Vijay Lobo1 , Bakrudeen Ali Ahmed Abdul1*
, Bakrudeen Ali Ahmed Abdul1* , Shenbagavarshini Sivasankar1
, Shenbagavarshini Sivasankar1 , Abdul Hakeem K1
, Abdul Hakeem K1 , Mahmood Pasha1 and Ram Arun Kumar2
, Mahmood Pasha1 and Ram Arun Kumar2
1Department of Biochemistry and Biotechnology, Research Development Cell (RDC), PRIST Deemed University, Thanjavur, India.
2Department of Microbiology, Research Development Cell (RDC), PRIST Deemed University, Thanjavur, India.
Corresponding Author E-mail: bakru24@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3054
Abstract
For the past few years cancer is the second most primary reason for death among human. There are several treatment techniques are raised till now. But they all have side effects and they do not completely eradicate the cancer. Therapy resistant and progression of cancer are frequently caused by the inadequate and generalized targets of conventional therapeutic techniques in the treatment of cancer. Hence, the treatment of the cancer has a challenging one. Over the last decade, stem cell based therapy techniques have become increasingly appealing treatment choices. Although stem cells can be used for regenerative therapy, therapeutic transporters for drugs, biomedical applications, drug targeting and immune cell production, they also show remarkable biological behaviors such as self-renewal, direct motility, differentiating, and immuno regulatory methods. Stem cells are recently being used as delivery vehicles for a variety of specific proteins and viruses, particularly in cancer therapy. The goal of this chapter is i). To highlight a number of research that have effectively used these techniques to combat distinct cancer kinds; ii) With a focus on a variety of factors which are crucial to the victory of future in cancer stem cell therapy and; iii) Focuses on stem cells, is types, properties and stem cell therapies.
Keywords
Cancer stem cells; Stem cell; Stem cell therapy; Transplant; therapeutics
Download this article as:| Copy the following to cite this article: Lobo V, Abdul B. A. A, Sivasankar S, Hakeem K. A, Pasha M, Kumar R. A. Stem Cell Therapies approach for Treating Cancers: Opportunity Progress and Challenges. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Lobo V, Abdul B. A. A, Sivasankar S, Hakeem K. A, Pasha M, Kumar R. A. Stem Cell Therapies approach for Treating Cancers: Opportunity Progress and Challenges. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3ZbNBxE |
Introduction
Benchmark studies in stem cell research
1981 — From mice, embryonic stem cells were isolated
1989 – The first knockout mouse was created.
1998 – In an in vitro laboratory dish, stem cells from a human embryo are grown for the first time.
2001 – Outside the body stem cells are used to make beating heart cells.
2002 – Embryonic stem cells are shown to be capable of producing new heart muscle.
2003 – Cardiac stem cells are discovered.
2004 – Stem cells commit to a future of fat with one signal stem cells and progenitor cells reside in various tissues of the body and are capable of generating daughter cells of different lineages. Fat stem cells, in contrast, appear to be particularly well-suited for the job, as they are capable of transforming into fat, heart, bone, or muscle tissue.
2007 – Discovered that human skin cells can be transformed to iPSCs quickly and efficiently. These iPSCs will be used to create new cardiac cells.
2010-Thymosin beta-4, a protein, encourages stem cells to migrate to wounded tissue and assists in the growth of new blood vessels and muscle cells.
2013- Identified compounds (polymers) that are derived from bacterial culture and can be used to repair damaged heart tissues in the body.
2016 – An endless supply of clean blood for transfusion has been provided by stem cell-derived red blood cells. This could aid those who have lost blood as a result of surgery or an injury.
2022 – Glyoxlase 1 as a therapeutic target in cancer and cancer stem cells
2023 – ALPL-1 target for chimeric antigen receptor therapy in osteosarcoma.
In both industrialized and developing countries, cancer has the main cause of mortality1. Cancer is mainly treated using surgery, chemotherapy and radiotherapy, etc. Yet, therapeutic side effects, antibiotic resistance and mishit impact limits the adequacy of the treatment. Further metastatic cancers are not completely eradicated by traditional therapies. As a result, scientists have attempting to discover novel and effective treatments which do not affect the normal cells. And finally they discovered the stem cell therapy, which is secure and efficient treatment2. Researchers are looking at using stem cells to rebuild the injured organs such as the heart, skin, bone, spinal cord, liver, pancreas and cornea, as well as to cure blood and rigid tissue cancer. They have two main properties: Renewing and differentiate into specific types of cells3.
Stem cells are indistinct biological cells, which have the ability to develop into specific forms of cells/tissues and multiply (via mitosis) indefinitely. Multicellular creatures contain them. All the cells in our body are differentiated cells and only replicate a limited number of times and limited function. However, after development, somatic or adult stem cells are undifferentiated and found among differentiated cells throughout the body. These cells are responsible for facilitating the healing, growth, and replacement of cells that are lost each day4. At different times, stem cells can be discovered in different areas in the body. Stem cells are present in every organ of our body; which has the ability to restore that organ. As we age, stem cells become less active and in some organs they are inactive for the majority of our lives. We may be able to heal or repair the damage that is caused as a result of aging or disease; if we understand how to stimulate or reactivate stem cells that are unique to a tissue5.
Somatic or adult stem cells are undifferentiated and found among differentiated cells in the whole body after development. The function of these cells is to enable the healing, growth, and replacement of cells that are lost each day. In collection of stem cells there are spare embryo – stem cells taken from embryos that have been preserved at reproductive clinic lab; but have not yet been implanted as well as special purpose embryo-an embryo generated by means of in vitro fertilization solely for the aim of obtaining stem cells followed that cloned embryo – an embryo that has been cloned in a laboratory utilizing the somatic nucleus transfer procedure in addition Aborted fetus – stem cells from fetuses that were aborted during the early stages of development and also Adult tissue or organ-obtained by surgery6.
According to scientists, stem cells can help to treat and understand the diseases better. They can be converting into a distinct of other types of cells because they had the ability to do so. They are employed for various purposes such as
Restore damaged tissues or organs with fresh cells that grow in the lab.
Reconstruct any malfunctioning organ parts.
Look into the causes of cellular genetic anomalies.
Look into the causes of diseases or the reasons why some cells develop into cancer cells.
To assure the safety and effectiveness of new medications rigorous testing is done7-9.
Stem cells are categorized as follows based on their potential differentiation: Totipotent cells can form all the cell types in a body that have evolved into all types of cells. The placenta along with the merger of an egg and sperm cell (embryo) produces for further processes. It has zygote formed at egg fertilization and pluripotent states that with the exception of placental tissue, the capability to modify into practically all forms. Ex: cells obtained from 3 germ layers. Whereas, the multipotent ability to modify into a varieties of unique cells belonging to a closely related cell family. These are generalized stem cells with the capacity to self-restoration and modify into specialized cells with specified function over a lengthy period of time ex: hematopoietic stem cells. Oligo-potent is the ability to divide into a few varieties of cell kinds ex: lymphoid and myeloid stem cells. Finally the Unipotent states that the ability to create only one cell type, but with the capacity to self-renew, distinguishing stem cells from non-stem cells ex: muscle stem cells, progenitor cells10.
In general stem cells were classified into ESC’s (embryonic stem cells) and SSC’s (somatic stem cells). SSCs are often referred as adult stem cells, that are commonly multipotent also had the capacity to modify into any type of cells with a specified origin. However, stem cell divided into the neural stem cells (NSCs), mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs) and so on11.
Embryonic Stem Cells (ESCs) are pluripotent stem cells generated from blastocyst (inner cell mass), an embryo in its pre stages. After 4-5 days of fertilization, human embryos attain the stage of blastocyst, which contains 50-150 cells. The fertilized human embryo is demolished when the embryo-blast (inner cell mass) is detached. They are harvested during in-vitro fertilization. They can renew themselves indefinitely and differentiate into almost all types of cells12. These ESCs provide the wide range of therapeutic applications. Because of ethical concerns, ESCs applications in research studies and human trails are restricted. Instead induced pluripotent stem cells (iPSCs) are used13.
Adult Stem Cells (ASCs) couldn’t modify into many other cell types like ESCs. Adult tissues, organs, blood, cord blood and other tissues are used to isolate stem cells. Multipotent stem cells can be used in a various ways. After development they are located throughout the body multiplying by cell proliferation to renewal of died cells and heals injured tissues. They can renew themselves a number of times but not indefinitely. These are widely used because they are free of ethical issues.
Literature Review
Neural stem cells (NSCs)
NSCs had been used to medicate brain, breast, prostate and lung malignancies because they can self-renew and develop into astrocytes, neurons or oligodendrocytes14-17.
Mesenchymal stem cells (MSCs)
MSCs are obtained from the bone marrow and can differentiate cartilage, bone, adipose tissue, stroma, muscle, connective tissue and tendon among other mesodermal cells. They are similar to NSCs in that they’re easy to isolate and are commonly employed to treat cancer18-20.
Hematopoietic stem cells (HSCs)
HSCs are utmost primordial blood cells, develop only in bone marrow and produce grown blood cells by the process of differentiation and proliferation. They produce billions of new blood cells every day. They replace worn out and older blood cells in our body21-22.
Induced Pluripotent Stem Cells (iPSCs) that can be created from somatic cells are known as induced pluripotent stem cells. These are altered form of adult stem cells. They had the capacity to modify into a various forms of specialized cells throughout the body. Mechanism shows that they have the ability to produce new cells for any condition of an organ or a piece of tissue. iPSCs are identical to ESCs, however they don’t have the same immune free and have no ethical concerns. As a result they may be more clinically useful than ESCs13.
Umbilical cord stem cells states that the Cord blood is taken, stored and frozen at the time of delivery. Two types of stem cells are present in umbilical cord blood. They are: Haematopoietic stem cells (HSCs), Mesenchymal stem cells (MSCs). It can be used to cure blood diseases including sickle cell disease, thalassemia and leukaemia23.
Amniotic fluid stem cell comes from Amniotic fluid which contains multipotent stem cells. Adipogenic, osteogenic, myogenic, endothelial, hepatic and also neuronal cell lines can all be differentiated from amniotic stem cells24.
Fetal stem cells are found in the aborted fetal tissue. They had a limited ability for self-restoration. Normal tissue formation from these cells is more challenging. Properties of stem cells are they have the capacity to proliferate and self-restore themselves for a long time; they are generalized; they can modify into specific cell types in response to internal and external stimuli4, 5. Stem cell therapy is the application of healthy adult stem cells to cure diseases or injury in tissue that has been healed or damaged. It has the capacity to self-renovate and modify into specific types of cells. This will potentially replace the diseased and damaged body parts with little risk of rejection or negative consequences. Healthy stem cells are needed to live. During cancer treatments healthy stem cells are destroyed. So stem cell transplantation is the best medication option. There are various stem cell therapies available, however the most are still in the experimental stage, are expensive or are contentious25, 26.
There are three type of stem cells sources such as bone marrow followed that the bloodstream (peripheral blood), and umbilical cord blood from newborn27.
Stem Cells roles in Cancer Therapy
Pluripotent Stem Cells were cultured to form the somatic cells. These stem cells have same as ESCs but also lack ethical concerns. However, T cells and NK cells were the essential resources of iPSCs28-30 and anticancer vaccines are made from these stem cells31, 32.
Adult stem cells were developing into many tissue and organ cell types. HSCs, MSCs and NSCs are commonly used for cancer treatment. All adult blood cells were formed in the body by HSCs and are seen in bone marrow. Until date, the FDA has only approved the use of HSCs obtained from cord blood to treatment of multiple myeloma, leukemia and other blood related diseases33. MSCs are found in a variety of tissues and organs as well as play an important role in healing of tissue and also regeneration. They can multiply quickly and produce a variety of specialized types of cells in a short time. Osteocytes, adipocytes and chondrocytes are examples of cells that can be cultured in in vitro. MSCs are biologically distinct from other forms of stem cells qualities and they’ve been frequently used to complement or deliver therapeutic substances in the treatment of several forms of malignant cancers34, 35. NSCs are self-renewing cells that arise from central nervous system and had the ability to create new neurons and glial cells. They had been thoroughly tested in mice models for the treatment of both primary and secondary breast, lung and prostate cancers36-38.
Cancer stem cells were called as Normal stem cells or CSC precursor/progenitor cells have epigenetic alterations. These are also known stem-like cells, immature progenitors of tumor cells or tumor initiating cells. CSCs can be identified in tumor tissues and play a crucial role in cancer proliferation, migration and renovation. As a result focusing on CSCs could be beneficial and provides a promising treatment for a variety of solid tumors39. Stem cells involve cancer cells have special properties are Karyotype is abnormal; Progeny with a wide range of phenotypes; Capacity to cause tumors; Self-renewal potential that is vast and endless; Within tumors, it’s uncommon and unusual; Mitotic activity is less than other cancerous cells; Self-renewal and differentiation both are highly dysregulated40.
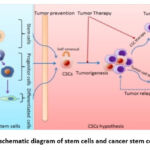 |
Figure 1: schematic diagram of stem cells and cancer stem cells (CSCs) |
Left panel: normal stem cell proliferation and differentiation. Right panel: CSCs and tumorigenesis as well as implications of CSCs for cancer therapy. (Source: Wenjing J, Jianhua P, Yue Z, William C S C, Kunlin J. (2012). The implications of cancer stem cells for cancer therapy. International Journal of Molecular Sciences. 13(12):16636-57. Doi: 10.3390/ijms131216636).
Types of Stem Cell Transplant
Depending on the source of the stem cells, the stem cell transplant were classified into, Bone marrow transplant; Peripheral blood stem cell transplant; Cord blood transplant. They are also called as hematopoietic stem cell transplants. Extremely the high doses of chemotherapy, frequently in combination with radiation therapy, were used to destroy all the cancer cells in a normal cancer stem cell transplant. This therapy also destroys the stem cells in our bodies. Myeloablation or myeloablative therapy is the term used for this. Stem cells are then given (transplanted) to repair those that were destroyed before. Identical to blood transfusion, backup stem cells are injected into a vein. The intention could be for the cells to become normal stem cells after entering the bone marrow. Engraftment is the procedure of grafting cells onto another person’s body over time41, 42.
Transplants are classified as follows: Autologous, auto means self. Here the patient has their own donor. Stem cells for transplant are comes from the same person and transplant comes from the other person either a similar or dissimilar match43.
Autologous Stem Cell Transplant
In some cases the patient is treated with radiation therapy or chemotherapy. Stem cells and our immune system are damaged due to these types of treatments. As a result, before starting cancer treatment doctors extract or harvest our stem cells from our peripheral blood or bone marrow. And it is kept frozen. After the chemotherapy or radiation therapy, the stem cells are returned to our body like blood transfusion. This helps to restore our body’s immune system and helps to make blood cells and fight illness. This procedure is also known as auto transplantation or stem cell rescue.
PRO’S: it avoids the rejection of engrafted cells or graft by our body because they are our own stem cells.
CON’S: In some cases the graft can fail by they do not reach the bone marrow and make blood cells; while harvesting stem cells from our body, cancer cells are also harvested along with them and which is inserted into our body after cancer treatment. This leads to the growth of cancer again; the immune system of our body remains the same before and after the transplant. So cancer cells have the ability to evade our immune system’s attack; this is used to cure leukemia, multiple myeloma and lymphoma. It also sometimes utilised to treat cancers like testicular cancer and neuroblastoma44.
Tandem Transplant
Tandem transplant (double autologous) is that the two autologous transplant are performed within a period of time and does not exceeds more than six months. Some cancers require two doses of chemotherapy, each accompanied by a stem cell transplant. Here the recipient also acts as a donor. This type of transplant shows good result in neuroblastoma and multiple myeloma. The outcomes of this transplant are higher than that of single transplant. So, doctors don’t agree to this transplant45, 46.
Allogenic Stem Cell Transplant
Allogenic stem cell transplant were the given stem cells through transplant. The stem cells are derived from a giver whose tissue form is similar to that of the recipient. Family member, sister or brother is the best donor. If this isn’t matched donor might be found in common people. This transplant is also known as MUD (matched unrelated donor) transplant. MUD transplant are more dangerous than using a family member as a donor. This transplant procedure is same as the autologous transplant.
PRO’S: Donor stem cells develop its own immune cells, which will help to eradicate any cancer cells that sustain the chemotherapy or radiation therapy. The graft-versus cancer or graft versus tumor effect is a term used to describe this phenomenon; if necessary, donor will be asked or requested to contribute extra stem cells or even more white blood cells. Because they are free of cancer cells.
CON’S: The transplant, or graft, will fail if the stem cells of donor die or removed by the body of patient or receiver before settles in the bone marrow; Another concern is that the immune cells of the giver will not only kill the cancer cells, but also other healthy and normal cells in the patient body. The medical term for this condition is graft-versus-host disease; also a minor chance of despite the fact that donors are checked before they are used, certain illnesses from the donor cells are donated into the recipient body; Infections that you’ve had before and that your immune system hasn’t recovered from put you at a higher risk. The immune system has been brought under control. Following an allogeneic transplant, these infections may resurface. Because drugs called immune suppressants keep your immune system in check (suppress it). Infections of this nature can be fatal47-50.
Syngenic Stem Cell Transplant:
Syngenic stem cell transplant is a unique form of transplant reserved for patients who have identical siblings (twins or triplets). PRO’S: Graft versus host disease does not occur; the implanted stem cells do not contain any cancer cells. CON’S: The immune system of the donor and recipient are identical, there is no graft versus cancer impact. To prevent cancer from returning after the transplant, every effort should be made to kill all cancer cells51.
Half Matched Stem Cell Transplant:
Half matched stem cell transplant is the utilization of family members as donors has improved in recent years. This form of transplant is utilized for patients who do not have a completely matched or identical family member. And this is called as half matched (haplo identical) transplant. This is an additional option for MUD transplant52. Demerits that occur after the stem cell transplant are Fever or chills; Shortness of breath; A feeling of heaviness in the chest; Blood pressure becomes low; Coughing; Chest discomfort; Urine is reduced; Faint and feels sick 53.
Stem Cell Resutation in Cancer Therapy
Multiple techniques can be used to modify stem cells, most notably NSCs and MSCs for use in cancer therapy. The following are examples of common modifications: Enzyme / prodrug therapy: NSCs and MSCs can be genetically engineered to produce enzymes which transform non-toxic prodrugs into cytotoxic drugs. When transformed stem cells are implanted into tumour-bearing mice, the foreign enzyme transforms prodrug into a deadly compound, causing the tumor cells to die. From this we conclude that, release of drug can be controlled in terms of quantity, timing, and location. Suicide gene therapy, also termed as enzyme/prodrug therapy, was the first designed NSC therapeutic use to reach clinical trials54,55. The enzyme cytosine deaminase (CD) is a common enzyme utilized in enzyme/prodrug treatment. The prodrug 5-fluorocytosine (5-FC) is converted to the harmful Variant 5-fluorouracil by CD. Glioblastoma (GBM) cell development was suppressed by a pair of CD-bearing mouse NSCs and 5-FC. 5-FC injection of CD-expressing MSCs into the brain Tumor development was also slowed in Human HB156. F3 cells are modified to exhibit CD (HB1.F3.CD) in one of the most often utilized cytotoxic therapies57. HB1.F3.CD/5-FC therapy was currently used in the first human therapeutic trial (ClinicalTrials.gov identifier: NCT01172964), in which patients received oral 5-FC and HB1.F3. CD cells have been implanted into the cavity wall succeeding GBM abscission.
The research has been finished, but the results have not yet been made public. In October 2018, a new trial utilizing engineered NSCs to cure glioma (ClinicalTrials.gov identifier: NCT02015819) will be finished. Suicide gene therapy has also used the herpes simplex virus-thymidine kinase (HSV-TK)58. HSVTK phosphorylates the monophosphorylate Ganciclovir (GCV), a prodrug, to create cytotoxic triphosphate ganciclovir (GCV-TP). During cell division, GCV-TP integrates into the DNA of surrounding cells, causing cell death by inhibiting DNA polymerase. The intra-tumoral HSV-TK-transduced NSC (NSC-TK) injection succeeded by intraperitoneal GCV injection daily for 10 days (two 15 mg/kg doses/day) efficiently cured C6 gliomas in rats. Six of nine rats survived 100 days after being injected with no evidence of tumor. NSCs-TK inserted into the brain moved to the contra lateral hemisphere, co-localized with U87 cells, and provided prolonged viability on GCV-treated animals, according to another study59, 60.
Secreted Agents
Stem cells are able to behave as in situ drug industries, secrets anti-cancer drugs for long periods of time and overpower some of the constraints of cancer therapy, such as raised systemic virulence and low drug half-life. TNF-related apoptosis-inducing ligand (TRAIL) is a frequently utilized secreted therapeutic drug that promotes apoptosis in tumor cells61. In vivo, however, its short half-life decreases its therapeutic efficacy. Encapsulating TRAIL-expressing stem cells in a synthetic extracellular matrix (sECM) which is injected into the GBM resection cavity following surgical debulking should help to attenuate this. At the resection margins, the encapsulated cells might continuously release therapeutic chemicals. In mice, this method reduces the redevelopment of metastatic and intrusive brain tumors and improves survival62.
Table 1: Methods of strategies on Cancer and Application in stem cell research
|
Strategies |
Cancer Types |
Stem Cell Applications |
|
Stem cell modifications |
Glioma |
MSCs (lentiviral and retroviral transduction with S-TRAIL and HSV-TK)18-20 |
|
Enzyme/prodrug therapy |
Colon Adenocarcinoma Metastatic lung cancer Primary lung cancer Glioma |
NSCs (retroviral transduction with CD)33 NSCs (baculoviral transduction with HSV-TK)58 MSCs (retroviral transduction with CD )34-35 NSCs (adenovirus transduction with a rabbit CE)65 NSCs (manipulated to express CE)14-17 |
|
Secreted agents |
Breast cancer brain Metastases Breast cancer Glioma |
NSCs (retrovirus transduction with IL-4 )58 NSCs (adenovirus transduction with TRAIL)59 NSCs (encapsulated in sECM after being modified to express S-TRAIL)60 NSCs (lentivirus transduction with antiHER2Ab)15
|
|
Viral therapy |
Hepatocellular carcinoma Solid tumor Glioma |
MSCs (modified to over express IFN-beta)51 NSCs (affected with CRAd-S-pk7)63 MSCs (armed with oHSV)64 MSCs (affected with measles virus)65 |
|
Nanoparticle carriers |
Hematologic malignancies Liver disease Solid tumor |
NSCs (armed with gold nanorods) 68-70 MSCs (armed with poly-lactic acid nanoparticles and lipid nanocapsules)69-70 |
|
Regenerative medicine |
Lymphomas Hematologic malignancies |
MSCs (armed with nanoparticles)71 HSCs (allogeneic transplantation)72-73 |
|
Immunotherapy |
Liver disease Solid tumor Lymphomas Melanoma Glioma |
iPSCs (engraftment of patient-specific iPSCs)74-75 HSCs (trigger of graft vs. tumor effect )76-77 HSCs (allogeneic transplantation)78-79 iPSCs (produce T cells)80-81 HSCs (genetically modified HSCs to produce antigen-specific CD8 T cells)82-83 |
|
Targeting CSCs |
Glioma |
HSCs (engineering the proteome profile of HSCs )84 |
|
Anticancer drug screening |
Glioma
|
Cancer tissue-derived iPSCs (allocate cellular targets)85 |
Viral Therapy
Unlike standard attenuated viruses, oncolytic viruses (OVs) conditionally reproduce in cancer cells. OVs has proliferated throughout the body and are able to conceal from the immune system. NSCs that have been infected with OVs can still home to cancer cells, and OVs supplied via NSCs had stronger anticancer effects in vivo than the viruses alone against GBMs63. Similarly, NSC-delivered OVs improved viability in glioma-bearing mice after radiation and temozolomide treatment64. Former medical trial for adenovirus-based anti-glioma gene therapy indicated adequate tolerability and no significant side effects65. MSC-mediated virus delivery is also an optimistic method for cancer therapy. The study revealed that the combination of attenuated measles virus’s powerful oncolytic activity and MSCs’ unique immune privileged and tumor tropic features could combat hepato-cellular cancer66. MSCs infected with the measles virus were supplied systemically to tumor injected ortho-topically in the liver, where they transported MV infectivity to cancer cells via hetero-fusion and inhibited tumor development. MSC-mediated administration of oncolytic Herpes simplex virus (oHSV) in a GBM resection mice model increased the virus’ anticancer impacts. oHSV generated by MSC dynamically infected GBM cells in this method, destroying cancer cells in vitro and in vivo. Combining oHSV with TRAIL might be useful in preventing tumor resistance. In mice with oHSV- and TRAIL-resistant GBMs, oHSV/TRAIL-loaded MSCs successfully triggered cancer cell death and increased median viable time67.
Nanoparticle carriers
Nanoparticle carriers (NPs) are used in delivery systems to preserve high-concentration insoluble chemotherapeutic chemicals from decay in a hostile biotic habitat. The use of stem cells as NP delivery agents can overcome constraints such as failed to focus micrometastatic abrasion, ineffective distribution in solid tumors, and others. Stem cells can also protect therapeutic molecules from host immune-surveillance by reducing the uncontrolled absorption of nanoparticles by mononuclear cells68,69. MSCs effectively absorbed NPs and can operate as NP delivery agent in brain malignancies. For tumor-tropic therapy, MSC cell membranes could be armed with doxorubicin-containing porous silica nanorattles70. Compared to free drug or drug delivery systems by silica nano-rattles alone, our technique boosted and extended intra-tumoral drug distribution and accelerated tumor cell death. As a result, stem cell-mediated NP-based medication delivery holds a lot of promise for cancer treatments and deserves more research71.
Other Applications of Stem Cells in Cancer Therapy
Regenerative medicine: Stem cells can be employed to heal human tissues following chemotherapy betwixt of their self-restoration and modification capacities. Later medication of cancers along with elevated-dose radiation or chemotherapy, HSC transplantation has been frequently used in clinical trials to aid long-term haematological recovery. This treatment acts by delivering stem cells which specialize into a required kind of blood cell in order to restore bone marrow in marrow malfunction disorders (e.g., aplastic anemia) and to cure blood cell related genetic diseases. Only one HSC can restore hematopoiesis in patients after transplantation and effective engraftment72,73. Healthy iPSCs produced from patient tissues might hypothetically be utilised to renew tissues which had been injured by tumors or treatment. iPSCs can be used to create different tissues in regenerative medicine. Cancer patients’ iPSCs may benefit from iPSC therapy to repair or replace those that have been destroyed by chemotherapy, radiation, or surgery. In vivo engraftment of iPSC-derived tissues is required for regenerative treatment mediated by human iPSCs. Merely a certain types of human iPSC-derived cells (for example, hepatocytes) had been effectively implanted in animal models to yet74,75.
Immunotherapy
Some hematological malignancies may be cured solely by an immune-mediated anticancer response succeeding allogeneic HSC transplantation76,77. HSCs are promising for use in Cancer immunotherapy because they can be genetically modified to produce chimeric antigen receptors (CARs) or T-cell receptors (TCRs) which target tumor-associated antigens78,79. Immunotherapy techniques could also benefit from patient-specific iPSCs80, 81. T lymphocyte-derived human iPSCs contain the pre-rearranged TCR gene that can be additionally stimulated to develop into functioning active T cells. By reorganizing chosen T cells to become iPSCs and then develop again into T lymphocytes for insertion into victims, functional, tumor antigen-specific T lymphocytes could be created in vitro. The safety of T cell-derived human iPSCs is required to be confirmed82,83.
Targeting CSCs
CSCs are multipotent, self-renewing, and multiplicative, allowing tumor invasion and metastasis to occur quickly. CSCs must therefore be targeted appropriate to achieve increased treatment efficacies and avoid tumor reoccurrence. Normal stem cells could be utilized to target CSCs in cancer therapy since CSCs can attract them. Normal stem cell interactions with CSCs minimize inflammation and death while suppressing tumor proliferation, angiogenesis, and metastasis. It has compared the capability of NSCs and HSCs in anti-glioblastoma therapy84, concluding that HSCs are more suited for creating techniques to control glioblastoma CSC activities than NSCs because HSCs are rarely exposed to neoplastic conversion in neural tumors than NSCs. In the same way, modified HSCs may make it easier to create cell systems that can be used in a variety of applications. Engineered HSCs may also make it easier to create cell systems that can trigger targeted CSC death.
Anticancer drug screening
iPSCs could be utilized to monitor potential antitumor medicines as well as treat malignancies directly. Patient cancer tissue-derived iPSCs can be differentiated to produce cell forms which are further biologically similar to human tumor than currently accessible drug scanning ideas, such as classical cancer cell lines, mice xenograft models, and mouse tumors. Hepatotoxicity may also suppress numerous potential antitumor medicines out from arriving the health care, and also it might be tested by using hepatocytes obtained out of human iPSCs of varied genetic backgrounds85.
Innovation of Stem Cell Research
Molecule with little toxicity reduces the effects of cancer cells
SVC 112 (SuviCa, Inc.), a compound firmed on the chemical bouvardin, was discovered to be effective against neck and head cancer stem cells by researchers86. They came to the conclusion that the cancer stem cells operate as tumour growth controllers, and disrupting this group of cells will cause the cancer to slow or cease87.
Inhibiting Progeny Stem Cells
To treat stomach malignancies, researchers are using osteopontin inhibitors and CD44+ antibodies to see if they can limit the growth of Lgr5-CD44+ cells88.
A Protein Vital for Leukemic Stem Cell function is Identified
The protein HBO1 is needed for leukemic stem cell survival. HBO1 is a protein that aids in the high expression of critical genes that assist leukemic stem cells maintain their functional features89.
Targeting Cancer Stem Cell Energy Supply
Even if only a few survive chemotherapy, tumors can recur if cancer stem cells are not present. As a result, by inhibiting the glutamine satisfy and forcefully starve the tumor, targeting CD9 could provide a treatment option for the disease90-92.
Biomarker and Drug Target for Cancer Stem Cells
Plectin could become a more common pharmacological target than the ones already available. Plectin has the potential to be both a drug lead and a drug delivery agent. Plectin is mostly expressed intracellularly, however it is connected to tumor invasion and metastasis when it is translocated to the cell surface93.
Conclusion
Stem cell therapy is only hope for cancer treatment. It may open new doors for the cancer treatment. Not only stem cell therapy be used to treat cancer but it can also be used to treat a variety of other diseases also. In this thesis, we focus on the stem cell, its types, cancer stem cells, stem cell therapy, stem cell modifications and provide update on the other applications of stem cells in cancer therapy. Different types of stem cells are used in anti-cancer therapy depending on their inherent capabilities. Despite the fact that clinical trials were successful, there are still many obstacles to overcome. Outcomes of stem cell therapy in cancer are highly encouraging but still it needs to enhance the treatment’s safety and efficacy.
Acknowledgement
The authors would like to extend their sincere appreciation to the Research and Development Cell (RDC) at PRIST Deemed University for their support during Project works.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Conflict of Interests
The authors do not have any conflict of interest
Data Availability Statement
This statement does not apply to this article
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required
Permission to reproduce material from other sources
Figure.1 schematic diagram of stem cells and cancer stem cells (CSCs)
Wenjing J, Jianhua P, Yue Z, William C S C, Kunlin J. (2012). The implications of cancer stem cells for cancer therapy. International Journal of Molecular Sciences. 13(12):16636-57. Doi: 10.3390/ijms131216636).
Authors’ Contribution
Bakrudeen Ali Ahmed Abdul – Conceptualization, Writing – Supervision.
Vijay Lobo – First author, Data Collection, Draft, Writing,
Shenbagavarshini Sivasankar – Visualization, Data typing, writing part.
Abdul Hakeem K – Resources, Editing, Rewriting , Data collection
Mahmood Pasha – Editing, Writing, Resources, Table, References
Arun Kumar R– Rewriting, Co-supervision, Editing, Data collection
References
- Siegel R. L, Miller K. D, Ahmed J. Cancer statistics. CA Cancer Journal for Clinicians., 2016; 66: 7–30.
CrossRef - Cheng L. Z, Ting H, Bi-Li W, Wen-Xi H, Dong L. Stem cells in cancer therapy: opportunities and challenges. Oncotarget., 2017; 8: 75756-75766.
CrossRef - Reya T, Morrison S. J, Clarke M. F, Irving L. W. Stem cells, cancer, and cancer stem cells. Nature., 2001; 414: 105-11.
CrossRef - Tran C, Damaser M. S. Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Advance Drug Delivery Reviews., 2015; 82-83: 1–11.
CrossRef - Seita J, Rossi D. J, Weissman I. L. Differential DNA damage response in stem and progenitor cells. Cell Stem Cell., 2010; 7: 145–147.
CrossRef - National Institutes of Health (NIH). Stem cell basics. Access at https://stemcells.nih.gov/info/basics.html on March 17, 2020.
- Lee R. H, Oh J. Y, Choi H, Nikolay B. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. Journal of Cellular Biochemistry., 2011; 112: 3073– 3078.
CrossRef - Fakunle E. S, Loring J. F. Ethnically diverse pluripotent stem cells for drug development. Trends in Molecular Medicine., 2012; 18 (12): 709-716.
CrossRef - Guye P, Ebrahimkhani M. R, Kipniss N, Velazquez J. J, Schoenfeld E, Kiani S, Griffith L. G, Weiss R. Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nature Communications., 2016; 7: 10243.
CrossRef - Fortier L. A. Stem cells: classifications, controversies, and clinical applications. Veterinary surgery., 2005; 34(5): 415-423.
CrossRef - Xiao J, Mu J, Liu T, Xu H. Dig the root of cancer: targeting cancer stem cells therapy. Journal of Medical Discovery., 2017; 2(2): 1-7. JMD17003.
CrossRef - Lin H. T, Otsu M, Nakauchi H. Stem cell therapy: an exercise in patience and prudence. Philosophical transactions of the Royal Society B: Biological Sciences., 2013; 368(1609): 20110334.
CrossRef - Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell., 2006; 126: 663–676.
CrossRef - Bago J. R, Sheets K. T, Hingtgen S. D. Neural stem cell therapy for cancer. Methods., 2016; 99: 37-43.
CrossRef - Kanojia D, Balyasnikova I. V, Morshed R. A, Frank R. T, Yu D, Zhang L, Spencer D. A, Kim J. W, Han Y, Yu D, Ahmed A. U, Aboody K. S, Lesniak M. S. Neural stem cells secreting anti-her2 antibody improve survival in a preclinical model of her2 over expressing breast cancer brain metastases. Stem Cells., 2015; 33(10): 2985–2994.
CrossRef - Lee H. J, Doo S. W, Kim D. H, Cha Y. J, Kim J. H, Yun Seob Song Y. S, Kim U. S. Cytosine deaminase-expressing human neural stem cells inhibit tumor growth in prostate cancer-bearing mice. Cancer Letters., 2013; 335(1): 58–65.
CrossRef - Yi B. R, Kim S. U, Choi K. C. Co-treatment with therapeutic neural stem cells expressing carboxyl esterase and CPT-11 inhibit growth of primary and metastatic lung cancers in mice. Oncotarget., 2014; 5(24): 12835–12848.
CrossRef - Bernardo M. E, Fibbe W. E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell., 2013; 13(4): 392–402.
CrossRef - Milwid J. M, Elman J. S, Li M, Shen K, Manrai A, Gabow A, Yarmush J, Jiao Y, Fletcher A, Lee J, Cima M. J, Yarmush M. L, Parekkadan B. Enriched protein screening of human bone marrow mesenchymal stromal cell secretions reveals MFAP5 and PENK as novel IL-10 modulators. Molecular Therapy., 2014; 22(5): 999–1007.
CrossRef - Motaln H, Gruden K, Hren M, Schichor C, Primon M, Rotter A, Lah T. T. Human mesenchymal stem cells exploit the immune response mediating chemokines to impact the phenotype of glioblastoma. Cell Transplant., 2012; 21(7): 1529-1545.
CrossRef - Khurana S, Margamuljana L, Joseph C, Schouteden S, Buckley S. M, Verfaillie C. M. Glypican-3-mediated inhibition of CD26 by TFPI: a novel mechanism in hematopoietic stem cell homing and maintenance. Blood., 2013; 121(14): 2587-2595.
CrossRef - Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C. Mobilization and homing of hematopoietic stem cells. Advances in Experimental Medicine and Biology., 2012; 741: 152–170.
CrossRef - Kawabata A, Ohta N, Seiler G, Pyle M. M, Ishiguro S, Zhang Y. Q, Becker K. G, Troyer D, Tamura M. Naive rat umbilical cord matrix stem cells significantly attenuate mammary tumor growth through modulation of endogenous immune responses. Cytotherapy., 2013; 15(5): 586-597.
CrossRef - Bitsika V, Roubelakis M. G, Zagoura D, Trohatou O, Makridakis M, Pappa K. I, Marini F. C, Vlahou A, Anagnou P. N. Human amniotic fluid-derived mesenchymal stem cells as therapeutic vehicles: A novel approach for the treatment of bladder cancer. Stem Cells and Development. 2012; 21(7): 1097-1111.
CrossRef - American Society of Clinical Oncology (ASCO). What is a stem cell transplant (bone marrow transplant)? Accessed at https://www.cancer.net/navigating-cancercare/how-cancer-treated/bone-marrowstem-cell-transplantation/what-bone-marrowtransplant-stem-cell-transplant on March 17, 2020.
- Din D. C, Chang Y. H, Shyu W. C, Lin S. Z. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplantation., 2015; 24, 339–347.
CrossRef - Diana K, Agnieszka K. B, Wojciech Łabus W, Kraut M, Glik J, Kawecki M, Kuźma A. Amniotic and umbilical cord of transgenic pigs as an alternative sources of stem cells. Transplantation proceedings., 2020; 5(7): 2193-2197.
CrossRef - Li Y, Hermanson D. L, Branden S Moriarity B. S, Kaufman D. S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell., 2018; 23(2): 181–192.
CrossRef - Ruella M, Kenderian S. S. Next-Generation Chimeric Antigen Receptor T-Cell Therapy: Going off the Shelf. BioDrugs., 2017; 31(6): 473–481.
CrossRef - Timmermans F, Velghe I, Vanwalleghem L, De Smedt M, Coppernolle S. V, Taghon T, Moore H. D, Leclercq G, Langerak A. W, Kerre T, Plum J, Vandekerckhove B. Generation of T Cells from Human Embryonic Stem Cell-Derived Hematopoietic Zones. Journal of Immunology., 2009; 182(11): 6879-6888.
CrossRef - Kooreman N. G, Kim Y, de Almeida P. E, Termglinchan V, Diecke S, Shao N, Wei T, Yi H, Dey D, Nelakanti R, Brouwer T. P, Paik D. T, Sagiv-Barfi I, Han A, Quax P. H. A, Hamming J. F, Levy R, Davis M. M, Wu J. C. Autologous iPSC-Based Vaccines Elicit Anti-tumor Responses In Vivo. Cell Stem Cell., 2018; 22(4): 501–513.
CrossRef - Ouyang X, Telli M. L, Wu J. C. Induced Pluripotent Stem Cell-Based Cancer Vaccines. Frontiers in Immunology., 2019; 10: 1510.
CrossRef - Copelan EA. Hematopoietic Stem-Cell Transplantation. New England Journal of Medicine., 2006; 354: 1813–1826.
CrossRef - Christodoulou I, Goulielmaki M, Devetzi M, Panagiotidis M, Koliakos G, Zoumpourlis V. Mesenchymal stem cells in preclinical cancer cytotherapy: A systematic review. Stem Cell Research and Therapy., 2018; 9: 336.
CrossRef - Lin W, Huang L, Li Y, Fang B, Li G, Chen L, Xu L. Mesenchymal Stem Cells and Cancer: Clinical Challenges and Opportunities. BioMed Research International., 2019; 2019: 2820853.
CrossRef - Kanojia D, Balyasnikova I. V, Morshed R. A, Frank R. T, Yu D, Zhang L, Spencer D. A, Kim J. W, Han Y, Yu D, Ahmed A. U, Aboody K. S, Lesniak M. S. Neural Stem Cells Secreting Anti-HER2 Antibody Improve Survival in a Preclinical Model of HER2 Over expressing Breast Cancer Brain Metastases. Stem Cells., 2015; 33: 2985-2994.
CrossRef - Lee H. J, Doo S. W, Kim D. H, Cha Y. J, Kim J. H, Song Y. S, Kim S. U. Cytosine deaminase-expressing human neural stem cells inhibit tumor growth in prostate cancer-bearing mice. Cancer Letters., 2013; 335: 58–65.
CrossRef - Yi B. R, Kim S. U, Choi K. C. Co-treatment with therapeutic neural stem cells expressing carboxyl esterase and CPT-11 inhibit growth of primary and metastatic lung cancers in mice. Oncotarget., 2014; 5: 12835–12848.
CrossRef - Chang J. C. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine., 2016; 95 (Suppl. 1); S20-S25.
CrossRef - Wei G, Joseph L. L, Hong W. Cancer stem cells. Pediatric Research., 2006; 59(4 Pt 2): 59R-64R.
CrossRef - Atilla P. A, Demirer T. A review of myeloablative vs reduced intensity/nonmyeloablative regimens in allogeneic hematopoietic stem cell transplantations. Balkan Medical Journal., 2017; 34(1): 1-9.
CrossRef - Alessandro I, Federica L, Visani G, Chiarucci M, Musto P, Kubasch A, Platzbecker U, Vinchi F. Iron toxicity and chelation therapy in hematopoietic stem cell transplant. Transplantation and cellular therapy., 2021; 27(5): 371-379.
CrossRef - Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T, Hayashi T, Onoe H, Shiina T, Yamanaka S, Takahashi J. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem Cell Reports., 2013; 1(4): 283-292.
CrossRef - Delemarre E. M, Van D. B. T, Mijnheer G, Jenny Meerding J, Wehrens E. J, Olek S, Boes M, van Herwijnen M. J. C, Broere F, Royen A. V, Wulffraat N. M, Prakken B. J, Spierings E, Wijk F. V. Autologous stem cell transplantation AIDS autoimmune patients by functional renewal and TCR diversification of regulatory T cells. Blood., 2016; 127: 91-101.
CrossRef - Nico G, Diderik J. E, Linda K, Caillot D, Pioltelli P, Lleonart J. B, Reményi P, Blaise D, Schaap N, Trneny M, Passweg J, Porras R. P, Cahn J. Y, Musso M, Poiré X, Fenk R, Itälä-Remes M, Pavone V, Fouillard L, Maertens J, Bron D, Pouli A, Schroyens W, Schönland S, Garderet L, Yakoub-Agha I, Kröger N. Tandem Autologous Stem Cell Transplantation Improves Outcomes in Newly Diagnosed Multiple Myeloma with Extramedullary Disease and High-Risk Cytogenetics: A Study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. American Society for Transplantation and Cellular Therapy., 2019; 25(11): 2134-2142.
CrossRef - Thompson P. A, Perera T, Marin D, Oran B, Popat U, Qazilbash M, Shah N, Parmar S, Rezvani K, Olson A, Kebriaei P, Anderlini P, Rondon G, Alousi A, Ciurea S, Champlin R. E, Bajel A, Szer J, Shpall E. J, Ritchie D, Hosing C. M. Double umbilical cord blood transplant is effective therapy for relapsed or refractory Hodgkin lymphoma. Leukemia and Lymphoma., 2016; 57(7): 1607-1615.
CrossRef - Gaballa S, Palmisiano N, Alpdogan O, Carabasi M, Filicko-O’Hara J, Kasner M, Kraft W. K, Leiby B, Martinez-Outschoorn U, O’Hara W, Pro B, Rudolph S, Sharma M, Wagner J. L, Weiss M, Flomenberg N, Grosso D. A two-step haploidentical versus a two-step matched related allogeneic myeloablative peripheral blood stem cell transplantation. Biology of Blood and Marrow Transplantation., 2016; 22(1): 141-148.
CrossRef - Wang Y, Chen F, Gu B, Chen G, Chang H, Wu D. Mesenchymal stromal cells as an adjuvant treatment for severe late-onset hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Acta Haematologica., 2015; 133(1): 72-77.
CrossRef - Nauta A. J, Westerhuis G, Kruisselbrink A. B, Lurvink E. G. A, Willemze R, Fibbe W. E. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a non-myeloablative setting. Blood., 2006; 108: 2114–2120.
CrossRef - Baron F, Lechanteur C, Willems E, Bruck F, Baudoux E, Seidel L, Vanbellinghen J, Hafraoui K, Lejeune M, Gothot A, Fillet G, Beguin Y. Cotransplantation of Mesenchymal Stem Cells Might Prevent Death from Graft-versus-Host Disease (GVHD) without Abrogating Graft-versus-Tumor Effects after HLA-Mismatched Allogeneic Transplantation following Non-myeloablative Conditioning. Biology of Blood and Marrow Transplantation., 2010; 16: 838–847.
CrossRef - Ling X, Marini F, Konopleva M, Wendy Schober W, Shi Y, Burks J, Clise-Dwyer K, Wang R, Zhang W, Yuan X, Lu H, Caldwell L, Andreeff M. Mesenchymal stem cells Over expressing IFN-beta Inhibit Breast Cancer Growth and Metastases through Stat3 Signaling in a Syngeneic Tumor Model. Cancer Microenvironment., 2010; 3(1): 83-95.
CrossRef - Hanna R, Majhail N. S. HLA-identical siblings versus haploidentical donors: full match still beats half match. Bone marrow transplantation., 2016; 51(3): 344-345. DOI: 10.1038/bmt.2015.291
CrossRef - American Society of Clinical Oncology (ASCO). Side effects of a bone marrow transplant (stem cell transplant). Accessed at https://www.cancer.net/navigatingcancer-care/how-cancer-treated/bone-marrowstem-cell-transplantation/side-effectsbone-marrow-transplant-stem-cell-transplant on March 17, 2020.
- Aboody K. S, Brown A, Rainov N. G, Bower K. A, Liu S, Yang W, Small J. E, Herrlinger U, Ourednik V, Black P. M, Breakefield X. O, Snyder E. Y. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proceedings of the National Academy of Sciences of the United States of America., 2000; 97: 12846–12851.
CrossRef - Aboody K. S, Najbauer J, Metz M. Z, D’Apuzzo M, Gutova M, Annala A. J, Synold T. W, Couture L. A, Blanchard S, Moats R. A, Garcia E, Aramburo S, Valenzuela V. V, Frank R. T, Barish M. E, Brown C. E, Kim S. U, Badie B, Portnow J. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Science Translational Medicine., 2013; 5: 159r–184r.
CrossRef - Altaner C, Altanerova V, Cihova M, Ondicova K, Rychly B, Baciak L, Mravec B. Complete regression of glioblastoma by mesenchymal stem cells mediated pro-drug gene therapy simulating clinical therapeutic scenario. International Journal of Cancer., 2014; 134: 1458–1465.
CrossRef - Aboody K. S, Najbauer J, Danks M. K. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Therapy., 2008; 15: 739-752.
CrossRef - Martinez-Quintanilla J, Bhere D, Heidari P, He D, Mahmood U, Shah K. Therapeutic efficacy and fate of bimodal engineered stem cells in malignant brain tumors. Stem Cells., 2013; 31: 1706–1714.
CrossRef - Li S, Tokuyama T, Yamamoto J, Koide M, Yokota N, Namba H. Bystander effect-mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Therapy., 2005; 12: 600–607.
CrossRef - Zhao Y, Lam D. H, Yang J, Lin J, Tham C. K, Ng W. H, Wang S. Targeted suicide gene therapy for glioma using human embryonic stem cell-derived neural stem cells genetically modified by baculoviral vectors. Gene Therapy., 2012; 19: 189-200.
CrossRef - Stuckey D. W, Shah K. TRAIL on trial: preclinical advances in cancer therapy. Trends in Molecular Medicine., 2013; 19: 685-694.
CrossRef - Kauer T. M, Figueiredo J. L, Hingtgen S, Shah K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nature Neuroscience., 2011; 15: 197–204.
CrossRef - Wollmann G, Ozduman K, van den Pol A. N. Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates. Cancer Journal., 2012; 18: 69–81.
CrossRef - Tobias A.L, Thaci B, Brenda Auffinger B, Rincon E, Balyasnikova I. V, Kim C. K, Han Y, Zhang L, Aboody K. S, Ahmed A. U, Lesniak M. S. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Translational Medicine., 2013; 2: 655-666.
CrossRef - Pulkkanen K. J, Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Molecular Therapy., 2005; 12: 585–598.
CrossRef - Ong H. T, Federspiel M. J, Guo C. M, Ooi L. L, Russell S. J, Kah-Whye Peng K, Kam M Hui K. M. Systemically delivered measles virus infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. Journal of Hepatology., 2013; 59: 999–1006.
CrossRef - Duebgen M, Martinez-Quintanilla J, Tamura K, Hingtgen S, Redjal N, Wakimoto H, Shah K. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. Journal of National Cancer Institute., 2014; 106: u90.
CrossRef - Auffinger B, Morshed R, Tobias A, Cheng Y, Ahmed A. U, Lesniak M. S. Drug-loaded nanoparticle systems and adult stem cells: a potential marriage for the treatment of malignant glioma? Oncotarget., 2013; 4: 378–396.
CrossRef - Mooney R, Roma L, Zhao D, Haute V. D, Garcia E, Kim S. U, Annala A. J, Aboody K. S, Berlin J. M. Neural stem cell mediated intratumoral delivery of gold nanorods improves photothermal therapy. ACS Nano., 2014; 8: 12450–12460.
CrossRef - Roger M, Clavreul A, Venier-Julienne M, Passirani C, Sindji L, Schiller P, Montero-Menei C, Menei P. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials., 2010; 31: 8393–8401.
CrossRef - Li L, Guan Y, Liu H, Hao N, Liu T, Meng X, Fu C, Li Y, Qu Q, Zhang Y, Ji S, Chen L, Chen D, Fangqiong Tang Silica nanorattledoxorubicin-anchored mesenchymal stem cells for tumortropic therapy. ACS Nano., 2011; 5: 746.
CrossRef - Okabe M, Otsu M, Ahn D. H, Kobayashi T, Morita Y, Wakiyama Y, Onodera M, Eto K, Ema H, Nakauchi H. Definitive proof for direct reprogramming of hematopoietic cells to pluripotency. Blood., 2009; 114: 1764–1767.
CrossRef - Barriga F, Rojas N, Wietstruck A. Alternative Donor Sources for Hematopoietic Stem Cell Transplantation. Innovations in Stem Cell Transplantation. Intech., 2013.
CrossRef - Liu H, Kim Y, Sharkis S, Marchionni L, Jang Y. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Science Translational Medicine., 2011; 3: 39r-82r.
CrossRef - Choi S. M, Kim Y, Liu H, Chaudhari P, Ye Z, Jang Y. Liver engraftment potential of hepatic cells derived from patient specific induced pluripotent stem cells. Cell Cycle., 2011; 10: 2423–2427.
CrossRef - Renga M, Pedrazzoli P, Siena S. Present results and perspectives of allogeneic non-myeloablative hematopoietic stem cell transplantation for treatment of human solid tumors. Annals of Oncology., 2003; 14: 1177–1184.
CrossRef - Bertz H, Illerhaus G, Veelken H, Finke J. Allogeneic hematopoetic stem-cell transplantation for patients with relapsed or refractory lymphomas: comparison of highdose conventional conditioning versus fludarabine-based reduced-intensity regimens. Annals of Oncology., 2002; 13: 135–139.
CrossRef - Gschweng E, De Oliveira S, Kohn D. B. Hematopoietic stem cells for cancer immunotherapy. Immunological Reviews., 2014; 257: 237-249.
CrossRef - Vatakis D. N, Koya R. C, Nixon C. N, Wei L, Kim S. G, Avancena P, Bristol G, Baltimore D, Kohn D. B, Ribas A, Radu C. G, Galic Z, Zack J, A. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America., 2011; 108: E1408–E1416.
CrossRef - Karan D, Dubey S, Veldhuizen P. V, Holzbeierlein J. M, Tawfik O, Thrasher J. B. Dual antigen target-based immunotherapy for prostate cancer eliminates the growth of established tumors in mice. Immunotherapy-UK., 2011; 3: 735-746.
CrossRef - Serwold T, Hochedlinger K, Swindle J, Hedgpeth J, Jaenisch R, Weissman I. L. T-cell receptor-driven lymphomagenesis in mice derived from a reprogrammed T cell. Proceedings of the National Academy of Sciences of the United States of America., 2010; 107: 18939–18943.
CrossRef - Brown M. E, Rondon E, Rajesh D, Mack S, Lewis R, Feng X, Zitur L. J, Learish R. D, Nuwaysir E. F. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLOS One., 2010; 5:e11373.
CrossRef - Loh Y. H, Hartung O, Li H, Chunguang Guo C, Sahalie J. M, Manos P. D, Urbach A, Heffner G. C, Grskovic M, Vigneault F, Lensch M. W, Park I, Agarwal S, Church G. M, Collins J. J, Irion S, Daley G. Q. Reprogramming of T cells from human peripheral blood. Cell Stem Cell., 2010; 7: 15–19.
CrossRef - Bryukhovetskiy I. S, Dyuizen I. V, Shevchenko V. E, Bryukhovetskiy A. S, Mischenko P. V, Milkina E. V, Khotimchenko Y. S. Hematopoietic stem cells as a tool for the treatment of glioblastoma multiforme. Molecular Medicine Reports., 2016; 14: 4511–4520.
CrossRef - Wilke R. A, Lin D. W, Roden D. M, Watkins P. B, Flockhart D, Zineh I, Giacomini K. M, Krauss R. M. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nature Reviews Drug Discovery., 2007; 6: 904–916.
CrossRef - Yoshida Y, Nagaishi C, Hasuda T, Park H, Takeya K, Hitotsuyanagi Y, Hitotsuyanagi Y. Synthesis and Cytotoxicity Evaluation of Tyrosine-5 Fluorinated Analogues of RA-VII, An Antitumor Bicyclic Hexapeptide. Synlett., 2023; 34: 16; 1905-1910.
CrossRef - Keysar S. B, Gomes N, Miller B, Jackson B. C, Le P. N, Morton J. J, Reisinger J, Chimed T, Gomez K. E, Nieto C, Frederick B, Pronk G. J. Inhibiting translation elongation with SVC112 suppresses cancer stem cells and inhibits growth in head and neck squamous carcinoma. Cancer Research., 2020; 80: 5; 1183-1198.
CrossRef - Tao D, Lou S, Huang W, Kaidi L.J, Wang Z, Pi Y, Zhao Y, Wen J, Xie Q, Meng F, Lou G. Clinical and prognostic significance of FBXL6 expression in ovarian cancer. Gene., 2025; 93315: 1, Article number 148978.
CrossRef - Yokoyama A, Niida H, Kutateladze T. G, Cote jacques. HBO1, a MYSTerious KAT and its links to cancer. Biochimica et Biophysica Acta – Gene Regulatory Mechanism., 2024; 1867: 3, Article number 195045.
CrossRef - Hasoglu I, Karatug K. A. The therapeutic effects of exosomes the first time isolated from pancreatic islet-derived progenitor cells in the treatment of pancreatic cancer. Protoplasma., 2024; 261: 2; 281-291.
CrossRef - Kim J. Y, Jung J. H, Lee S J, Han S S, Hong S H. Glyoxalase 1 as a Therapeutic Target in Cancer and Cancer Stem Cells. Molecules and Cells., 2022; 45(12): 869–876.
CrossRef - Pajenda S, Gerges D, Wagner L, O’Connell D, Aiad M, Imre R, Mechtler K, Zimprich A, Schmidt A, Sengoelge G, Winnicki W. Identification of Pathogenic Pathways for Recurrence of Focal Segmental Glomerulosclerosis after Kidney Transplantation. Diagnostics., 2024; 14: Article number 1591.
CrossRef - Tan Z, Tian L, Luo Y, Ai K, Zhang X, Yuan H, Zhou J, Ye G, Yang S, Zhong M, Li G, Wang Y. Preventing postsurgical colorectal cancer relapse: A hemostatic hydrogel loaded with METTL3 inhibitor for CAR-NK cell therapy. Bioactive Materials., 2025; 44: 236-255.
CrossRef








