Shagufta Bi1* , Rashi Srivastava
, Rashi Srivastava and Tanzeel Ahmad
and Tanzeel Ahmad
School of Biotechnology, IFTM University, Lodhipur Rajpoot, Delhi road, Moradabad, Uttar Pradesh, India
Corresponding Author E-mail:shaguftabt@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3053
Abstract
This research outlines that the manufacture of palladium nanoparticles (PdNPs) as being straight forward, inexpensive, and environmentally friendly. Rosa damascena flower extract was successfully used in this investigation to reduce palladium nanoparticles (PdNPs). UV-VIS, FTIR, XRD, TEM, EDX, DLS, and SEM were used to the characterized of the biologically formed of PdNPs. Upon UV-visible irradiation of physico-chemically produced PdNPs, the SPR peak was measured at 360 nanometers. The TEM investigation indicated that the produced palladium nanoparticles had a spherical form and a diameter of around 50 nm. The biologically synthesized PdNPs demonstrated notable antimicrobial activity, including antifungal activity. PdNPs shows antifungal activity against some fungal species such as Aspergillus niger, Aspergillus flavus and Candida albicans. Based on findings, zone of inhibition of fungal strains is less than from fungal strains with Fluconazole. Bio-inspired production of PdNPs allows for adaptability to fungus strains. This makes PdNPs more suitable for biomedical applications.
Keywords
Antifungal Activity; FTIR; Green synthesis; Palladium nanoparticles; TEM; UV-Visible spectroscopy
Download this article as:| Copy the following to cite this article: Bi S, Srivastava R, Ahmad T. The Potential Antifungal Activity of the Developed Palladium Nanoparticles. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Bi S, Srivastava R, Ahmad T. The Potential Antifungal Activity of the Developed Palladium Nanoparticles. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/4fnql5k |
Introduction
A new discipline of nanobiotechnology was recently formed due to the merging of nanometers-scale technologies with biological technologies. This newly developed area concentrates on the production, control, and application of nano-sized materials for emerging biotechnology1. Globally, studies and research in nanotechnology have rapidly advanced quickly. Although the science of nanotechnology has the potential to expand, some people are still concerned on the possible risks and effects of nanoparticles on human health and the environment2, 3, 4. A primary function of nanoparticles is to act as a link connecting larger particles and molecular or atomic structures5. The multidisciplinary fields encompassing research and technology from physics, chemistry, and biology, usually known as nanotechnology, was initially introduced in 1974 by Prof. Norio Taniguchi.
In the last twenty years, the synthesis of nanoparticles has ushered in nanotechnology, nanoparticles synthesis, and the invention of materials with a variety of uses6, 7. Among these, palladium, platinum, gold, and silver nanoparticles have been extensively studied to better understand their size and shape-dependent physicochemical properties and their interactions with various substances and molecules8, 9. Much work has gone into creating innovative and promising processes for making noble metal nanoparticles. Overall, top-down to bottom-up procedures are able of producing of nanoparticles. Top-down methods involve slicing original materials to create nanoparticles10. The bottom-up approach entails creating nanoparticles from smaller compounds like atoms and molecules, which then develop into nano-sized particles using different biological and chemical techniques11.
The three most crucial requirements are choosing a safe material for stabilization, an appropriate non-toxic oxidizer, and an eco-friendly liquid (the most popular ones are ethanol, water, and their mixtures)12. Many synthetic approaches have been utilized in order to produce nanoparticles; the most common method is biophysical, molecular, and biological. Biochemical processes usually contain the use of dangerous reagents. They are also quite expensive13. Among the most throughout comparison from the other methods like physical and chemical, plant- mediated processes offer a secure, non-toxic, and eco-friendly way to synthesis nanoparticles for a variety of functions such as physiological ones14, 15. The above synthesis process has been accomplished using algae, fungi, bacteria, and plants16, 17. However, plant extracts may be used to create nanoparticles with certain sizes, shapes, and contents. Furthermore, the range of physicochemicals present in their material might function as organic stabilizing and reducing agents for the nanoparticles production process18, 19. The biological formation of palladium nanoparticles has been investigated in types of plant species, such as Annona squamosa20. In a well-diffusion assay, palladium nanoparticles were shown to have antimicrobial activity against anti-fungal strains21. A few species of the common, filamentous, mycotoxigenic fungus Aspergillus, including A. niger and A. flavus, are referred to as pathogens that like advantages of their surroundings22. Candida species cause chronic mycosis, which affects the mouth, throat, skin, ovaries, and the gut23, 24, 25. The potential for harm from these species to humans also depends on how well-developed their immune systems when inhaled or injected with spores. They are responsible for 90-100% of infections, including invasive aspergillosis (IA)26, which has a high fatality rate in critically ill and immunocompromised persons.As a result, they can spread locally or disseminate to other locations.Delaying the initiation of antifungal medication due to delayed diagnosis worsens the prognosis of IA27. 28. As a result, it is still difficult to definitively diagnose fungal infections, especially in immuno-compromised hosts. Furthermore, if any fungal infection symptoms exist, they are typically non-specific29. The identification of the etiological agent may result from culture-based diagnostics, which has the potential to be a laborious procedure. Furthermore, the place of collection may have an impact on the sample’s sensitivity, and contamination may go unnoticed30. The abuse of currently available antifungal medications, such as fluconazole, azoles, echinocandins, and polyenes, has caused the appearance of clinical drug-resistant Aspergillus species in recent years31, 32. As an illustration, azole resistance is uncommon and results from a shift in the amount of ergosterol or from the creation of other forms of sterols that make up the membranes. Without impairing regular cell function, this process might be able to explain the resistance. Nevertheless, studies have shown that resistance to azole medications can have major clinical consequences; individual with aspergillosis that is resistant to therapy have a 25% increased risk of death33. On the other hand, current research suggests that metallic nanoparticles be employed to treat Aspergillus sp.-caused fungal infections34. Systemic candidaemia, a potentially fatal condition, can also result from candida infections35. The yearly occurrences of Candida infections are estimated to be approx 4 million cases globally36, with a death rate of roughly 40%37. This leads to a substantial financial strain due to the high cost of medications and extended hospital stays38. Candida albicans is the main pathogen regarding the pathogenic agents of Candidiasis due to its phenotypic flexibility and high infection frequency39. Fungal virulence factors impact C. albicans colonization and infection. These variables comprise the capacity to develop at 37 degree C, structure transition, hemolytic activity, and release of hydrolytic enzymes, tissues adhesion, immune system evasion, filamentation ability, and biofilm formation40, 41. Recent years have seen a rise in research on nanotechnology, and the utilization of nanoparticles in clinical and diagnostics has been crucial to this field’s expansion, essentially replacing traditional treatment modalities42. The size, shape, and metallic element of the particles all play a role. Biosynthetic NPs offer several beneficial characteristics, including reduced toxicity and remarkable catalytic and physicochemical capabilities43, 44. With significant efficacy against the growth of A. niger45, Aspergillus flavus 46, and Candida albicans 47, PdNPs are particularly well-known for their antifungal activities. Since Aspergillus sp. and Candida albicans resistance to standard antifungal therapy essentially provides a principle obstacle to the prevention of diseases, the introduction of NPs as microbial drugs could significantly enhance the control of Aspergillus along with other species infections. Compared to chemical, and physical processes, biosynthesis procedure has several benefits, such as low environmentally impact, more affordable, free from pollutants, and high sustainable. They have numerous applications in material science, medicine, and agriculture. Bio-inspired palladium nanoparticles from Rosa damascena plant extract increase antifungal impact and making nanoparticles suitable for site-specific administration. PdNPs also display improved biological recognition, enabling specific interaction with fungal cell components. Belonging to the family of Rosaceae, Rosa damascena uses in the various fields like pharmacological characteristics encompass antibacterial, antifungal, etc. This research investigates the green manufacturing of palladium nanoparticles used extract of R. damascena plant. Extract from flower is used in the manufacturing of palladium nanoparticles as a moderating agent. After green procedure of PdNPs in-vitro study was done, such as checking the activity of palladium nanoparticles against antifungal strains.
Materials and Methods
Chemicals and Plant
The high-level analytical chemicals substances utilized all of the materials used in this work were bought by Sigma-Aldrich Co. The deionized water used in every part of the studies. The flowers petals of the R. damascena were collected from the campus in the IFTM University in Moradabad 244102, U.P, and India.
Preparation of Flower Extract
Flower of R. damascena were plucked from campus of IFTM University and gently washed with distilled water. Petals of flower was washed and then dried in air for 14-15 days in the air on wet paper. After removal of moisture from petals, crush and make a powder. A 10-gram powder was combined with 90 milliliters of distilled water and boiled for 20 minutes at 40℃ on a heating mantle. The extract was cooled at room temperature after 20 minutes, and the Whatman filter paper was used to remove any leftover components. For later usage, the flower extract was maintained in a refrigerator at 40 degree C.
Biosynthesis of R. damascena capped PdNPs
According to our prior research, the formation of PdNPs was executed utilized the method describes in our investigation48. The formation of PdNPs through metal ions (PdCl2) 90 ml of a 1mM aqueous palladium chloride (PdCl2) solution combines in 10 ml of R. damascena flower extract. A magnetic stirrer was used to mix this solution; after 15 minutes reaction, mixture color turn into brown. After 24 hours, the mixture of solution turns brown to black color, which shows the formation of palladium nanoparticles through flower extract49.
Anti-fungal Study of PdNPs
The anti-fungal efficacy of PdNPs including Aspergillus niger, Aspergillus flavus, and Candida albicans was investigated. The antifungal study has been done through the Well diffusion method. According to the findings of the antifungal research conducted on PdNPs, the size of the nanoparticles had a significant impact in determining their antifungal activity, with smaller particles seeming to be more effective. The antifungal activity may also be affected by other parameters in addition to size, such as the fine-scale, the content of the nutrients, and the organisms that are being targeted.
Results and Discussion
According to the findings of the research, floral extracts from R. damascena have the potential to produce PdNPs, which are metal nanoparticles that are safe for the environment, according to the research findings. R. damascena flowers have the potential to produce a wide range of secondary metabolites, which could potentially aid in the production of PdNPs. This approach is more effective than the conventional ones, which involve the use of hazardous chemicals and solvents. The findings demonstrated exceptional antifungal effectiveness in opposition to antifungal strains, with the mechanism of action being connected to the emergence of disintegration.
UV-Visible analysis of PdNPs
A distinctive characteristic of PdNPs, Surface Plasmon Resonance peak at 360 nm has been studied in our previous research50, shown in the UV-Visible graph, is excited as the biosynthesis of PdNPs in Fig 1. Turning the solution color change yellow to black after 24 hours that visualized formation of PdNPs. This variation in color denotes the initial formation of PdNPs.
FTIR Characterization of PdNPs
Fourier Transform Infrared spectrophotometer for the R. damascena extract performed significantly as stabilization and reducing agent. The plant extract and the PdNPs were analyzed using the FTIR spectra shown in Fig.2. The FTIR analysis revealed a variety of peaks in the characteristic areas that indicate the phytochemicals in plant extract which have ability to reduce palladium ions into nanoparticles. Strong peaks in the hydroxyl group typical to alcoholic compounds were found at 3333 cm-1, according to the floral extract analysis51. The C-H bond is located at 2932 cm-1, while the C=O bond at the peak of 2152 cm-1. C=O bond at 1958 cm-1, and 1708 cm-1. Aromatic ring of OH at 1606 cm-1, and 1364 cm-1 at C-N, 779 cm-1 at C-H region.
XRD Characterization of PdNPs
Fig.3. displays the X-Ray image of originally produced PdNPs derived from R. damascena floral extract. The crystalline shape of PdNPs was analyzed and confirms using XRD pattern spectroscopy. X-ray diffraction, which was performed employed through “X’Pert PRO” spectroscopy, a technique that was often used for determining the crystalline organization of nanoparticles. A spectrophotometer used to measure PdNPs synthesized by plant extract indicates the existence of different regions at 2ϴ. XRD pattern shows great purity because the nanocrystalline PdNPs are free of crystallographic imperfections.
TEM and EDX of PdNPs
Transmission Electron Microscopy was used for the synthesized PdNPs for analyze particles size. Biologically synthesized PdNPs were found at 50 nm with a spherical shape shown in Fig.4 (a). Energy Dispersive X-Ray Spectrophotometer used for the examined of elements in the solution of synthesized PdNPs that confirm the palladium is presently shown in Fig.4 (b).
DLS Characterization of PdNPs
Dynamic light scattering instrument name (DynaPro-TC-04) analysis of the particles size distribution of the nanoparticles is shown in Fig.5. PdNPs ranged from 20 nm to 100 nm. The DLS pattern shows the major particles as 50, 60 and 70 nm. This estimated through the intensity gain during the dynamic light scattering evaluation.
SEM Characterization of PdNPs
The size of the biologically produced PdNPs via R. damascena flower extract was approximately 42-47 nanometers through the used of the ZEISS EVO 18 equipped with an acceleration voltage ranging from 0.2 to 30 kV. Scanning electron microscopy tests were also used to establish the diameter and structure of PdNPs. The structure of the PdNPs was barley spherical, and mostly PdNPs aggregates non-spherical illustrate in Fig. 6.
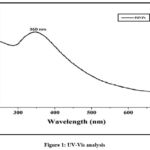 |
Figure 1: UV-Vis analysis |
 |
Figure 2: FTIR analysis |
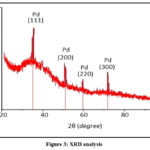 |
Figure 3: XRD analysis |
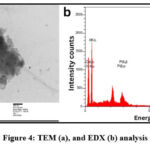 |
Figure 4: TEM (a), and EDX (b) analysis |
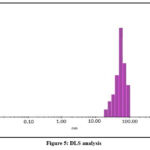 |
Figure 5: DLS analysis |
 |
Figure 6: SEM analysis |
Antimicrobial Efficacy of PdNPs
The antifungal action of the biosynthesized PdNPs from the R. damascena plant was evaluated with the well-diffusion process. The process was employed for perform the antifungal action in opposition to the fungal strain chosen: Aspergillus niger, Aspergillus flavus, and Candida albicans shown in Fig 7 (a, b). There are 4 wells in which each well has a distinct zone of inhibition against fungal strains illustrated in Table.1 shows the zone of inhibition in diameter that was calculated. 25ml of SDA agar media was added to every petri plate which was sterile and was left to set. For positive control of fungal strains, 1000 ppm Fluconazole was used.
For the showing fungal strain’s inhibition zone, plates were incubated in incubator for 1 day. Plates were incubated, and the inhibitory zone was measured after. The inhibit area of Fluconazole were 15 mm for Aspergillus niger, 15mm for Aspergillus flavus, and Candida albicans 14mm, and the inhibition zones of PdNPs against Aspergillus niger 13mm, Aspergillus flavus 8mm, and12mm for Candida albicans. The PdNPs’ inhibitory zones with fluconazole were measured in opposition to as 18 mm for Aspergillus niger, 15 mm for Aspergillus flavus, and 15 mm for Candida albicans respectively.
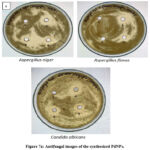 |
Figure 7a: Antifungal images of the synthesized PdNPs. |
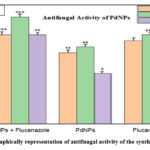 |
Figure 7b: Graphically representation of antifungal activity of the synthesized PdNPs. |
Table 1: Inhibition zone in mm
|
S.No. |
Fungal Strain |
Inhibition Zone (mm) |
||
|
Positive control |
PdNPs |
PdNPs + Fluconazole |
||
|
1. |
Aspergillus niger |
15± 0.12 |
16 ±0.24 |
18±0.30 |
|
2. |
Aspergillus flavus |
15 ±0.43 |
8 ± 0.42 |
15±0.55 |
|
3. |
Candida albicans |
14 ±1.0 |
16 ±0.22 |
19±0.48 |
Conclusion
The use of the R. damascena plant extract stabilizes and reduction of Pd ions to PdNPs production. The study investigated the production of PdNPs using a range of techniques, including FTIR, UV-Visible spectrophotometer, XRD, TEM-EDX, DLS, and SEM. The Pd content was validated by EDX analysis, which revealed that the green-produced PdNPs were spherical and about 50 nanometers in size. The extract’s phytochemicals create nanoparticles with potent antifungal activity against a wide range of fungus. More research is needed to identify the individual phytochemicals employed to decrease palladium chloride and produce PdNPs, which have biological antifungal properties and hence merit additional treatment studies. The green manufacture of PdNPs is an alternative to conventional chemical procedures that is both safe for the environment and sustainable. By making use of plant extracts, enzymes, and microbes, this method reduces the amount of waste produced, reduces the amount of harmful chemicals that are used, and reduces the expenses. PdNPs that have been synthesized using environmentally friendly methods possess potent antifungal properties, which make them appropriate for usage in antifungal drugs. The fact that they are so small and have such a large surface area allows them to come into contact with the membranes of fungal cells. This causes the integrity of the cells to be compromised, which in turn makes it more difficult for the fungal cells to reproduce. Due to the fact that these nanoparticles are biocompatible, it is quite unlikely that they will have a significant influence on either people or the environment.
Future Prospective
The use of nanostructures for targeted drug delivery, detection, diagnostics, and bioimaging has recently shown encouraging results. In this instance, nanoparticles are likely to affect medicine positively. Different factors affected the synthesis of PdNPs from plant of R. damascena extract. Palladium nanoparticles have been used against antifungal activities. Green syntheses of PdNPs can be used in nano-pharmaceuticals soon join the nano-medical as quickly as possible. In order to improve efficiency and scalability, future research could concentrate on optimizing the synthesis process, which could result in more environmentally friendly ways to produce nanoparticles. Evaluating the benefits and drawbacks of biologically generated vs conventionally synthesized PdNPs could yield important information.
Acknowledgment
We are thankful to the Cytogene laboratory (Lucknow) for check the antifungal activity of the sample.
Funding Sources
The authors received monetary support from UGC NEW DELHI for NFPWDs, grant number: 201819-NFPWDs-2018-20-UTT-8128.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
The manuscript incorporates all datasets examined throughout in this research study.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
Shagufta Bi: Methodology, Data curation, Writing—draft manuscript, Writing—original draft, Writing—review and editing.
Rashi Srivastava: Review & Supervision.
Tanzeel Ahmad: Supervision.
References
- Nasrollahzadeh, M., Sajadi, M. S., Atarod, M., Sajjadi, M., & Isaabadi, Z. An introduction to green nanotechnology. Academic Press. 2019. https://doi.org/10.1016/B978-0-12-813586-0.00001-8.
CrossRef - Chokkareddy, R., & Redhi, G. G. Green synthesis of metal nanoparticles and its reaction mechanisms. Green metal nanoparticles: synthesis, characterization and their applications, 2018,113-139.
CrossRef - Shah, M., Fawcett, D., Sharma, S., Tripathy, S. K., & Poinern, G. E. J. Green synthesis of metallic nanoparticles via biological entities. Materials, 2015, 8(11), 7278-7308.https://doi.org/10.3390/ma8115377
CrossRef - Phan, T. T. V. A Review of the Green Synthesis of Palladium Nanoparticles for Medical Applications. Journal of Cluster Science, 2024,1-17.
- Jadoun, S., Arif, R., Jangid, N. K., & Meena, R. K. Green synthesis of nanoparticles using plant extracts: A review. Environmental Chemistry Letters, 2021,19(1), 355-374. https://doi.org/10.1007/s10311-020-01074-x
CrossRef - Woldeamanuel, K. M., Kurra, F. A., & Roba, Y. T. A review on nanotechnology and its application in modern veterinary science. International Journal of Nanomaterials, Nanotechnology and Nanomedicine, 2021, 7(1), 026-031. DOI: https://dx.doi.org/10.17352/2455-3492.000041
CrossRef - Samuel, M. S., Ravikumar, M., John J, A., Selvarajan, E., Patel, H., Chander, P. S., … & Chandrasekar, N. A review on green synthesis of nanoparticles and their diverse biomedical and environmental applications. Catalysts, 2022, 12(5), 459. https://doi.org/10.3390/catal12050459
CrossRef - Ghosh, S., Nitnavare, R., Dewle, A., Tomar, G. B., Chippalkatti, R., More, P., … & Chopade, B. A. Novel platinum–palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: anticancer and antioxidant activities. International journal of nanomedicine, 2015, 7477-7490.doi: 10.2147/IJN.S91579
CrossRef - Piermatti, O. Green synthesis of Pd nanoparticles for sustainable and environmentally benign processes. Catalysts, 2021,11(11), 12-58.https://doi.org/10.3390/catal11111258
CrossRef - Siddiqi, K. S., & Husen, A. Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanoscale research letters, 2016, 11, 1-13.DOI 10.1186/s11671-016-1695-z
CrossRef - Hulkoti, N. I., & Taranath, T. C. Biosynthesis of nanoparticles using microbes—a review. Colloids and surfaces B: Biointerfaces, 2014,121, 474-483.. https://doi.org/10.1016/j.colsurfb.2014.05.027
CrossRef - Phan, T. T. V., Huynh, T. C., Manivasagan, P., Mondal, S., & Oh, J. An up-to-date review on biomedical applications of palladium nanoparticles. Nanomaterials, 2019, 10(1), 66.. https://doi.org/10.3390/nano10010066
CrossRef - Vishnukumar, P., Vivekanandhan, S., & Muthuramkumar, S. Plant‐Mediated Biogenic Synthesis of Palladium Nanoparticles: Recent Trends and Emerging Opportunities. ChemBioEng Reviews, 2017, 4(1), 18-36.. https://doi.org/10.1002/cben.201600017
CrossRef - Rokade, S. S., Joshi, K. A., Mahajan, K., Tomar, G., Dubal, D. S., Singh, V., … & Ghosh, S. Novel anticancer platinum and palladium nanoparticles from Barleria prionitis. Global journal of nanomedicine, 2017, 2(5), 555-600.. https://doi.org/10.1155/2018/4924186
CrossRef - Liang, Y., Demir, H., Wu, Y., Aygun, A., Tiri, R. N. E., Gur, T., … & Vasseghian, Y. Facile synthesis of biogenic palladium nanoparticles using biomass strategy and application as photocatalyst degradation for textile dye pollutants and their in-vitro antimicrobial activity. Chemosphere, 2022, 306, 135-518.. https://doi.org/10.1016/j.chemosphere.2022.135518
CrossRef - Ovais, M., Khalil, A. T., Islam, N. U., Ahmad, I., Ayaz, M., Saravanan, M., … & Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Applied microbiology and biotechnology, 2018, 102, 6799-6814.https://doi.org/10.1007/s00253-018-9146-7
CrossRef - Vinodhini, S., Vithiya, B. S. M., & Prasad, T. A. A. Green synthesis of palladium nanoparticles using aqueous plant extracts and its biomedical applications. Journal of King Saud University-Science, 2022, 34(4), 102-017. https://doi.org/10.1016/j.jksus.2022.102017
CrossRef - Azizi, S., Shahri, M. M., Rahman, H. S., Rahim, R. A., Rasedee, A., & Mohamad, R. Green Synthesis Palladium Nanoparticles Mediated by White Tea (Camellia sinensis) Extract with Antioxidant, Antibacterial, and Antiproliferative Activities Toward the Human Leukemia (MOLT-4) Cell Line [Retraction]. International Journal of Nanomedicine, 2022, 17, 1227-1228.. doi: 10.2147/IJN.S149371
CrossRef - Bi, S., & Srivastava, R. Rosa damascena leaf extract mediated palladium nanoparticles and their anti-inflammatory and analgesic applications. Inorganic Chemistry Communications, 2024,162, 112-122.https://doi.org/10.1016/j.inoche.2024.112122
CrossRef - Roopan, S. M., Bharathi, A., Kumar, R., Khanna, V. G., & Prabhakarn, A. Acaricidal, insecticidal, and larvicidal efficacy of aqueous extract of Annona squamosa L peel as biomaterial for the reduction of palladium salts into nanoparticles. Colloids and Surfaces B: Biointerfaces, 2012, 92, 209-212.. https://doi.org/10.1016/ j.colsurfb.2011.11.044
CrossRef - Fahmy, S. A., Preis, E., Bakowsky, U., & Azzazy, H. M. E. S. Palladium nanoparticles fabricated by green chemistry: Promising chemotherapeutic, antioxidant and antimicrobial agents. Materials, 2020, 13(17), 3661.https://doi.org/10.3390/ ma13173661
CrossRef - Navale, V., Vamkudoth, K. R., Ajmera, S., & Dhuri, V. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicology reports, 2021, 8, 1008-1030.https://doi.org/10.1016/j.toxrep.2021.04.013 .
CrossRef - Darwazeh, A. M., & Darwazeh, T. A. What makes oral candidiasis recurrent infection? A clinical view. Journal of Mycology, 2014,(1), 758-394.
CrossRef - Ghaddar, N., El Roz, A., Ghssein, G., & Ibrahim, J. N. Emergence of vulvovaginal candidiasis among Lebanese pregnant women: prevalence, risk factors, and species distribution. Infectious diseases in obstetrics and gynecology, 2019(1), 5016810.
CrossRef - Taverne-Ghadwal, L., Kuhns, M., Buhl, T., Schulze, M. H., Mbaitolum, W. J., Kersch, L., … & Groß, U. Epidemiology and prevalence of oral candidiasis in HIV patients from chad in the post-HAART era. Frontiers in Microbiology, 2022,13, 844-069.
CrossRef - Achilonu, C. C., Davies, A., Kanu, O. O., Noel, C. B., & Oladele, R. Recent advances and future perspectives in mitigating invasive antifungal-resistant pathogen Aspergillus fumigatus in Africa. Current Treatment Options in Infectious Diseases, 2024,16(1), 14-33.. https://doi.org/ 10.1007/s40506-023-00269-4
CrossRef - Kanj, S. S., Omrani, A. S., Al-Abdely, H. M., Subhi, A., Fakih, R. E., Abosoudah, I., … & Dimopoulos, G. Survival outcome of empirical antifungal therapy and the value of early initiation: A review of the last decade. Journal of Fungi, 2022, 8(11), 11-46.https:// doi.org/10.3390/jof8111146
CrossRef - Achilonu, C. C., Gryzenhout, M., Marais, G. J., Johar, D., Ghosh, S., & Hassanin, S. O. Antifungal activity of Carya illinoinensis extracts against Alternaria alternata pathogen and their cytotoxicity effects on HEK-293T cells: HPLC analysis of bioactive compounds. Discover Applied Sciences, 2024,6(2), 67..https://doi. org/10.1007/s42452-024-05721-8
CrossRef - Sanguinetti, M., Posteraro, B., Beigelman-Aubry, C., Lamoth, F., Dunet, V., Slavin, M., & Richardson, M. D. Diagnosis and treatment of invasive fungal infections: looking ahead. Journal of Antimicrobial Chemotherapy,2019,74, ii27-ii37..https://doi.org/10.1093/jac/dkz041
CrossRef - Zhang, S. X., Babady, N. E., Hanson, K. E., Harrington, A. T., Larkin, P. M., Leal Jr, S. M., … & Lockhart, S. R. Recognition of diagnostic gaps for laboratory diagnosis of fungal diseases: expert opinion from the Fungal Diagnostics Laboratories Consortium (FDLC). Journal of clinical microbiology, 2021, 59(7), 10-1128..https://doi.org/ 10.1128/jcm.01784-20
CrossRef - Wiederhold, N. P. Emerging fungal infections: new species, new names, and antifungal resistance. Clinical chemistry, 2022,68(1), 83-90..https://doi.org/10.1093/CLINCHEM/HVAB217
CrossRef - Campbell, C. A., Osaigbovo, I. I., & Oladele, R. O. Triazole susceptibility of Aspergillus species: environmental survey in Lagos, Nigeria and review of the rest of Africa. Therapeutic Advances in Infectious Disease, 2021, 8, 20499361211044330..https:// doi.org/10.1177/20499361211044330
CrossRef - Lestrade, P. P., Bentvelsen, R. G., Schauwvlieghe, A. F., Schalekamp, S., van der Velden, W. J., Kuiper, E. J., … & Verweij, P. E. Voriconazole resistance and mortality in invasive aspergillosis: a multicenter retrospective cohort study. Clinical Infectious Diseases, 2019, 68(9), 1463-1471..https://doi.org/ 10.1093/CID/CIY859
CrossRef - Joshi, K. M., Shelar, A., Kasabe, U., Nikam, L. K., Pawar, R. A., Sangshetti, J., … & Chaskar, M. G. Biofilm inhibition in Candida albicans with biogenic hierarchical zinc-oxide nanoparticles. Biomaterials Advances, 2022, 134, 112-592.
CrossRef - Bhattacharya, S., Sae-Tia, S., & Fries, B. C. Candidiasis and mechanisms of antifungal resistance. Antibiotics, 2020,9(6), 312.
CrossRef - Bongomin, F., Gago, S., Oladele, R. O., & Denning, D. W. Global and multi-national prevalence of fungal diseases—estimate precision. Journal of fungi, 2017, 3(4), 57.
CrossRef - Benedict, K., Jackson, B. R., Chiller, T., & Beer, K. D. Estimation of direct healthcare costs of fungal diseases in the United States. Clinical Infectious Diseases, 2019, 68(11), 1791-1797.
CrossRef - Mba, I. E., Nweze, E. I., Eze, E. A., & Anyaegbunam, Z. K. G. Genome plasticity in Candida albicans: A cutting-edge strategy for evolution, adaptation, and survival. Infection, Genetics and Evolution, 2022,99, 105-256.
CrossRef - Padmavathi, A. R., P, S. M., Das, A., Priya, A., Sushmitha, T. J., Pandian, S. K., & Toleti, S. R. Impediment to growth and yeast-to-hyphae transition in Candida albicans by copper oxide nanoparticles. Biofouling, 2020, 36(1), 56-72..
CrossRef - Haleem, A., Javaid, M., Singh, R. P., Rab, S., & Suman, R. Applications of nanotechnology in medical field: a brief review. Global Health Journal, 2023,7(2), 70-77..https://doi.org/10.1016/j. glohj.2023.02.008
CrossRef - Joseph, T. M., Kar Mahapatra, D., Esmaeili, A., Piszczyk, Ł., Hasanin, M. S., Kattali, M., … & Thomas, S. Nanoparticles: Taking a unique position in medicine. Nanomaterials, 2023, 13(3), 574.. https://doi.org/10.3390/nano13030574
CrossRef - Arsiya, F., Sayadi, M. H., & Sobhani, S. Green synthesis of palladium nanoparticles using Chlorella vulgaris. Materials Letters, 2017,186, 113-115.
CrossRef - Phan, T. T. V. A Review of the Green Synthesis of Palladium Nanoparticles for Medical Applications. Journal of Cluster Science, 2024, 1-17.
- Tiri, R. N. E., Aygun, A., Bekmezci, M., Gonca, S., Ozdemir, S., Kaymak, G., … & Sen, F. Environmental Energy Production and Wastewater Treatment Using Synthesized Pd Nanoparticles with Biological and Photocatalytic Activity. Topics in Catalysis, 2024, 67(9), 714-724.
CrossRef - Nasr Azadani, F., Madani, M., Karimi, J., & Sepahvand, S. Green Synthesis of Silver Nanoparticles by Fusarium oxysporum and its Function Against Aspergillus and Fusarium Fungi. Indian Journal of Microbiology, 2024, 64(1), 213-224.
CrossRef - Liu, J., Chen, H., Lv, Y., Wu, H., Yang, L. J., Zhang, J., … & Wang, W. Synthesis, Characterization, and Antifungal Activity of Benzimidazole-Grafted Chitosan against Aspergillus flavus. Journal of Agricultural and Food Chemistry, 2024, 72(19), 11185-11194.
CrossRef - Vaghela, H., Shah, R., & Pathan, A. Palladium nanoparticles mediated through bauhinia variegata: Potent in vitro anticancer activity against mcf-7 cell lines and antimicrobial assay. Current Nanomaterials, 2018, 3(3), 168-177.. https:// doi.org/10.2174/2405461504666190131142303.
CrossRef - Bi, S., & Ahmad, N. Green synthesis of palladium nanoparticles and their biomedical applications. Materials Today: Proceedings, 2022, 62, 3172-3177.. https://doi.org/10.1016/j.matpr.2022.03.441
CrossRef - Mallikarjuna, K., Bathula, C., Buruga, K., Shrestha, N. K., Noh, Y. Y., & Kim, H. Green synthesis of palladium nanoparticles using fenugreek tea and their catalytic applications in organic reactions. Materials Letters, 2017,205, 138-141.https://doi.org/10.1016/j.matlet.2017.06.081
CrossRef - Bi, S., & Srivastava, R. Rosa damascena flower mediated phytofabrication of palladium nanoparticles, in-vitro and in-vivo applications. Materials Today: Proceedings, 2023. https://doi.org/10.1016/j.matpr.2023.07.305
CrossRef - Rokade, S. S., Joshi, K. A., Mahajan, K., Patil, S., Tomar, G., Dubal, D. S., & Ghosh, S. Gloriosa superba mediated synthesis of platinum and palladium nanoparticles for induction of apoptosis in breast cancer. Bioinorganic chemistry and applications, 2018,(1), 4924186.https://doi.org/10.1155/2018/4924186
CrossRef








