committee of Pratiksha Institute of Pharmaceutical Sciences, Panikhaiti, Chandrapur road, Guwahati, Kamrup (M), Assam, pin: 781026 for their contribution to experimental work, chemicals, instrument and equipment.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Conflict of Interest
The author(s) do not have any conflict of interest
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required
Clinical Trial Registration
This research does not involve any clinical trials
Author contributions
Methodology and Investigation: Ratnali Bania, Sofiqul Islam, Nekibur Rahman;
Writing- Original draft preparation: Ratnali Bania Debabrata Nath, Madhusmita Kumari, Abhishek Parasar;
Conceptualization: Ratnali Bania;
Validation: Satyendra Deka; Software: Ratnali Bania, Debabrata Nath; Reviewing and Editing: Madhusmita Kumari, Ratnali Bania
References
- Alegbejo J. Nutritional Value and Utilization of Amaranthus (Amaranthus spp.) – A Review. Bayero J Pure Appl Sci. 2014;6:136.
CrossRef - Chen M. X, Alexander K. S and Baki G. Formulation and Evaluation of Antibacterial Creams and Gels Containing Metal Ions for Topical Application. J Pharm. 2016;2016:e5754349.
CrossRef - Silwal A, Paudel N, Gyawali R and Shrestha S. Formulation and Evaluation of Antibacterial and Antioxidant Polyherbal Lotion. J Inst Sci Technol. 2016;21:148-156.
CrossRef - Shukla K. V, Choudhary N and Pathak R. Formulation and Evaluation of Topical Polyherbal Antiacne Gels Containing Luffa Acutangula, Amaranthus Spinosus and Morus Alba. J Drug Deliv Ther. 2019;9(4-s):439-444.
CrossRef - Rodrigues L. L. O, De Oliveira A. C. L,Tabrez S, Shakil S, Khan M. I, Asghar M. N, Matias B. D, Batista J. M. A. S, Rosal M. M, Fulgencio de Lima M. M. D, Gomes S. R. F, Mendes de Carvalho R, Pinho de Moraes G, Barros de Alencar M. V. O, Islam M. T and Melo-Cavalcante A. A. C. Mutagenic, antioxidant and wound healing properties of Aloe vera. J Ethnopharmacol. 2018;227:191-197.
CrossRef - Sharifi-Rad J, Hoseini-Alfatemi S. M, Sharifi-Rad M and Teixeira da Silva J. A. Antibacterial, antioxidant, antifungal and anti-inflammatory activities of crude extract from Nitraria schoberi fruits. 3 Biotech. 2015;5(5):677-684.
CrossRef - Sekar M, Jalil N. S. A. Formulation and evaluation of novel antibacterial and anti-inflammatory cream containing Muntingia calabura leaves extract. Asian J Pharm Clin Res. Published online December 1, 2017:376-379.
CrossRef - Kumar Bs. A, Lakshman K, Jayaveera K. N, Devaangam S, Kumar A and Manoj B. Antioxidant and antipyretic properties of methanolic extract of Amaranthus spinosus leaves. Asian Pac J Trop Med. 2010;3:702-706.
CrossRef - Ganjare A and Raut N. Nutritional and medicinal potential of Amaranthus spinosus. J Pharmacogn Phytochem. 2019;8(3):3149-3156.
- Ahmed S. A, Hanif S and Iftkhar T. Phytochemical Profiling with Antioxidant and Antimicrobial Screening of Amaranthus viridis L. Leaf and Seed Extracts. Open J Med Microbiol. 2013;3(3):164-171.
CrossRef - Kumar R. P, Shammy J, Nitin G and Rinu R. An inside review of Amaranthus spinosus linn: a potential medicinal plant of India. Int J Res Pharm Chem. 2014;4(3):643-653.
- Bang J. H, Lee K. J, Jeong W.T, Han S, Ick-Hyun J, Seong H. C, Cho H, Hyun T. K, Sung J, Lee J, So Y.S and Chung J. W. Antioxidant Activity and Phytochemical Content of Nine Amaranthus Species. Agronomy. 2021;11(6):1032.
CrossRef - Majumder R, Shahriar S. A, Simu S. Y, Hamad A. F and Alam M. B. Qualitative Analysis of the In vitro Antioxidant Activity of Amaranthus spinosus. Food Pharma Int. 2017;1(2):59-66.
CrossRef - S H. V. In-vitro antibacterial activity of Amaranthus spinosus root extracts. Pharmacophore. 2011;2(5-2011):229-243.
- Nasir R, Alhassan H, Abubakar A, Ridwan N, Hasssan A, Abdulraheem A. Nasir R, Alhasan H, Abubakar A and Ibrahim Y. Phytochemical analysis and antimicrobial activity of leave extract of Amaranthus spinosus. 2020;5(1):42-45.
- Paswan S. K, Srivastava S and Rao C. V. Wound healing, antimicrobial and antioxidant efficacy of Amaranthus spinosus ethanolic extract on rats. Biocatal Agric Biotechnol. 2020;26:101624.
CrossRef - Amabye T. G. Evaluation of Physiochemical, Phytochemical, Antioxidant and Antimicrobial Screening Parameters of Amaranthus spinosus Leaves. Nat Prod Chem Res. 2015;04(01):1000199.
CrossRef - Evans W. C, Trease GE, Evans D. Trease and Evans Pharmacognosy. 16. ed. Saunders/Elsevier; 2009.
CrossRef - Bhavani M. S, Naveena Ch, Nagamani P and Sowmya B. Formulation and Evaluation of Herbal Face Cream. Int J Pharm Sci Rev Res. 2023;83(2):74-78.
CrossRef - Gyawali R, Paudel N, Shrestha S and Silwal A. Formulation and Evaluation of Antibacterial and Antioxidant Polyherbal Lotion. J Inst Sci Technol. 2016;21(1):148-156.
CrossRef








