Manuscript accepted on :16-10-2024
Published online on: 29-10-2024
Plagiarism Check: Yes
Reviewed by: Dr. Md.Sarwar Hossain
Second Review by: Dr. M. Mohan Varma
Final Approval by: Dr. Gul Ozcan
Rama Rao Nadendla1 , Harish Narayanan2
, Harish Narayanan2 , Roopa Murgod3
, Roopa Murgod3 , Khalid Suliman Alboloi4
, Khalid Suliman Alboloi4 , Maya Savira5
, Maya Savira5 and Pichandy Muthuprasanna6
and Pichandy Muthuprasanna6 *
*
1Chalapathi Institute of Pharmaceutical Sciences, Chalapathi Nagar, Lam, Guntur, Andhrapradesh, India.
2Department of Community Medicine, Sree Balaji Medical College and Hospital, Bharath Institute of Higher Education and Research, Tamil Nadu, India.
3Consultant Biochemist, Manipal Hospital, Old Airport Road, Bangalore, India.
4Department of Clinical Laboratory and Blood Bank, Ohud Hospital, Medina, Saudi Arabia.
5Department of Physiology, Faculty of Medicine, Universitas Sumatera Utara, Indonesia.
6Department of Pharmaceutical Biotechnology, Surya School of Pharmacy, Vikiravandi, Villupuram Dt. Tamil Nadu, India.
Corresponding Author E-mail: muthuprasanna78@gmail.com
Abstract
Acute coronary syndrome (ACS) represents a spectrum of heart muscle oxygen deprivation (ischemia) which ranges from temporary tissue damage in angina to unstable angina with possible minor cell death and finally to a full-blown heart attack (myocardial infarction) with extensive tissue necrosis. Biochemical indicators of damage to the heart muscle remain crucial in the comprehensive evaluation and management of individuals with various forms of ACS. Diagnosing ACS accurately is crucial for optimal treatment. While existing biochemical markers play a vital role, searching for an ideal biomarker continues. Biochemical markers of myocardial injury are critical for the global assessment and treatment of individuals with these syndromes. The objective of the review, is to primarily evaluate the current understanding of ACS biomarkers rather than specifically to assess which biomarkers are most promising. The review emphasizes the importance of existing biochemical markers, the ongoing search for an ideal biomarker, and how these markers are crucial for the diagnosis and management of ACS. This review examines various biochemical markers associated with atherosclerosis, including matrixins, PAPP-A metalloproteinase, myeloperoxidase, microalbuminuria, cystatin, P-selectin, glycogen phosphorylase-BB, C-reactive protein marker, cluster of differentiation 40 (CD40L) marker, creatine kinase-myocardial band (CK-MB), and ischemia-modified albumin. Traditional biomarker Tn levels may not rise immediately during ischemia, but MPO levels increase earlier, aiding early ACS detection. MPO, produced during inflammation, offers predictive data surpassing troponins. IMA also rises faster than troponins, signaling myocardial ischemia before irreversible damage. hs-CRP provides insights into inflammation and cardiovascular risk, complementing troponins. MicroRNAs serve as precise biomarkers for ACS, surpassing troponins in detecting molecular changes linked to the disease. These biomarkers enhance early diagnosis and treatment. Clinically, Tn levels may not have significantly risen at the early stage of ischemia; however, other biomarkers should be considered for early detection and risk stratification. Elevated MPO and IMA levels can indicate myocardial ischemia and inflammation earlier than troponins. hs-CRP offers additional insights into systemic inflammation and cardiovascular risk. MicroRNAs provide detailed molecular data and may outperform troponins in identifying ACS, offering a more sensitive diagnostic approach. Further monitoring and treatment adjustments based on these biomarkers are advised. In conclusion, while troponins remain crucial in diagnosing ACS, novel biomarkers like MPO, IMA, hs-CRP, and microRNAs offer earlier detection and better risk assessment. These markers provide additional insights into ischemia and inflammation, improving diagnostic accuracy and patient outcomes when used alongside traditional biomarkers.
Keywords
Acute Coronary Syndrome; C-reactive protein marker; Creatine Kinase-Myocardial Band (CK-MB); Ischemia Modified Albumin; Matrixins; Myocardial Infarction
| Copy the following to cite this article: Nadendla R. R, Narayanan H, Murgod R, Alboloi K. S, Savira M, Muthuprasanna P. Current and Prospective Biochemical Markers for The Identification of Acute Coronary Syndrome-A Review. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Nadendla R. R, Narayanan H, Murgod R, Alboloi K. S, Savira M, Muthuprasanna P. Current and Prospective Biochemical Markers for The Identification of Acute Coronary Syndrome-A Review. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3C4ZIUk |
Introduction
Managing individuals with chest discomfort is a significant obstacle in emergency care, including identifying and treating their condition. Rapid diagnosis and initial risk assessment are crucial for identifying individuals for early therapy. The first evaluation of individuals experiencing chest pain relies on the analysis of clinical symptoms, physical-examination, electro-cardiogram (ECG), and determination of biochemical markers indicating damage to the heart muscle. The first diagnostic marker for myocardial infarction (MI), aspartate aminotransferase, was initially documented in 19541. Advancements in immunoassay technology, together with the use of more precise markers of myocardial damage, have made it possible to identify even lower amounts of myocardial necrosis. Biomarkers may provide other methods of categorizing individuals based on their risk, even if there is no increase in markers indicating damage to the heart muscle. This can allow for proactive treatment of these individuals. ACS include unbalanced angina, NSTEMI (non-ST elevation myocardial infarction), and STEMI (ST-elevation-myocardial-infarction)2. These disorders might take diverse clinical manifestations and outcomes. Hence, it is vital to classify ACS into subcategories with distinct prognosis and treatment approaches at an early stage. Biomarkers are crucial and unquestionably essential in the identification and treatment of patients with ACS. In addition, they offer insights into the underlying mechanisms of disease and can be valuable in enhancing therapeutic approaches for patient management. Acute-myocardial-infarction (AMI) and unstable-angina (UA) are two substantial factors that contribute to mortality and impairment on a global scale. Early detection is crucial due to the highest risk of death and the greatest benefit from early revascularization occurring within the first few hours. Although clinical assessment, which includes a thorough review of chest pain features, and the 12-lead ECG are essential tools3, they lack sufficient accuracy when used individually. Consequently, it is essential to test a biomarker that indicates and measures damage to heart muscle cells in all individuals who show signs of ACS. Cardiac biomarkers are crucial in diagnosing ACS, especially when there is a lack of additional diagnostic evidence, for example unusual cardiac chest discomfort or non-specific ECG abnormalities. Numerous bio-markers, including troponin, CK, CK-MB, LDH, and myoglobin4, have been identified as valuable diagnostic tools in ACS. Troponins serve as indicators of myocardial necrosis, whereas CRP and MPO replicate the inflammatory progression. Vasodilator peptides, on the other hand, suggest neuro-hormonal initiation and hemo-dynamic anxiety. The development of cardiac-biomarkers exhibits not only offered a distinct understanding of the illness progression, but also serves as a crucial factor in defining ACS. Biochemical markers are critical for evaluating most individuals with nonspecific or unclear symptoms and an inconclusive ECG. Analysis of ECG for a suspected MI individual, reveals significant changes in the ST-segment elevation. Individuals with 1millivolt (mV) in adjacent leads, exhibit symptoms, or have a new left bundle extend block should receive immediate reperfusion therapy5. These markers are one of the two criteria used to diagnose a MI. The classification of the MI definition depends on the cardiac symptoms, ECG abnormalities, and biomarker response exhibited by the patients. ST elevation MI refers to an abnormal increase in the ST segment of the ECG together with characteristic chest pain and an increase in cardiac enzymes. A NSTEMI is characterized by cardiac chest discomfort accompanied by elevated cardiac enzymes, but without an ST-segment-elevation. However, it might exhibit ST depression, T inversion, or ambiguous alterations. UA refers to the occurrence of cardiac chest discomfort while at rest, without any increase in enzymes, but with a significant likelihood of experiencing a heart attack in the future6. Cardiac troponin is a biomarker that is now utilized to diagnose ACS. Troponin is a cardiac protein secreted into the bloodstream in response to myocardial injury, that occurs after a heart attack. This marker is both sensitive and selective for detecting heart damage. High-sensitivity troponin assays are highly valuable in the diagnosis of ACS due to their ability to detect even minuscule quantities of troponin in the bloodstream7. This enables earlier identification and intervention. Troponin is a trimeric multifaceted residing of three regulatory proteins (Troponin-C, Troponin-I, and Troponin-T) that are existing in both skeletal and cardiac muscle. It has a vital function in controlling muscular contraction. Troponin plays a role in controlling muscle contraction in the setting of cardiac muscle, specifically in relation to the regulation of calcium-mediated contraction. Troponin-I (TnI) and Troponin-T (TnT) are exclusively found in cardiac muscle. Cardiac muscle cells produce troponin into the bloodstream when they are damaged, for example, after a heart attack (MI). Troponins are often regarded as the most reliable biomarker for identifying MI. The levels of troponin start to increase within a few hours following the start of damage to the heart muscle, reaching their highest point after 24-48 hours. This enables the timely identification of cardiac muscle injury. Elevated levels of troponin are linked to bigger instances of MI and an increased likelihood of sequelae, such as heart failure and mortality8. In addition to troponin, other biomarkers such as creatine kinase-MB (CK-MB) and myoglobin can be evaluated to aid in the diagnosis and treatment of ACS. However, troponin is typically regarded as the main biomarker due to its superior sensitivity and specificity in detecting cardiac damage. Biomarkers aid healthcare providers in evaluating the magnitude and seriousness of cardiac injury and informing therapy choices9. This comprehensive analysis examines a range of biomarkers that surpass the traditional troponins commonly employed in the diagnosis of ACS. The following markers in this review have been investigated: CRP Marker, CD40 L Marker, Brain-type Natriuretic Peptide (BNP), Plasma N-terminal pro-BNP, Creatine kinase-myocardial band (CK-MB), and Ischemia modified albumin (IMA). These biomarkers offer valuable information about several aspects of ACS pathophysiology, including inflammation, necrosis, cardiac pump malfunction, and ischemia. While troponins are still crucial in diagnosing ACS, exploring other biomarkers has the potential to improve diagnostic accuracy and increase risk evaluation.
Cardiac Muscle Contraction Constituents
Muscle cells consist of several components, each playing a critical role. While certain components have a physiological function, others primarily serve a structural purpose. Myoglobin, with a molecular weight of 17.8 kDa, is responsible for transporting oxygen. Cell enzymes enable energy-related processes, while troponins (Tns) and their related compounds have a structural and physiological function in muscle movement. The process of muscle retrenchment at the molecular level is driven by the hydrolysis of adenosine triphosphate (ATP)10. Actin is the second most prevalent contractile protein, behind myosin, among all myofibrillar proteins. The troponin complex consists of three subunits, namely TnI, Troponin C (TnC), and TnT. TnT attaches the assembly to the tropomyosin filament, TnI hinders the actomyosin ATPase, and TnC binds calcium and functions to control the complex formation. Tropomyosin, the Tn subunits, and myosin exist in various isoforms. There are distinct isoforms of TnT and TnI, specifically for slow twitch skeletal muscle fibers, fast twitch skeletal muscle fibers, and cardiac muscle (cTnI and cTnT)11. There is also a specific proportion of TnC in cardiac muscle called cardiac TnC. While it is true that there are different isoforms of troponin subunits for different muscle fibers, the presence of distinct isoforms does not necessarily mean that they play a significant role in controlling complex formation or muscle function. Additionally, the specific proportion of TnC in cardiac muscle may not be as crucial as other factors in regulating muscle contraction.
Ideal Biomarkers
Before adopting a novel biomarker for ACS, it is essential to assess certain crucial parameters to ascertain its efficacy in clinical settings. This includes examining three primary factors: analytical performance, clinical originality, and therapeutic influence12. Analytical performance is essential, and the biomarker measurement needs to be precise and capable of being replicated. This necessitates well established analytical techniques and a meticulous assessment of pre-analytical variables such as sample durability. It is also necessary to establish reference ranges for the whole population and certain groups of individuals13. Clinical originality refers that the biomarker should not just replicate or duplicate current information. It should exhibit a robust and consistent correlation with ACS outcomes across several investigations and enhance or augment current diagnostics for identifying or categorizing ACS individuals. The use of verified decision limits (Normal ranges), which are predetermined thresholds for interpretation, is essential for precise clinical implementation14. Therapeutic influence refers that the biomarker should facilitate improved individual care and treatment. It should surpass current diagnostic tests regarding precision and the ability to identify risks. It is necessary to provide data demonstrating the related risk altered via treatment and improves outcomes15. Through a thorough evaluation of these three factors, we can ascertain if a suggested biomarker is ideal for enhancing the identification and treatment of ACS. In addition, the biomarker should also be easily measurable and reproducible in different clinical settings to ensure widespread use and effectiveness. Furthermore, it is important to consider the cost-effectiveness of implementing the biomarker in routine clinical practice. By carefully evaluating these criteria, healthcare providers can determine the true value of a potential biomarker for ACS and its impact on patient care. Ultimately, the successful integration of a biomarker into clinical practice can lead to more accurate diagnoses, personalized treatment plans, and improved outcomes for patients with ACS.
Inflammatory Biomarkers
C-reactive Protein Marker (CRP)
CRP’s prognostic and severity determining ACS have been extensively investigated across several contexts. The main underlying physiological event in ACS is believed to be the rupture of a plaque in the artery, followed by a blood clot (thrombus). For individuals who have survived acute myocardial infarction (AMI), measuring blood levels of CRP and SAA might help predict the likelihood of another cardiac episode while still in the hospital16. The serum CRP levels in the early stages of an AMI may provide information about the severity of the heart muscle damage and the body’s response. Hence, it is recommended to postpone the assessment of CRP levels for a minimum of four to six weeks after MI to allow for the subsidence of the acute phase response. Elevated levels of CRP upon admission and before being discharged from the hospital indicate a more unfavorable short and long term probability for ACS17. Measuring CRP may provide additional valuable information for individuals with normal cardiac troponins. Specifically, an elevated CRP level is linked to a possible outcome, regardless of troponin elevated levels. To stratify risk early, it is advisable to collect blood samples for CRP measurement as soon as possible ideally within 8 hours after the symptoms appear. Those who have high levels of CRP in their blood when they leave the hospital are more likely to have recurring symptoms or a second MI compared to those with normal CRP levels at discharge18. Hepatocytes are the primary producers of CRP, which is mostly regulated by inflammatory cytokines. CRP, an acute-phase reactant, has been suggested as a possible marker for ACS. The advancement of high sensitivity CRP (hsCRP) examines has facilitated research on the utilization of CRP for forecasting heart artery disease. These assays can extent short levels of CRP in the bloodstream even when there is no obvious inflammatory event. The initial CRP level was a momentous extrapolative factor for both premature and delayed mortality death in individuals with ACS undergoing an initial hostile approach, particularly in individuals who tested positive for cTnT19.
Cluster of differentiation 40 (CD40 L) Marker
CD40L is a marker for CD 40. The CD40-CD40L complex is crucial in ACS’s genesis, progression, and prognosis. ACS individuals have increased plasma concentrations of the proinflammatory and prothrombotic cytokine CD40 ligand (sCD40L)20. Higher levels of soluble CD40 ligand (sCD40L) in the blood suggest a greater likelihood of experiencing cardiovascular events in the future among women who seem to be in good health. Preeminent plasma stages of sCD40L classify individuals with ACS at higher hazard of demise and recurrent MI, autonomous of other extrapolative variables, including cTnI and CRP. Elevated levels of blood-soluble CD40L in individuals with unstable coronary artery disease are indicative of an independent and more possibility of significant contrary cardiovascular events. Initiating statin treatment promptly after ACS mitigates the risk linked to increased sCD40L21.
Necrosis Biomarkers
Troponin(Tn)
TnT enables increased ability to identify myocardial injury and exhibits more specificity for myocardial tissue compared to classic “cardiac enzymes” like creatine kinase (CK) or its CKMB. cTnI and cTnT are polypeptides that regulate the communication between actin and myosin in the heart, via modulation of calcium signaling22. Tn I is a slighter molecule than Tn T. Their use in diagnosing AMI is vital. The coronary variants of these polypeptides are derived from distinct genetic factor, making them potentially exclusive to the heart. To ensure precise readings, every laboratory needs to verify the accuracy and reproducibility of the troponin test during the first analysis and periodically thereafter to confirm the performance of the assay23. Existing tests quantify troponin levels in nano-grams per milliliter, but upcoming examines can identify amounts in pico-grams per milliliter. Tns are widely recognized as the most reliable method for diagnosing ACS. The presence of elevated cTn levels and corresponding clinical evidence serve as a reliable indicator of myocardial injury due to the high specificity of cTn. Several researches have proven the predictive significance of increased troponins in individuals with ST segment elevation in MI. The GUSTO-III study examined 12,666 individuals diagnosed with STEMI who were treated with thrombolytic treatment24.
Limitations of Tn
Tn levels can increase in situations other than ACS, including myocarditis, heart failure, pulmonary embolism, renal failure, and severe sepsis.
Chronic renal disease patients may exhibit persistently high levels of troponin, even when there are no acute cardiac events occurring. It can be difficult to differentiate between sudden and long-lasting increases and appropriately interpret troponin data.
Patients diagnosed with NSTEMI experience a delayed increase in troponin levels. This delay might cause troponin measurements taken too soon after the onset of symptoms to produce false-negative readings.
Individual heterogeneity in troponin levels results in greater baseline values for some individuals, necessitating the establishment of universal standard troponin levels.
Haemolysis can disrupt troponin tests, hence it is crucial to handle and treat samples correctly to reduce the chance of interference.
One important drawback of traditional Tn assays is their limited ability to detect low levels of Tn at the time when a patient first presents, due to a delayed rise in circulating levels. This often necessitates several blood samples taken over a period of 6-9 hours for a considerable number of patients.
Cardiac Pump Dysfunction Biomarkers
Brain type Natriuretic Peptide (BNP)
BNP is a neurohormone fabricated in the left ventricle’s myocardium and is released into the bloodstream when the ventricle becomes enlarged and experiences excessive pressure25. Increased levels of BNP in the blood, which may be detected with a rapid bedside test, are significant for predicting both short-term and long term pump dysfunction in individuals with a non-ST-elevation of ACS26. The efficacy of plasma BNP as a predictor was most evident in individuals with plasma BNP levels elevated approximately 40 hours after the dysfunction symptoms begins. Elevated plasma BNP levels were also linked to an amplified hazard of developing or experiencing a repeat MI and the onset or deteriorating of cardiac failure. Retrospectively, blood samples were collected from individuals with a non-ST elevation ACS within 24 hours of the beginning of symptoms and were then analyzed for NT-proBNP. Individuals with the low NT-proBNP levels had a marked reduced death rate in two years compared to those with the highest value. The correlation with mortality is higher for this measure than other markers tested, such as cTnT and CRP. Plasma NT-proBNP peaks during the acute phase and declines gradually throughout six to seven months27. Higher levels at given interval period are linked to an augmented risk of heart failure. Monitoring NT-proBNP levels could provide valuable prognostic information for individuals with non-ST elevation ACS. The gradual decline in NT-proBNP levels over six to seven months suggests a potential window of opportunity for intervention to prevent the development of heart failure. Thus early identification and monitoring of NT-proBNP levels in individuals with acute coronary syndrome is critical for improved outcomes and management of the condition.
Plasma N-terminal proBNP
The BNP gene is on the chromosome and may be promptly stimulated in rejoinder to indication propagation since the myocyte cell membrane. Proteolytic cleavage of the first two amino acid residues at the N-terminus of BNP occurs either in the bloodstream or just after blood is collected28. It is important to consider these enzymatic cleavages when selecting epitopes for antibody production and designing immunoassays. Problems may arise with analytical specificity when detecting BNP or NT-pro-BNP due to pro-BNP in human plasma. Cardiomyocytes produces biologically active BNP in response to increased strain on the heart wall. BNP levels often indicate the fluctuating wall tension that the left chamber undergoes and has a dumpier half duration than NT-proBNP due to its clearance via distinct pathways29.
When selecting epitopes for antibody production and designing biomarker immunoassays for BNP and NT-proBNP, it’s crucial to consider enzymatic cleavage and pro-BNP forms in human plasma. Pro-BNP can complicate analytical specificity, as it affects accurate measurement of these biomarkers. Cardiomyocytes release biologically active BNP in response to increased heart wall strain, with BNP levels reflecting fluctuating wall tension, particularly in the left ventricle. Unlike NT-proBNP, BNP has a shorter half-life due to its clearance through distinct pathways, such as natriuretic peptide receptors and enzymatic degradation. These factors can influence the sensitivity and specificity of diagnostic assays. The proportion of NT-pro-BNP to BNP rises multiplicatively as the phase of nephric illness progresses, indicating that nephric function needs to be considered. Natriuretic peptides are released in retort to higher petition and may not oscillate about a physiological baseline like other substances. Various cardiac pump dysfunction biomarkers feature listed in Figure 1.
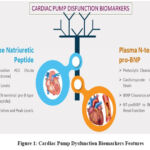 |
Figure 1: Cardiac Pump Dysfunction Biomarkers Features |
Creatine Kinase-myocardial Band (CK-MB)
CK-MB is the regular biochemical marker for assessing myocardial damage and is the reference point for comparing other markers. While CK-MB indicates myocardial damage, skeletal muscle has higher overall CK activity per gram of tissue and may include up to 3.2% CK-MB30. The occurrence of CK-MB in skeletal muscle complicates the interpretation of CK-MB levels in individuals with suspected myocardial infarction. It is particularly important in skeletal muscle damage or injury cases, as it can lead to false-positive results and misdiagnosis. Therefore, alternative markers more specific to myocardial damage have been investigated to improve diagnostic accuracy and individual management. Collecting samples for 8 to 12 hours is necessary for a high accuracy and precision and diagnosis. CK-MB is a crucial factor in evaluating the occurrence of a second heart attack or the expansion of ACS31. For example, an individual who has experienced a heart attack may also have skeletal muscle damage due to physical exertion during the event. If only CK-MB levels are measured, indication of severe myocardial damage or misdiagnose the individual with multiple heart attacks can be determined. In such cases, alternative markers like troponin levels can be measured along with CK-MB to assess myocardial damage and aid in proper individual management. Although CK-MB demonstrates exceptional performance, it is not the optimal indicator because of its delayed increase of 8-12 hours after the beginning of symptoms, making it less suitable for diagnosis32. Troponin is highly sensitive and specific for cardiac injury, often rising within a few hours of ischemic events, whereas CK-MB typically shows a delayed increase of 8-12 hours after symptom onset. This delay can hinder timely diagnosis and intervention. Although CK-MB can still offer valuable information, particularly for detecting reinfection, its slower response limits its effectiveness in acute settings. Therefore, combining both markers enhances diagnostic accuracy and helps guide treatment decisions in patients with suspected acute coronary syndrome. CK-MB levels can also be influenced by factors other than myocardial damage, such as skeletal muscle injury or strenuous physical activity.
Ischemia Modified Albumin (IMA)
Albumin’s capacity to bind cobalt was previously utilized to indicate coronary ischemia. The proposed appliance suggests that the amino-terminal end of albumin under ischemic conditions experiences structural alterations, including modifications to a sequence of aspartate alanine histidine lysine caused by acid-base imbalance and low oxygen33. These modifications would decrease the attraction of cobalt, which is essential for assessing ischemia-modified IMA. Another theory is that fatty acids are released during cardiac ischemia and bind to albumin. Consequently, this would moderate the capacity of albumin to absorb cobalt. Readings have raised doubts about the diagnostic accuracy of IMA due to a significantly insufficient degree of accuracy and a high proportion of incorrect positive results of specificity34. Additional research indicates that IMA is an indicator of acute ischemic events that are not exclusively related to cardiac ischemia. This suggests that IMA is not effective in distinguishing between people with and without AMI who are experiencing ischemia35. Studies on forearm activity in hypoxic circumstances or activity provoked ischemia in persons with external arterial illness have demonstrated that levels of IMA undergo a transient decrease following exercise before recovering to their initial levels. It was theorized that higher quantities of naturally occurring lactate decreases concentrations of IMA36.
This IMA may not always perform as expected due to factors like individual patient variability and co-existing conditions. For instance, renal dysfunction, inflammation, or other non-cardiac conditions can alter IMA levels, reducing its specificity for myocardial ischemia. Differences in age, sex, and genetic factors can significantly influence patient’s response biochemically to ischemia, impacting the reliability of biomarkers like IMA. Older patients may have co-existing conditions, like diabetes, that alter biomarker levels, while women, especially postmenopausal, may show different responses due to hormonal changes. Genetic variability affects the production and regulation of proteins involved in ischemia, leading to differing biomarker responses. Ethnic background can influence baseline cardiovascular risks, further complicating diagnostic accuracy. These variations highlight the need for personalized diagnostics and the potential benefits of combining multiple biomarkers. Using multiple biomarkers together, such as combining IMA with troponins or hs-CRP, can improve diagnostic accuracy. This multi-marker approach provides a broader view of both ischemic damage and inflammation, leading to better clinical outcomes by overcoming individual limitations of single markers.
Superiority of Novel Biomarkers than Tn37,38
While Tn levels may not show an immediate increase once the symptoms start, MPO levels may rise earlier, which allow for early detection and treatment. MPO, an enzyme produced by activated leukocytes during inflammation, offer an additional predictive data in patients with ACS, surpassing troponins. Elevated levels of MPO is allied with an amplified probability of encountering adverse heart related events, such as mortality.
In the early stages of ischemia, troponin levels may not exhibit any anomalies. However, levels of IMA may increase at a faster rate, providing a prompt means of detecting and evaluating the risk. IMA serves as a marker for ischemic stress, signaling myocardial ischemia before irreversible damage occurs. IMA levels can rapidly rise during a heart attack, perhaps allowing for faster detection compared to troponins.
While troponin levels may not be a reliable indicator of inflammation in ACS, hs-CRP can provide useful insights into the inflammation and cardiovascular risk. hs-CRP is a biomarker that indicates the presence of inflammation throughout the body and is related with an augmented risk of heart related events. Furthermore, it may provide supplementary predictive insights that go beyond the scope of troponins.
Tns are not sensitive enough to identify small variations in gene appearance that are associated with the development of ACS. In contrast, microRNAs provide a more detailed sympathetic of the molecular mechanisms entangled. Certain microRNAs have a precise and reliable biomarker for ACS, and they outperform troponins in promptly identifying the illness.
Platelet Activating Factor (PAF)
PAF are a group of phospholipids that cause inflammation. They are produced in the body when certain cell types, including thrombocytes, immune cells, mononuclear phagocytes, eosinophils, granulocytes, and endothelial cells, are specifically stimulated39. It is a powerful phospholipid mediator that plays a critical function in regulating inflammation, thrombosis, and vascular permeability. Within the context of ACS, the activation of endothelial cells and the aggregation of platelets at the location of arterial plaque rupture or erosion lead to an elevated release of PAF. This, in turn, triggers a series of events characterized by inflammation and blood clot formation. The presence of platelet hyperaggregability to PAF in N-STEMI is marked through a notable level of distinctiveness and responsiveness. This suggests that it could potentially play a role in the development of ACS40. PAF is produced by platelets and facilitates the process of platelet aggregation, as well as the release of mediators involved in inflammation and platelet activity. Simultaneously, the proclamation of thromboxane-A2 (Tx-A2) and the coarse innards of thrombocytes, including platelet-factor-4 (PF-4) and β-thromboglobulin (β-TG), occurs. PAF does not function as an autonomous intermediary of platelet accumulation; rather, it operates in a similar way as Tx-A2 and adenosine di-phosphate. Activated platelets contribute to the evolution of atherosclerotic plaques and the creation of blood clots, which are important factors in the development of several cardiovascular disorders41.
PAF functions synergistically with other mediators like thromboxane A2 (Tx-A2) and adenosine diphosphate (ADP). Upon platelet activation, these substances work together to enhance platelet aggregation and degranulation, contributing to the formation of thrombi and the progression of atherosclerotic plaques. The activated platelets release additional inflammatory mediators, leading to vascular injury, plaque instability, and increased risk of cardiovascular events such as heart attacks and strokes. Thus, PAF’s interaction with these mediators underscores its importance in cardiovascular disease development.
Various animal model investigations have demonstrated that PAF plays a significant character in the development of cardiac diseases. Simply a limited number of studies have been conducted in humans to investigate the raised levels of PAF in patients who are at a higher risk of experiencing ischemic episodes due to coronary artery disease42.
Galectin-3 (Gal-3)
Gal-3 is a polypeptide that can bind to β-galactoside molecules and is released by activated macrophages. Galectin-3 has a crucial function in the advancement and growth of heart failure and atherosclerosis. Elevated expression of this hybrid lectin was detected in ACS, and it is linked to heightened fibrosis and inflammation. Gal-3 levels in ACS increase due to myocardial damage, endothelial dysfunction, and inflammatory processes caused by plaque rupture or erosion. It functions by attaching and stimulating the fibroblasts responsible for producing collagen and scar tissue, resulting in the gradual development of heart fibrosis and ACS. Galectin-3 levels are markedly elevated in chronic heart failure, regardless of whether it has an acute or non-acute beginning, and regardless of its cause43. Higher levels of Gal-3 are linked to a greater likelihood of experiencing negative cardiovascular events, such as repeated heart attacks, heart failure, and death. This suggests that Gal-3 could be a useful indicator for predicting outcomes in individuals with acute coronary syndrome. By regulating Gal-3 activity, therapies have the potential to alleviate myocardial damage, decrease fibrosis, and enhance heart function in patients with ACS. There is speculation that inhibiting galectin-3 could perhaps slow down the advancement of heart failure and potentially decrease the negative health outcomes and death associated with heart failure. Galectin-3, a mediator produced by macrophages, stimulates the growth of cardiac fibroblasts, the accumulation of collagen, and the impairment of ventricular function. This suggests that in order to address inflammatory responses in heart failure (HF), it is necessary to focus on the first phases of HF and may counteract the effects of galectin-3. The clinical specimens have a galectin-3 size range of 1.4-94.8 ng/mL. Social anti-murine immunoglobulins or rheumatoid factor can disrupt the gal-3 assay, leading to inaccurately high findings. N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP), a tetrapeptide found naturally, can directly inhibit galectin-344. This compound has the ability to prevent and contrary tenderness and collagen confession in the cardiac, particularly in cases of hypertension and cardiac failure following a heart attack. Patients with acute heart failure, regardless of the cause, as well as those with hypertrophic cardiac and aortic valve stenosis with systolic dysfunction, exhibited elevated levels of Gal-345. The gal-3 pathway, which is specifically associated with cardiac fibrosis, shows great potential for identifying high-risk patients. Gal-3 exhibits a significantly high predictive value for both short term and long term projection, and it also adds importance to the assessment of vasodilator peptide. Incorporating Gal-3 assessment into clinical practice has the potential to enhance the ability to forecast risk, inform treatment decision-making, and eventually lead to improved outcomes in patients with ACS.
Soluble ST-2
Soluble ST-2 is a constituent of the pro-inflammatory cytokine transducer family, present in two forms: interface bound (ST-2L) and soluble (sST2). Soluble ST-2 functions as a receptor that distracts interleukin-33 (IL-33), a cytokine that reduces inflammation and is involved in maintaining cardiovascular balance. The ST-2 gene is situated on the 2q12 region of the chromosomal unit of humans and produces two primary polypeptide isoforms: an integral membrane receptor known as ST-2 L and a shorter soluble receptor known as sST2. The IL-33 and ST-2 L interaction facilitates inflammation-reducing effects and fibrosis-reducing effects46. Increased levels of sST-2 have repeatedly been linked to negative outcomes in individuals with ACS, such as a higher risk of death, repeated heart attacks, and heart failure. sST-2 has additional predictive value beyond conventional risk variables and known biomarkers such as troponin. Incorporating sST2 measurement into current risk assessment algorithms has the potential to improve the accuracy of risk prediction, enabling the implementation of more tailored patient care plans. Elevated sST2 levels serve as a robust indicator of heightened mortality risk and unfavorable cardiac events, including recurrent myocardial infarction. While sST2 may provide additional predictive value, incorporating it into current risk assessment algorithms may not necessarily improve accuracy if it does not significantly impact treatment decisions or outcomes. Additionally, focusing solely on sST2 levels may overlook other important factors that contribute to patient risk and prognosis. The levels of plasma sST-2 were markedly elevated in ACS individuals with multifarious lacerations compared to those with unpretentious lacerations, suggesting that sST-2 could serve as a novel indicator for evaluating the steadiness and intricacy of arterial plaques that contribute to ACS47. Due to its lower susceptibility to age and renal insufficiency compared to NT-proBNP and hs-TnT, sST2 has been included in heart failure guidelines. It is considered to offer additional prognostic value and can assist in making therapy decisions for heart failure48.
Unbound Free Fatty Acids Fraction (u-FFAF)
Maintaining a balanced diet with proper nutrient intake is crucial for overall health, reducing heart issues, and potentially lowering the risk of certain cancers49. Biomarkers are crucial indicators aiding diagnosis in various conditions. In diabetes, elevated blood sugar (glucose) levels and HbA1c serve as biomarkers. For heart attacks, troponin levels signify heart muscle damage. Free fatty acids indicate metabolic dysfunction, influencing diabetes and heart health50. Biomarkers like oxidized LDL cholesterol, elevated in heart disease, signal increased risk of heart attacks. Free fatty acids, released during lipid metabolism, impact LDL oxidation, contributing to atherosclerosis. Monitoring these biomarkers aids in assessing cardiovascular health, highlighting the link between oxidized LDL, free fatty acids, and the risk of heart attack51. While the majority of the fatty compounds in the serous fluid are coupled to albumin, a smaller portion remains unbound, often known as the ‘free’ fraction. The calculation of the u-FFAF in serum is based on the proportion of free fatty acids to the total serum albumin52. Elevated levels of blood catecholamines during ischemia indicate that elevated FFAF concentrations are caused by the increased release of free fatty acids via lipolysis. Heparin-induced lipolysis or direct infusion of heparin leads to elevated levels of FFA, leads to cardiac arrhythmias. These effects might be caused by an increase in the use of oxygen by the heart muscle owing to a decrease in the productivity of oxidizing FFA associated to glucose or by the direct harmful effects of FFA on the heart muscle. FFAF is restrained by using a fluorescently labeled transgenic FFA interacting enzyme. The attachment of a free fatty acid to the marked synthetic protein causes the displacement of the fluorescent tag since its obligatory site, leading to a transient change in the spectrum. This change may be quantified using a fluorometer53. FFAF increases precede conventional indicators of myocardial necrosis. Relationship between biomarker and ACS listed in Figure 2.
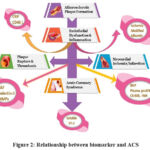 |
Figure 2: Relationship between biomarker and ACS |
Balancing Prognosis and Diagnosis in ACS
Prognostic indicators are essential in forecasting outcomes and informing treatment choices in cases of ACS. It is crucial to acknowledge that they should not always be considered as a replacement for the diagnostic indicators in this illness. Although prognostic markers such as sST2 or platelet-activating factor (PAF) offer useful information about the risk assessment and long-term outlook of patients with ACS, they may not always accurately indicate the extent of myocardial damage during the acute phase. However, these markers frequently signify future alterations in cardiac tissue, such as continuous inflammation or remodeling, which may lack the necessary sensitivity or specificity for rapid identification. Thus, depending exclusively on prognostic markers without proper diagnostic assessment may lead to a delay in prompt therapies that are vital for rescuing ischemic myocardium and preventing additional harm. Therefore, it is crucial to adopt a holistic approach that includes both diagnostic and prognostic markers in order to effectively manage patients with ACS. This comprehensive approach allows for timely and accurate identification of the underlying cause of the acute coronary syndrome, guiding appropriate treatment strategies. By combining diagnostic tests such as ECG, cardiac enzymes, and imaging studies with prognostic markers like troponin levels and risk scores, healthcare providers can make informed decisions to optimize patient outcomes and minimize complications. Ultimately, a multidisciplinary approach that considers both diagnostic and prognostic markers is essential for delivering the highest standard of care for patients with ACS. This approach will enable rapid diagnosis, risk assessment, and implementation of suitable treatment methods. Diagnosis, as well as the identification of biomarkers, should be primarily determined by the medical presentation of retro-sternal pain, supported by electro-cardio-graphic observations of ST segment nonconformities and cardiac imaging, including myocardial scans that reveal perfusion deficiencies. The treatment should take into account the use of antiischemic medications (such as nitrates and β blockers), antiplatelet pharmaceuticals (including aspirin, P2Y (12) inhibitors, and glycoprotein IIb/IIIa receptor blockers), as well as anticoagulants 54. Advantages of novel biomarkers are shown in Table 1.
Table 1: Advantage of Novel Biomarkers for ACS than conventional biomarkers55, 56
|
Features of Biomarkers |
Novel Biomarkers |
Conventional Troponin Biomarkers |
|
Early identification of cardiac damage |
hs-cTn |
The detection of myocardial damage may be delayed. |
|
Enhanced risk categorization |
Galectin-3, ST2 |
Restricted capacity for categorizing danger |
|
Increased precision |
Heart-type fatty acid-binding protein (H-FABP), Myeloperoxidase (MPO) |
May experience non-specific increases |
|
Improved differentiation between cardiac and non-cardiac causes |
BNP, CRP |
Restricted capacity to distinguish between cardiac and non-cardiac origins |
|
Potential for individualized medical treatment based on distinct biomarker profiles |
miRNAs |
Restricted customization determined by troponin levels |
|
Capability to identify persistent damage to the heart muscle in long-term medical situations |
IMA, Soluble ST2 |
Primarily suggestive of acute damage to the heart muscle |
|
Reduce the occurrence of incorrect positive results |
Heart-type fatty acid-binding protein (H-FABP), Myoglobin |
Elevated incidence of false positives in specific demographic groups |
|
Increased predictive significance |
Galectin-3 |
The prognostic value may be restricted in certain instances. |
|
Possibility of intervening sooner based on changes in biomarkers |
miRNAs |
Intervention decisions frequently depend on individual troponin values. |
Conclusion
Cardiac troponins are increasingly used as indicators due to their association with the heart and ability to identify even minor heart muscle damage. Traditional indicators of myocardial necrosis, such as CK-MB and myoglobin, provide limited data on an individual’s condition and risk. Diagnosing and assessing the risk level in ACS is closely interconnected and provides valuable guidance for treatment. This process involves combining ECG, physical examination, medical history, and cardiac biomarkers in individuals with indications of ACS. Recent studies have explored other biomarkers, including inflammation, platelet activation, and ventricular stress. It is anticipated that the quantity of new biomarkers will increase in the future, with the ultimate objective being the discovery of markers that could potentially enhance patient care by offering greater value and additional information. As the omics method continues to expand, doctors and researchers can utilize metabolomics to examine these processes at the molecular level, hence enhancing the development of tailored medicines that can be applied in clinical settings.
Acknowledgement
The authors would like to thank Chalapathi Institute of Pharmaceutical Sciences, Chalapathi Nagar, Lam, Guntur, India and Department of Biology, Faculty of Science and Technology, University of Relizane, Algeria for providing library and other facilities to draft this review
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Conflict of interest
The author(s) do not have any conflict of interest
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration-
This research does not involve any clinical trials
Author Contributions
RRN: concept, designed; MB and RM: reviewed the manuscript; KSA and MP: manuscript preparation, editing and designed the manuscript; MP: all image works. Finally, all authors had given approval for manuscript publication.
Reference
- Kemp M, Donovan J, Higham H, Hooper J. Biochemical markers of myocardial injury. British Journal of Anaesthesia. 2004; 93(1):63-73.
CrossRef - Munta AK, Raghavan V, Gorle RM, Basha SJ. Cardiac biomarkers and their importance in the diagnosis of myocardial ischemia and acute myocardial infarction. Indian Journal of Medical Biochemistry. 2018; 22(1):78-84.
CrossRef - Mueller C. Biomarkers and acute coronary syndromes: an update. European heart journal. 2014; 35(9):552-6.
CrossRef - Lindahl B, Venge P, Wallentin L. Relation between troponin T and the risk of subsequent cardiac events in unstable coronary artery disease. The FRISC study group. Circulation 93(9), 1651-1657 (1996).
CrossRef - Kaplangoray M, Toprak K, Cicek OF, Deveci E. Relationship between the fibrinogen/albumin ratio and microvascular perfusion in patients undergoing primary percutaneous coronary intervention for ST-elevated myocardial infarction: A prospective study. ABC Cardiol. 2023; 120(11):e20230002.
- Chacko S, Haseeb S, Glover BM, Wallbridge D, Harper A. The role of biomarkers in the diagnosis and risk stratification of acute coronary syndrome. Future Science OA. 2018; 4(1):FSO251.
CrossRef - Garg P, Morris P, Fazlanie AL, Vijayan S, Dancso B, Dastidar AG, Sven P, Christian M & Philip H. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Internal and Emergency Medicine. 2017; 12(2):147-55.
CrossRef - Collinson PO, Boa FG, Gaze DC. Measurement of cardiac troponins. Annals of Clinical Biochemistry. 2001; 38(5):423-49.
CrossRef - Kehl DW, Iqbal N, Fard A, Kipper BA, De La Parra Landa AD, Maisel AS. Biomarkers in acute myocardial injury. Translational Research. 2012; 159(4):252-64.
CrossRef - Solaro RJ, Rarick HM. Troponin and tropomyosin: proteins that switch on and tune in the activity of cardiac myofilaments. Circulation Research. 1998; 83(5):471-80.
CrossRef - Dowrick JM, Taberner AJ, Han J-C, Tran K. Methods for assessing cardiac myofilament calcium sensitivity. Frontiers in Physiology. 2023; 14:1323768.
CrossRef - Mueller C, Muench-Gerber TS, de Boer RA. Growth differentiation factor 15: a biomarker searching for an indication. European Heart Journal. 2023; 44(4):301-3.
CrossRef - Danese E, Montagnana M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Annals of Translational Medicine’s. 2016; 4(10):194.
CrossRef - Geaney A, O’Reilly P, Maxwell P, James JA, McArt D, Salto-Tellez M. Translation of tissue-based artificial intelligence into clinical practice: from discovery to adoption. Oncogene. 2023; 42(48):3545-55.
CrossRef - Forman DE, Kuchel GA, Newman JC, Kirkland JL, Volpi E, Taffet GE, Nir Barzilai MD, Ambarish Pandey, Dalane W, Peter Libby, Luigi Ferrucci. Impact of Geroscience on therapeutic strategies for older adults with cardiovascular disease: JACC scientific statement. Journal of the American College of Cardiology. 2023; 82(7):631-47.
CrossRef - Zamani B, Golabchi A, Ghadakkar N, Motedayyen H. C-reactive protein and uric acid roles in distinguishing ST-segment elevation myocardial infarction from non-ST-elevation acute coronary syndrome. Journal of Immunoassay and Immunochemistry. 2023; 44(1):66-75.
CrossRef - Tomoda H & Aoki N. Prognostic value of C-reactive protein levels within six hours after the onset of acute myocardial infarction. American Heart Journal. 2000; 140(2):324-8.
CrossRef - Heeschen C, Hamm CW, Bruemmer J & Simoons ML. Predictive value of C-reactive protein and troponin T in patients with unstable angina: a comparative analysis. CAPTURE Investigators. Chimeric c7E3 AntiPlatelet Therapy in Unstable angina REfractory to standard treatment trial. Journal of the American College of Cardiology. 2000; 35(6):1535-42.
CrossRef - Caixeta A, Stone GW, Mehran R, Lee EA, McLaurin BT, Cox DA, Michel E, Michael L, Jeffrey W, Harvey D, Magnus E, Tullio P, George S, Christos K, Martin F, Craig W, Alexandra J & George D. Predictive value of C-reactive protein on 30-day and 1-year mortality in acute coronary syndromes: an analysis from the ACUITY trial. Journal of Thrombosis and Thrombolysis. 2011; 31(2):154-64.
CrossRef - Tousoulis D, Androulakis E, Papageorgiou N, Briasoulis A, Siasos G, Antoniades C, Stefanadis C. From atherosclerosis to acute coronary syndromes: the role of soluble CD40 ligand. Trends in Cardiovascular Medicine. 2010; 20(5):153-64.
CrossRef - Rizzo G, Gropper J, Piollet M, Vafadarnejad E, Rizakou A, Bandi SR, Panagiota A, Tobias K, Nina D, Oliver D, Anahi P L, Marco P, Matthias M, Kai S, Christian H, Jean S, Alma Z, Antoine E, Clement C. Dynamics of monocyte-derived macrophage diversity in experimental myocardial infarction. Cardiovascular Research. 2023; 119(3):772-85.
CrossRef - Zaman GS, Mir MA, Bashir N, Kakaraparthi VN, Zaman FA. Comparison of creatine kinase-MB, troponin-I, troponin-T, triiodothyronine, thyroxine, apoprotein-B and homocysteine between non-smokers, smokers at high altitudes, and smokers at sea level. Cellular and Molecular Biology (Noisy-Le-Grand). 2023; 69(2):101-9.
CrossRef - Westermann D, Neumann JT, Sörensen NA, Blankenberg S. High-sensitivity assays for troponin in patients with cardiac disease. Nature Reviews Cardiology. 2017; 14(8):472-83.
CrossRef - Olukoga A, Donaldson D. An overview of biochemical markers in acute coronary syndromes. Journal of the Royal Society for the Promotion of Health. 2001; 121(2):103-6.
CrossRef - Sawatani T, Shirakabe A, Okazaki H, Matsushita M, Shibata Y, Shigihara S, Suguru N, Nozomi S, Kazutaka K, Nobuaki K, Wataru S, Kuniya A. Time-dependent changes in N-terminal pro-brain natriuretic peptide and B-type natriuretic peptide ratio during hospitalization for acute heart failure. International Heart Journal. 2023; 64(2):213-22.
CrossRef - de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Christian H, Christopher P, Eugene B. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. The New England Journal of Medicine. 2001; 345(14):1014-21.
CrossRef - Ferrannini G, Benson L, Lautsch D, Dahlström U, Lund LH, Savarese G, Carrero JJ. N‐terminal pro‐B‐type natriuretic peptide concentrations, testing and associations with worsening heart failure events. ESC Heart Failure. 2024; 11(2):759-71.
CrossRef - Nishikimi T, Nakagawa Y. B-type natriuretic peptide (BNP) revisited-is BNP still a biomarker for heart failure in the angiotensin receptor/neprilysin inhibitor era? Biology. 2022; 11(7):1034.
CrossRef - Koljonen E, Lappalainen L, Kotiranta S, Turpeinen A, Vepsäläinen V, Karkkainen S, Jarkko R, Tuomas S, Juha H & Jaana R. Plasma N-terminal pro-B-type natriuretic peptide in the detection of aortic valve stenosis. Scandinavian Journal of Clinical and Laboratory Investigation. 2023; 83(7):489-94.
CrossRef - Ingwall JS, Kramer MF, Fifer MA, Lorell BH, Shemin R, Grossman W, Allen PD. The creatine kinase system in normal and diseased human myocardium. The New England Journal of Medicine. 1985; 313(17):1050-4.
CrossRef - Ellis SG, Chew D, Chan A, Whitlow PL, Schneider JP, Topol EJ. Death following creatine kinase-MB elevation after coronary intervention: identification of an early risk period: importance of creatine kinase-MB level, completeness of revascularization, ventricular function, and probable benefit of statin therapy. Circulation. 2002; 106(10):1205-10.
CrossRef - Lott JA, Stang JM. Differential diagnosis of patients with abnormal serum creatine kinase isoenzymes. Clinics in Laboratory Medicine. 1989; 9(4):627-42.
CrossRef - Roy D, Quiles J, Gaze DC, Collinson P, Kaski JC, Baxter GF. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. Heart. 2006; 92(1):113-4.
CrossRef - Keating L, Benger JR, Beetham R, Bateman S, Veysey S, Kendall J, Pullinger R. The PRIMA study: presentation ischaemia-modified albumin in the emergency department. Emergency Medicine Journal. 2006; 23(10):764-8.
CrossRef - Talwalkar SS, Bon Homme M, Miller JJ, Elin RJ. Ischemia modified albumin, a marker of acute ischemic events: a pilot study. Annals of Clinical & Laboratory Science. 2008; 38(2):132-7.
- Zapico-Muñiz E, Santaló-Bel M, Mercé-Muntañola J, Montiel JA, Martínez-Rubio A, Ordóñez-Llanos J. Ischemia-modified albumin during skeletal muscle ischemia. Clinical Chemistry. 2004; 50(6):1063-5.
CrossRef - Mihaileanu FV, Popa SL, Grad S, Dumitrascu DI, Ismaiel A, Rus E, Vlad D, Alexandru M, Miruna O, Daria C, Traian A, Andrei V, Paolo B, Giuseppe C, Cristina P, Cristina M, Maria B, Claudia D, Bogdan S & Liliana D. The efficiency of serum biomarkers in predicting the clinical outcome of patients with mesenteric ischemia during follow-up: A systematic review. Diagnostics (Basel). 2024; 14(7):670.
CrossRef - Katsioupa M, Kourampi I, Oikonomou E, Tsigkou V, Theofilis P, Charalambous G, George M, Ioannis G, Konstantinos Z, Artemis A, Efstratios K, Konstantinos K, Ourania K, Manolis V, Gerasimos S & Dimitris T. Novel biomarkers and their role in the diagnosis and prognosis of acute coronary syndrome. Life (Basel). 2023; 13(10):1992.
CrossRef - Triggiani M, Schleimer RP, Warner JA, Chilton FH. Differential synthesis of 1- acyl-2- acetyl-sn-glycero-3-phosphocholine and platelet activating factor by human inflammatory cells. The Journal of Immunology. 1991; 147(2):660-6.
CrossRef - Michalis LK, Tambaki AP, Katsouras CS, Goudevenos JA, Kolettis T, Adamides K, Tselepis AD, Sideris DA. Platelet hyperaggregability to platelet activating factor (PAF) in non-ST elevation acute coronary syndromes. Current Medical Research and Opinion. 2002; 18(2):108-12.
CrossRef - de Jonge P, Rosmalen JG, Kema IP, Doornbos B, van Melle JP, Pouwer F, Kupper N. Psychophysiological biomarkers explaining the association between depression and prognosis in coronary artery patients: a critical review of the literature. Neuroscience & Biobehavioral Reviews. 2010; 35(1):84-90.
CrossRef - Musselman DL, Tomer A, Manatunga AK, Knight BT, Porter MR, Kasey S, Marzec U, Harker LA, Nemeroff CB. Exaggerated platelet reactivity in major depression. The American Journal of Psychiatry. 1996; 153(10):1313-7.
CrossRef - Hrynchyshyn N, Jourdain P, Desnos M, Diebold B, Funck F. Galectin-3: a new biomarker for the diagnosis, analysis and prognosis of acute and chronic heart failure. Archives of Cardiovascular Diseases. 2013; 106(10):541-6.
CrossRef - Christenson RH, Duh S-H, Wu AHB, Smith A, Abel G, deFilippi CR, Shunguang W, Aram Adourian, Carol A, Peter G. Multi-center determination of galectin-3 assay performance characteristics: anatomy of a novel assay for use in heart failure. Clinical Biochemistry. 2010; 43(7-8):683-90.
CrossRef - Liu Y-H, D’Ambrosio M, Liao TD, Peng H, Rhaleb N-E, Sharma U, Sabine A, Hans G & Oscar A. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. American Journal of Physiology-Heart and Circulatory Physiology. 2009; 296(2):H404-12.
CrossRef - Arora H, Javed B, Kutikuppala LVS, Chaurasia M, Khullar K, Kannan S, Golla V. ST2 levels and neurodegenerative diseases: is this a significant relation? Annals of Medicine and Surgery (Lond). 2024; 86(5):2812-7.
CrossRef - Zhang Y, Fan Z, Liu H, Ma J, Zhang M. Correlation of plasma soluble suppression of tumorigenicity-2 level with the severity and stability of coronary atherosclerosis. Coronary Artery Disease. 2020; 31(7):628-35.
CrossRef - Aimo A, Januzzi Jr JL, Vergaro G, Richards AM, Lam CSP, Latini R, Inder S, Jay N, Thor U, Lars G, Pal A, Hans-Peter B, Antoni B, Josep L, Rudolf A, Yasuchika T, Michael E, Ida G, Hanna K, Kai M, Kurt H, Greg D, Lieng H, Kui T, Poh S, Hean Y, Fazlur J, Tze P, Richard T, Robert N, Claudio P & Michele E. Circulating levels and prognostic value of soluble ST2 in heart failure are less influenced by age than N‐terminal pro‐B‐type natriuretic peptide and high‐sensitivity troponin T. European Journal of Heart Failure. 2020; 22(11):2078-88.
CrossRef - Bhupathiraju SN, Tucker KL. Coronary heart disease prevention: nutrients, foods, and dietary patterns. Clinica Chimica Acta. 2011; 412(17-18):1493-514.
CrossRef - Chakraborty T, Gupta S, Saini V, Talukdar A. Biomarkers: an important tool for diagnosing and treating diabetes mellitus. International Journal of Life science and Pharma Research. 2021; 11(2):123-9.
CrossRef - Oxidized kp [1-14 c] linoleic acid and hydrogen peroxide tone might promote anti-atherogenic action and prevent metabolic diseases. International Journal of Pharma and Bio Sciences. 2017; 8(4):112-8.
CrossRef - Ishikawa M, Uchida M, Asakawa T, Suzuki S, Yamazaki S, Shiko Y, Kawasaki Y, Suzuki T, Ishii I. A novel method for predicting the unbound valproic acid concentration. Drug Metabolism and Pharmacokinetics. 2023; 50:100503.
CrossRef - Richieri GV, Ogata RT, Kleinfeld AM. The measurement of free fatty acid concentration with the fluorescent probe ADIFAB: a practical guide for the use of the ADIFAB probe. Molecular and Cellular Biochemistry. 1999; 192(1-2):87-94.
CrossRef - Baghdasaryan P, Natarajan B, Nalbandian M, Varadarajan P, Pai RG. Myocardial infarction with nonobstructive coronary artery disease—definition, etiopathogenesis, diagnosis, and management. International Journal of Angiology. 2021.
CrossRef - Spencer, T.R., Sidhu, M.S., Bisaillon, J. Christopher King C. Novel Cardiac Biomarkers for Emergency Department Evaluation of Acute Coronary Syndrome: The Recent Evidence on Non-troponin Biomarkers and Their Limitations. Current Emergency and Hospital Medicine Reports 4, 99–106 (2016).
CrossRef - Maznyczka A, Kaier T, Marber M. Troponins and other biomarkers in the early diagnosis of acute myocardial infarction. Postgraduate Medical Journal. 2015 Jun;91(1076):322-30. doi: 10.1136/postgradmedj-2014-133129. Epub 2015.
CrossRef
Abbreviations
Acute coronary syndrome (ACS)
Creatine kinase-myocardial band (CK-MB)
electrocardiogram (ECG)
myocardial infarction (MI)
unstable angina (UA)
NSTEMI (non-ST elevation MI)
STEMI (ST elevation MI)
Acute myocardial infarction (AMI)
creatine kinase (CK)
C-reactive protein (CRP)
myeloperoxidase (MPO)
millivolt (mV)
cardiac Troponin I (cTnI)
cardiac Troponin T (cTnT)
brain-type natriuretic peptide (BNP)
ischemia modified albumin (IMA)
adenosine triphosphate (ATP)
troponin T (TnT)
high-sensitivity CRP (hs-CRP)
platelet-activating factor (PAF)
N-pro-BtNP (N-terminal pro-B-type Natriuretic Peptide)
BtNP – B type Natriuretic Peptide
Souluble suppression of tumorigenicity (SST-2)
Galectin-3 (Gal-3)







