Prasad Arvind Thakurdesai1* , Gayatri Veersing Shivsingwale2
, Gayatri Veersing Shivsingwale2 and Urmila Manoj Aswar2
and Urmila Manoj Aswar2
1Department of Scientific Affairs, Indus Biotech Limited, Pune, India.
2Department of Pharmacology, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, India.
Corresponding Author E-mail: prasad@indusbiotech.com
DOI : https://dx.doi.org/10.13005/bpj/3018
Abstract
Objective: To assess the effectiveness of nasal solution of Centella asiatica leaves standardized to triterpenoids (INDCA-NS) in preventing “chronic unpredictable mild stress” (CUMS) in rats. Methods: The study involved six groups of twelve rats each, with five groups receiving CUMS induction and one group without CUMS, serving as a vehicle control (VC). The other groups received intranasal administration of saline, buspirone, or INDCA-NS (10,30 and 100 µg/rat/day, 2.5, 7.5, and 10 µg/nostril/twice daily) from day 35 to day 49. Behavioral parameters were assessed using the marble burying test, Y-maze, Morris water maze, sucrose preference test, and resident intruder test on days 0, 35, 42, and 49, respectively. The levels of stress-related biomarkers, cortisol and “brain-derived neurotrophic factor” (BDNF) in the hypothalamus were measured using ELISA kits. The data was analyzed with analysis of variance followed by pairwise comparisons, with significance set at P < 0.05. Results: CUMS induction led to a significant increase in anxiety, anhedonia, aggression, and stress markers, as well as a reduction in working and spatial memory parameters (vs. VC). INDCA-NS and BUS administration for 14 days resulted in dose-dependent and significant prevention of CUMS-induced anxiety, working memory, anhedonia, and aggression, but not Morris’s water maze parameters and BDNF levels. Conclusion: Subacute intranasal INDCA-NS showed chronic stress-preventive potential in rats, indicated by the prevention of anxiety, anhedonia, and aggression in CUMS-induced rats, probably through cortisol reduction.
Keywords
Centella asiatica leaves; Chronic stress; Cortisol; Gotu kola; Intranasal administration
Download this article as:| Copy the following to cite this article: Thakurdesai P. A, Shivsingwale G. V, Aswar U. M. Nasal Treatment of Standardized Centella Asiatica Leaves Extract Ameliorates Chronic Unpredictable Mild Stress in Laboratory Rats. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Thakurdesai P. A, Shivsingwale G. V, Aswar U. M. Nasal Treatment of Standardized Centella Asiatica Leaves Extract Ameliorates Chronic Unpredictable Mild Stress in Laboratory Rats. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3YH5uEs |
Introduction
Individuals often encounter stressors in their daily lives, which could affect mood, focus, and performance. However, chronic exposure can lead to more serious consequences, including cognitive and behavioral changes, such as cognitive decline1. Chronic stress disrupts the body’s ability to maintain stability (allostasis) and activates many physiological changes, such as stimulation of the sympathetic nervous system and inflammation, ultimately compromising cardiovascular and immune functions2, 3. These physiological consequences significantly reduce quality of life, highlighting the need for interventions that target stress-induced physiological dysregulation to improve overall well-being4.
The symptoms of stress-induced behavioral and cognitive decline follow distinct temporal profiles. Within minutes to hours of stress induction, anxiety emerges as the initial response and is characterized by heightened worry, nervousness, and physical tension5. Activation of “hypothalamic-pituitary-adrenal” (HPA) axis promotes stress hormones, such as cortisol6. In the short term (hours to days), working memory becomes compromised, impacting the ability to conduct routine tasks, especially those that require manipulation information in the mind. This is thought to be due to stress-induced disruptions in the prefrontal cortex, a key region in working memory function7.
The intermediate stages (days to weeks) showed a decline in spatial memory and ability to navigate and remember locations. Chronic stress can disrupt the hippocampus, a critical brain structure for spatial navigation, potentially through mechanisms involving reduced neurogenesis (neurogenesis refers to the birth of new neurons)8. While long-term memory initially appears to be less affected, chronic stress can impair the consolidation of new information into long-term storage over weeks or months9. This extended stress exposure may further contribute to the development of depression, a more prolonged state characterized by low mood, anhedonia (loss of pleasure), and sleep and appetite disturbances10 . Chronic stress alters neurotransmitter systems, such as serotonin and norepinephrine, to pose a risk of depression development11. Finally, although less prevalent, chronic stress can also lead to increased aggression or irritability, highlighting the diverse behavioral consequences of prolonged stress exposure12.
Chronic stress is known to disrupt the HPA axis and the body’s stress response system13. Dysregulation of the HPA axis can have far-reaching effects on various physiological systems, including emotional regulation, eating habits, and the immune system14. HPA axis stimulation secretes glucocorticoids, such as cortisol, which play a crucial role in redirecting energy resources to cope with stress15. Increased cytokines lead to depression both directly (HPA axis) and indirectly (cytokines)16. Cytokines directly act on hypothalamic and pituitary cells and increase the production of CRF, ACTH, and cortisol17. Dysregulation of 5HT transmission in the cortex is also associated with chronic stress18. During chronic stress, microglial activation occurs, which leads to increased cytokines, serotonin, and inflammation19.
The management of stress-related disorders is limited to symptomatic treatment without addressing the underlying cause (stress) with undesirable side effects20. Medication options addressing HPA axis dysregulation, a cause and important target of stress-related disorders, with serotonergic efficacy and an excellent safety profile can address chronic stress, and related behavioral symptoms can offer efficient interventions and enhance patient outcomes21.
Considerable amount of evidence in last decade demonstrated the efficacy of Gotu kola, Centella asiatica (L.) Urban, (i.e. CA) leaves (Family: Apiaceae) against the symptoms of many stress-induced neuropsychological disorders22, 23 probably HPA axis, serotonergic neurotransmission, and regulation of brain cortisol levels24. The source of CA leaves from Madagascar region contains the major bioactive triterpenoids, such as namely asiaticoside, and madecassoside that are beneficial for the management of many nervous system disorders22, 25. In addition, triterpenoid-based standardized CA leaf extract (INDCA) oral supplementation has been reported to alleviate experimental depression26 and migraine27 via serotonin agonist action27.
Recently, nasal route has emerged as a promising choice for safer and more effective patient-compliant solutions against stress-related disorders owing to its many advantages, such as avoidance of first-pass metabolism28. The olfactory perineural space contains transporters that enhance drug transport into the brain29. The intranasal delivery of various compounds, including natural bioactive phytoconstituents such as quercetin, has been reported to deliver therapeutic agents to the brain30.
INDCA nasal solution (INDCA-NS) showed robust safety at doses of 100 µg/rat/day in rats31. In addition, INDCA-NS has been reported to alleviate pain caused by nitroglycerine-induced migraine27. However, the potential of INDCA-NS to provide relief from chronic stress has not been explored.
Hence, our study aimed to evaluate the efficacy of nasal INDCA-NS against “chronic unpredictable mild stress” (CUMS), a type of chronic stress with good reliability and clinical validity32.
Materials and Methods
Animals
The study utilized Sprague-Dawley rats (both genders, 200-250 g weights). Rats were purchased from Crystal Biological Solutions (Pune, India). This study was conducted in accordance with Indian guidelines on animal ethics33. The rats were housed in polypropylene cages in groups of six and maintained in a controlled environment, as recommended34. The rats were provided with unrestricted access to drinking water and feed pellets supplied by Nutrivet Life Sciences (Pune, India). The experimental protocols were sanctioned by the “Institutional Animal Ethics Committee” (IAEC) of “Poona College of Pharmacy, Bharati Vidyapeeth Deemed University,” Pune, India (approval number CPCSEA/ PCP/PCL33/2018-19). Each rat was used once and acclimatized to conditions before the experiment. To eliminate potential bias, the observer was blinded to the treatment. All observations were conducted between 9:00 AM and 4:00 PM.
Drugs and Chemicals
Buspirone hydrochloride (Cat No: B7148, Sigma-Aldrich Chemicals Pvt. Ltd., USA) was used as a positive control. ELISA kits for estimating brain-derived neurotrophic factor (BDNF; Catalog No: EK0308; Boster Immunoleader, Pleasanton, CA, USA) and cortisol (Catalog number CSB-E05112r; Cusabio, USA) were purchased from a local distributor (GK BioScience, Pune, India).
The test solution, INDCA-NS
INDCA-NS was provided by Indus Biotech Limited (Pune, India) as a stock solution (1 mg/ml, 1000 µg /1000 µL) containing INDCA. Because of intranasal route, INDCA-NS was administered at a volume of 25 µL/nostril or 50 µL per rat as a safe intranasal volume in rats 35. To maintain the same volume per nostril, the stock solution was diluted 10-, 3-, and 0-times to deliver final doses of INDCA-NS of 2.5, 7.5, and 10 µg/nostril/twice daily, that is, 10, 30, and 100 µg/rat/day, respectively.
The positive control, BUS
The buspirone hydrochloride solution (BUS) was prepared and used for nasal administration as per a previously reported procedure36. BUS was prepared by dissolving 15.5 mg of buspirone hydrochloride in 5 ml of sterile 0.5% sodium chloride solution and filtering through a sterile (0.2 mm) membrane filter for a final concentration of 3.1 mg/ml. The intranasal dose of 20 µL of BUS, which is equivalent to 0.25 mg/kg of BUS (considering maximum weight of rat = 250 g), was used as a positive control based on past report36.
Grouping and Treatment Schedule
Adult rats were randomly divided into six groups of 12 rats (G1–G6). G1 (vehicle control, VC) was treated with vehicle (25 µL/nostril, twice daily, intranasally) for 49 days. Groups G2–G6 were subjected to CUMS for 49 days (D0 to D49), and parameters were measured during D35–D49. G2 (stress control, SC) was treated with vehicle (25 µL/nostril, twice a day, intranasal). G3 (BUS group) was served as a positive control and was treated with buspirone (0.25 mg/rat, twice a day, intranasally). G4 to G6 were treated with INDCA-NS (5,15,50 microgram/rat/day, twice a day, intranasal) at final doses of 10, 30, and 100 microgram/rat/day. The CUMS induction, treatment details, and outcome measures are presented in Figure 1.
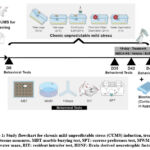 |
Figure 1: Study flowchart for chronic mild unpredictable stress (CUMS) induction, treatments, and outcome measures.
|
CUMS induction and outcome measures
Training Phase
CUMS was induced in rats as previously reported method37, 38. Briefly, CUMS was induced in the training phase of one week (1% sucrose solution with no food and water for 48 h and then without sucrose solution for an additional 18 h). Thereafter, the rats were housed individually, and sucrose consumption was measured for one hour on each of the following five consecutive days. Food and water were provided ad libitum for five hours. Sucrose consumption (intake) was calculated as the difference in bottle weights (pre- and post-training) and the animal’s body weight (g/kg) at the end of the training phase. Based on the sucrose consumption test, rats were allotted to non-stressed (G1:VC) and stressed (G2–G6) groups.
CUMS Induction Phase
After the first week (testing phase), all rats except those in the VC group were subjected to CUMS as a series of unpredictable stresses for a period of seven weeks (49 days) from D0 to D49 of the study, in which treatments were administered for the last 14 days (D36 to D49). After the first week, the sequence of mild and unpredictable stressors was randomly repeated. These stressors included exposure: 10 h – 45° tilted cage, 24 h – water /feed deprivation, 1 h – limited food, 1 h – empty bottles, 24 h – wet cages, and 24 h – continuous light. During the stress sessions, the animals were housed in groups of six per cage, except for sucrose consumption (single housing).
Measurements
Baseline measurements from behavioral tests, such as the marble burying test (MBT) for anxiety, Y-maze for worming memory, morris water maze (MWM) for special memory, and resident intrusion test (RIT) for aggression, were performed on D0, and sucrose preference tests (SFT) for anhedonia were performed on D5. These behavioral tests were conducted on D35, D42, and D49. On D49, rats were sacrificed, brains were removed, to prepare homogenate with phosphate buffer for cortisol and BDNF estimation.
Marble Burying Test (MBT)
The effects of treatments on early anxiety were assessed using an earlier reported method39. Briefly, the rats were placed in cages filled with husk bedding material cobs (5 cm height). The test was performed in two phases: habituation and testing. In habituation phase, rats were exposed to cages without marbles. During the testing phase, 20 glass marbles (plain black glass) were evenly placed in the cage on the bedding surface. The rats could explore cage for 20 min and were then removed. The number of marbles in two-thirds deep was counted.
Y maze
Working memory function was measured using the Y-maze, as described previously 40. The maze consisted of three Y-shaped arms. The three arms were randomly assigned: one was left open, the second was to keep the rat initially, and the third was blocked during the initial training period. During trials, visual cues were added to the maze. Before testing, rats were pre-trained with the novel arm closed. After a 1-hour break, the test proceeded with the novel arm open. The rats’ novelty versus familiarity was evaluated by comparing their behavior in all three arms. The number of visits and time spent in each arm were recorded for 5 min using a camera.
MWM test
Spatial memory measurement using the MWM test was performed as per an earlier procedure41. MWM, a circular water tank (diameter X height – 150 cm X 45 cm), filled with water (upto 35 cm at 20-22°C). The tank was divided into four equal sections and had a central round platform (15 cm diameter). Initially, training was given for five trials on five consecutive days, followed by a probe trial (memory retrieval trial) on D6. During the first 60 s, rats freely explored and searched for the hidden fixed platform (2 cm below the water surface), and the time to reach platform was noted as “escape latency.” If a rat was unsuccessful at reaching the platform, an additional 10 s were given before removal. On day 6, a probe trial was performed with opaque water using a nontoxic white color. The rats were permitted to explore the platform, and escape latency and path length were measured using a video tracking system (VJ Instruments, Karanja, India).
SPT
The SPT was performed using reported procedure37. Before the initiation of the experiments, the SPT was performed with overnight (12 h) fasted rats. At binging and every 7 days of study, the rats were individually placed in cages, where they were exposed to two 250 ml (one with 1% sucrose in distilled water and the second with distilled water). The animals were allowed to drink water for 1 h, with an interchange of bottles after 30 min (to avoid side preference). The percentage of sucrose preference was calculated as follows: [sucrose intake (mL)/(sucrose intake (mL) + water intake (mL)] × 100 42-44.
Resident Intruder Test (RIT)
As previously reported, RIT was used to evaluate the effects of the treatments on aggression45. This test was carried out in two phases: first, resident rats could explore the cage for 15 m (adaptation), and then, the intruder rat of lesser weight (100–150 g) was transferred to the resident’s cage for 5 m, and attack latency measurements were performed. Rats were considered aggressive when they displayed either a bite or lateral attack.
Cortisol and BDNF estimation in hypothalamus
After treatment, the rats were sacrificed, and brains were immediately isolated on ice slabs. The hippocampal region was separated, and homogenization was performed using phosphate buffer. The supernatants were divided and used for the estimation of cortisol and BDNF levels using an ELISA plate reader (LisaScan II, Erba Mannheim Diagnostic, London, United Kingdom), ELISA kits, and assay instructions. The results are expressed per gram as pg/g for BDNF and ng/g for cortisol.
Statistical Analysis
The data are expressed as mean ± standard error of means (SEM) using analyzed statistically with Prism 6 (GraphPad; La Jolla, USA) as follows (1) SPT, MWM, RIT, MBT, and OFT: two-way repeated measures analysis of variance (ANOVA) and Bonferroni’s test (2) Cortisol and BDNF: one-way ANOVA and Dunnett’s test. Statistical significance was set at P < 0.05.
Results
Effect on MBT Parameters
ANOVA of MBT data showed significant effects of time (F(2.445, 188.3) = 188.7, P < 0.001) and treatment (F(6, 77) = 24.52, P < 0.001) on the number of buried marbles (Table 1). Dunnett’s test revealed no significant difference in the number of buried marbles (between any pair of treatments on D0). The number of buried marbles was significantly higher in the SC group than in the VC group on D35, D42, and D49 (P < 0.001). On D35, there was no significant difference between buried marbles in the BUS, INDCA-NS (vs. SC). However, the number of buried marbles was significantly different (P < 0.01 or P < 0.01) in the BUS- and INDCA-NS-treated groups compared to SC on D42 and D49.
Table 1: Effects on early to short-term symptoms of CUMS
|
Day |
VC |
SC |
BUS |
INDCA- |
INDCA- |
INDCA- |
|
Anxiety – Number of marbles buried – MBT |
||||||
|
D0 |
3.17 ± |
3.50 ± |
2.25 ± |
1.33 ± |
2.67 ± |
2.67 ± |
|
D35 |
1.00 ± |
12.17 ± |
12.17 ± |
13.83 ± |
14.25 ± |
15.25 ± |
|
D42 |
1.67 ± |
12.08 ± |
3.83 ± |
6.92 ± |
5.08 ± |
4.58 ± |
|
D49 |
1.83 ± |
13.00 ± |
2.08 ± |
5.08 ± |
3.58 ± |
3.83 ± |
|
Working memory – Number of entries – Y-maze |
||||||
|
D0 |
3.67 ± |
3.00 ± |
2.33 ± |
2.83 ± |
2.83 ± |
3.33 ± |
|
D35 |
2.33 ± |
3.00 ± |
1.17 ± |
2.50 ± |
1.50 ± |
2.17 ± |
|
D42 |
1.83 ± |
3.50 ± |
2.17 ± |
2.50 ± |
1.67 ± |
1.83 ± |
|
D49 |
2.33 ± |
3.83 ± |
2.50 ± |
2.33 ± |
2.50 ± |
3.00 ± |
|
Working memory – Time spent (s) – Y-maze |
||||||
|
D0 |
66.83 ± |
96.17 ± |
88.33 ± |
101.00 ± |
91.50 ± |
86.67 ± |
|
D35 |
38.50 ± |
54.50 ± |
34.67 ± |
39.33 ± |
62.33 ± |
58.00 ± |
|
D42 |
30.50 ± |
50.50 ± |
28.67 ± |
28.83 ± |
18.17 ± |
44.33 ± |
|
D49 |
32.00 ± |
53.83 ± |
16.50 ± |
23.83 ± |
29.50 ± |
21.33 ± |
n = 12, Data as mean ± standard error of mean. Numbers in bracket indicate dose (microgram/day intranasal). VC: vehicle control, SC: CUMS control, BUS- Buspirone, MBT -= Marble burying test, ## P < 0.01, ### P < 0.001 (vs. VC), * P < 0.05, ** P < 0.01, *** P < 0.001 (vs. CS).
Effect on Y maze Parameter
Two-way repeated-measures ANOVA of working memory-related data on number of entries into novel arm (Table 1) showed significant effects with respect to time (F(2.561, 197.2) = 8.977, P < 0.001) and treatments (F(6, 77) = 4.533, P < 0.001). Dunnett’s test showed no significant effects of treatment between the groups on D0 (SC vs VC, BUS or INDCA-NS vs. SC). Number of entries in SC group (vs/ VC) was significant (P < 0.001 or P < 0.001) on D42 and D49, respectively; however, the difference between SC and VC was not statistically significant on D35. On D42, the number of entries in the BUS- and INDCA-NS (all doses)-treated groups was significantly lower. On D49, INDCA-NS (10 and 30)-treated groups showed significantly fewer entries (vs. SC), whereas the BUS- and INDCA-NS (100)-treated groups showed no significant changes (vs. SC).
Two-way repeated-measures ANOVA of time spent in the novel arm (Table 1) showed significant effects with respect to time (F(2.015, 155.1) = 70.95, P < 0.001) or treatment (F(6, 77) = 2.488, P < 0.05). Dunnett’s test revealed no significant effects of treatments, on D0, D35 or D49, between the groups (SC vs VC, BUS or INDCA-NS vs. SC). On D42, time spent signing was higher in SC (vs. VC) and lower in BUS and INDCA-NS (10 and 30), but not in INDCA-NS (100).
Effect on MWM Parameters
Two-way ANOVA of escape latency data from the MWM (Table 2) indicated significant effects of or treatment (F(6, 77) = 6.215, P < 0.001) or time (F(3, 231) = 70.84, P < 0.001) Dunnett’s test on D0, showed no effects in SC (vs VC) and BUS or INDCA-NS treated groups (vs SC) indicating uniform groupings. SC showed a significant increase in escape latencies compared with VC on D35, D42, and D49. Significant prevention of SC-induced enhanced escape latencies (vs. SC) was observed in the BUS (P < 0.05) and INDCA-NS groups at all tested doses on D49 but not on D35 or D42.
The ANOVA of time data spent near the target zone from the MWM indicated significant effects of time (F(2.409, 185.5) = 32.65, P < 0.001), but not of treatment (F(6, 77) = 1.877, not significant). In addition, none of treatments showed significant differences between groups on any of days.
Two-way ANOVA of the total distance travelled (path length) from the MWM indicated significant effects of treatment (F(6, 77) = 3.515, P < 0.01) or time (F(2.595, 199.8) = 58.84, P < 0.001). Dunnett’s test on D0 showed no effects in SC (vs. VC) and BUS or INDCA-NS treated groups (vs. SC), indicating uniform grouping. SC showed a significant increase in the total distance travelled on D35 (P < 0.01) and D42 (P < 0.05), but not on D49 (vs. VC). None of the treatments prevented SC-induced increase in total distance travelled on the day compared to SC.
Table 2: Effects of intermediate to long-term symptoms of CUMS
|
Day |
VC |
SC |
BUS |
INDCA- |
INDCA- |
INDCA- |
|
Spatial memory – Escape latency (s) – MWM |
||||||
|
D0 |
7.58 ± |
8.00 ± |
7.00 ± |
6.92 ± |
8.92 ± |
6.92 ± |
|
D35 |
7.67 ± |
35.92 ± |
40.83 ± |
42.25 ± |
43.17 ± |
36.83 ± |
|
D42 |
8.00 ± |
27.33 ± |
20.58 ± |
23.17 ± |
25.17 ± |
22.5 ± |
|
D49 |
7.58 ± |
32.25 ± |
17.00 ± |
12.75 ± |
11.75 ± |
9.33 ± |
|
Spatial memory – Time spend near target zone (s) – MWM |
||||||
|
D0 |
4.5 ± |
2.5 ± |
3.42 ± |
2.5 ± |
3.33 ± |
3.25 ± |
|
D35 |
4.42 ± |
11.25 ± |
11.67 ± |
9.75 ± |
6.58 ± |
11.25 ± |
|
D42 |
4.67 ± |
8.75 ± |
9.17 ± |
11.75 ± |
11.42 ± |
11.00 ± |
|
D49 |
4.00 ± |
5.92 ± |
6.83 ± |
4.00 ± |
3.75 ± |
4.25 ± |
|
Spatial Memory – Total distance travelled (cm) – MWM |
||||||
|
D0 |
158.47 ± |
143.7 ± |
240.34 ± |
190.7 ± |
251.49 ± |
169.99 ± |
|
D35 |
181.08 ± |
802.83 ± |
843.33 ± |
904.33 ± |
714.33 ± |
729.08 ± |
|
D42 |
171.92 ± |
422.83 ± |
366.58 ± |
446.58 ± |
511.58 ± |
423.58 ± |
|
D49 |
193.00 ± |
448.42 ± |
336.67 ± |
155.08 ± |
310.42 ± |
255.5 ± |
|
Anhedonia – % Sucrose consumption – SPT |
||||||
|
D35 |
51.78 ± |
47.88 ± |
59.53 ± |
28.50 ± |
35.81 ± |
32.49 ± |
|
D42 |
60.03 ± |
57.07 ± |
64.81 ± |
50.83 ± |
55.29 ± |
41.30 ± |
|
D49 |
63.66 ± |
34.35 ± |
70.12 ± |
71.21 ± |
66.46 ± |
59.48 ± |
|
Aggression – Attack latency (s) – RIT |
||||||
|
D0 |
300.00 ± |
300.00 ± |
300.00 ± |
300.00 ± |
300.00 ± |
300.00 ± |
|
D35 |
300.00 ± |
142.25 ± |
112.00 ± |
136.83 ± |
116.92 ± |
80.42 ± |
|
D42 |
300.00 ± |
123.08 ± |
261.25 ± |
261.83 ± |
262.00 ± |
269.17 ± |
|
D49 |
300.00 ± |
78.25 ± |
294.42 ± |
288.58 ± |
296.83 ± |
297.67 ± |
n = 12, Data as mean ± standard error of mean. Numbers in bracket indicate dose (microgram/day/rat intranasal). VC: vehicle control, SC: CUMS control, SPT: Sucrose preference test, BUS: buspirone, MWM: Morris water maze, RIT – Resident intruder test. # P < 0.05, ## P < 0.01, ### P < 0.001 (vs. VC), * P < 0.05, ** P < 0.01, *** P < 0.001 (vs. CS).
Effect on SPT Parameters
Sucrose consumption data during SPT, as shown in Table 2, indicated significant effects of treatment and time (F(6, 77) = 3.09, P < 0.01) and (F(1.912, 147.2) = 20.87, P < 0.001). Dunnett’s test showed a significant decrease in % sucrose consumption in SC (vs. VC) on D49, but not on D35 or D42. Treatment with BUS and INDCA-NS (all tested doses) significantly prevented the SC-induced decrease in % sucrose consumption on D49, but not on D35 or D42 (vs. SC).
Effect on RIT Parameters
Two-way ANOVA of the attack latency data (Table 2) indicated significant effects of time and treatment (F(1.805, 139.0) = 267.5, P < 0.001) and (F(6, 77) = 20.41, P < 0.01), respectively. Dunnett’s test showed significant (P < 0.001) reduction in attack latencies in SC (vs VC) on D35, D42 and D49. The BUS- and INDCA-NS (all tested doses)-treated groups showed significant (P < 0.001) prevention of SC-induced decline in attack latencies on D42 and D49, but not on D35.
Effect on Hypothalamic Cortisol
One-way ANOVA of cortisol level data (Table 3) showed significant effects (F(6, 21) = 24.10, P < 0.001). SC showed higher cortisol levels than those in the VC (P < 0.001), whereas BUS and INDCA-NS (30 and 100), but not INDCA-NS (10), significantly prevented SC-induced increases in cortisol levels.
Effect on Hypothalamic BDNF
One-way ANOVA of BDNF levels (Table 3) showed significant treatment effects (F(6, 49) = 2.938, P < 0.05). BDNF levels in the SC were higher, but not significantly different (vs. VC). In addition, BDNF levels in the BUS or INDCA-NS groups (at the tested doses) did not show a significant difference between the groups (vs. SC).
Table 3: Effects on biochemical markers of CUMS
|
Measure |
VC |
SC |
BUS |
INDCA- |
INDCA- |
INDCA- |
|
Cortisol |
0.44 |
38.93 |
19.82 |
30.31 |
1.01 |
0.66 |
|
BDNF |
204.60 |
311.50 |
325.60 |
356.30 |
268.70 |
213.70 |
n = 12, Data as mean ± standard error of mean. Numbers in bracket indicate dose (microgram/day/rat intranasal).VC – Vehicle control, SC- CUMS Stress control, BUS- Buspirone, # P < 0.05, ## P < 0.01, ### P < 0.001 (vs VC), * P < 0.05, ** P < 0.01, *** P < 0.001 (vs CS),
Discussion
The effects of INDCA-NS on early and short-term (anxiety and working memory) and intermediate-to long-term (spatial memory, anhedonia, and aggression) changes in CUMS-induced chronic stress in rats were explored. Subacute administration of INDCA-NS resulted in consistent and dose-dependent prevention of CUMS-induced anxiety, anhedonia, and aggression, but not cognitive decline, that is, loss of working and spatial memory. These findings are consistent with CUMS-preventive effects observed in rats treated with BUS, a serotonergic agent, suggesting a serotonergic mechanism of INDCA-NS. The findings of previous studies on the oral administration of INDCA to laboratory rats have provided further support27.
Recently, triterpenoids containing CA extracts (unstandardized form) showed stress-relieving efficacy in animal models of chronic stress in zebrafish24 and CUMS induced rats46. The present results are not only in line with these reports, but also extend to a triterpenoid-based standardized version of INDCA-NS with intranasal administration against chronic stress-related symptoms.
The CUMS paradigm was used to mimic everyday stress (unpredictable) and its effects on psychological well-being in a reliable and clinically validated manner in rats47, 48. These stressors included social stressors (isolation, cage mate changes, and crowding), environmental stressors (cage tilting, damp bedding, and altered light/dark cycles), and physiological stressors (food/water deprivation and restraint), which are presented at random intervals and durations48, 49. Because CUMS protocol prevents habituation (limitation of acute stress), it produces clinically relevant behavioral changes, such as anxiety, depression, cognitive decline, anadenia, and aggression associated with psychological stress48, 50, 51.
The efficacy of triterpenoids of INDCA-NS is suggested to involve serotonergic receptors27 for efficacy against stress disorders, including depression and anxiety26, 52. Concurrently, the role of serotoninergic agonist action has been confirmed in the mechanism of buspirone, a drug with anxiety prophylaxis53 and management of stress-induced disorders54. Therefore, BUS was used as a positive control.
In current investigation, CUMS for 35 days induced anxiety-like behavior during marble MBT, with an increased number of buried marbles, in agreement with previous reports55. MBT has been extensively used in rodents to model anxiety and other stress-related behaviors56, 57. These anxiety-like symptoms have been reported as early or intermediate features of CUMS-induced pathology50. The timelines of anxiety in CUMS align with clinical observations, indicating suitability of CUMS for modeling the complex interplay between anxiety and depression50. Subacute INDCA-NS dose-dependently prevented the stress-induced increase in the number of buried marbles, indicating its anxiolytic efficacy against CUMS.
Short-term spatial and working memory deficits are crucial components of executive function and have been linked to various neurodegenerative and psychiatric disorders. The Y-maze test is often utilized to evaluate the effects of treatments on working memory58. Chronic stress impairs spatial recognition memory, as evidenced by decreased performance in the Y-maze test59. In the present study, a higher number of entries and time in novel arms indicated working memory deficit in SC group (because of CUMS), whereas significant prevention of SC-induced increase in Y-maze parameters indicated protection from working memory deficit by subacute nasal administration of INDCA-NS.
In this study, the effects on spatial memory were assessed using the MWM, which is the most reliable and widely accepted tool60. The increased escape latency, time spent near the target zone, and decreased distance travelled during MWM in the SC group indicated impaired long-term spatial memory with CUMS, which is in line with earlier reports50. However, INDCA-NS and BUS did not show any preventive effects on spatial memory decline in this study.
Subacute intranasal INDCA-NS and BUS reversed the CUMS-induced decrease in sucrose consumption during the SPT on D49 in a dose-dependent manner. Stress-induced depression-like behavior and structural plasticity in the brain are characteristic of CUMS model61. Moreover, SPT involves measuring the preference of animals for a sucrose solution over water (a pleasurable activity) and a reduced sucrose preference, indicating an anhedonia state, which is common in chronic stress-related disorders62.
Previously, a correlation between serotonergic system enhancement and reversal of CUMS-induced anhedonia has been reported. For example, CUMS-induced anhedonia decreases hippocampal serotonin transporter protein levels, whereas non-anhedonia increases them63. In addition, the serotonergic agent fluoxetine has been reported to prevent CUMS-induced decreases in serotonin transporter levels63. Upon nasal treatment, INDCA-NS and BUS (5HT1A agonist) not only prevented CUMS-induced anhedonia but also increased sucrose preference compared to VC (without CUMS). The similarity in efficacy between INDCA-NS and BUS reveals the function of serotonergic mechanisms in INDCA-NS action against CUMS54, 64.
Aggression is an intermediate-to long-term symptom associated with chronic stress-related depression and is correlated with a decrease in serotonin and a rise in dopamine65. CUMS mimics many aspects of stress-related depression, including61. We used RIT, most acceptable measure of aggression66. CUMS induction increased attack latencies (increased aggression) in the SC group from D35, when intranasally presented with INDCA-NS and BUS.
The role of the serotonergic system has been reported in stress-induced67 and aggressive behaviors in rodents68. For examples: 5HT1A agonists (BUS) and reuptake inhibitors (fluoxetine) have been reported to reverse isolation stress-induced aggression69. The role of serotonin receptors, especially 5-HT1A/1 B receptors, in the anti-migraine mechanism of INDCA-NS has been reported27. These reports strongly indicate a role for serotonergic receptors in the anti-stress mechanism of CUMS.
In present study, CUMS induction in rats of the CS group caused a significant elevation in cortisol in the hypothalamus, which was prevented by subacute intranasal INDCA-NS and BUS. Cortisol, a glucocorticoid hormone, has been proposed as a promising biomarker for chronic stress assessment17, 70 because of activated HPA axis71. This notion is supported by involvement of HPA axis and elevated cortisol in the mechanism of action of INDCA-NS in stress-related neuropsychiatric conditions22.
In recent years, chronic stress has been reported to decrease brain BDNF72. Stress is a significant contributor to depression, especially anhedonia73. Many antidepressants may exert their effects by regulating BDNF74. Moreover, BDNF plays the foremost role in HPA axis72. However, present study showed elevated levels of BDNF in the hypothalamus of CUMS-induced rats, although the difference was not significant. These effects are in agreement with previous reports of increased BDNF in the dorsal and ventral hippocampi of CUMS-induced rats75 and chronically restrained rats76. Moreover, INDCA-NS and BUS did not affect BDNF levels in CUMS-induced rats.
Existing evidence indicates a strong relationship between BDNF and serotonin levels 77 especially under chronic stress78. The enhanced BDNF levels (in the present study) and serotoninergic properties of INDCA-NS27 indicate a synergistic role of two seemingly distinct signaling systems79 in anti-stress mechanism of INDCA-NS. In the past, enhanced BDNF levels were reported to be involved in CA’s neuroprotective80-82 and anti-stress83 properties of CA.
Conclusions
The intranasal subacute administration of INDCA-NS was effective in preventing early to short-term (anxiety and working memory) and intermediate to long-term (anhedonia and aggression) symptoms of CUMS in laboratory rats, probably through serotonin and cortisol involvement. The potential of INDCA-NS can be explored with suitable clinical studies for management stress-related disorders such as tension-type headache or migraine.
Acknowledgments
The authors would like to acknowledge Dr. K.R. Mahadik, Ex-Principal, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, India.
Funding Sources
The study was supported by Indus Biotech Limited, Pune, India (Grant Project No: IBS432).
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability
The manuscript incorporates all datasets produced or examined throughout this research study
Ethics Statement
The experimental protocols were sanctioned by “Institutional Animal Ethics Committee” (IAEC) of Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, India (Approval number CPCSEA/ PCP/PCL33/2018-19)
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Authors’ Contributions
Each author mentioned has significantly and directly contributed intellectually to the project and has given their approval for its publication.
Prasad Thakurdesai: Conceptualization, Methodology, Writing – review and editing, Visualization, Supervision, Funding acquisition;
Gayatri Shivsingwale: Investigation, Formal analysis;
Urmila Aswar: Methodology, Software, Validation, Formal analysis, Resources, Data curation, Writing – original draft, Writing – Review and editing, Supervision, Project administration.
References
- Lee T, Jarome T, Li S-J, Kim JJ, Helmstetter FJ. Chronic stress selectively reduces hippocampal volume in rats: A longitudinal magnetic resonance imaging study. NeuroReport. 2009;20(17):1554-8.
CrossRef - Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: A critical review. Learn Mem. 2015;22(9):411-6.
CrossRef - Suo L, Zhao L, Si J, Liu J, Zhu W, Chai B, Zhang Y, Feng J, Ding Z, Luo Y, Shi H, Shi J, Lu L. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology. 2013;38(8):1387-400.
CrossRef - Parsaei R, Roohafza H, Feizi A, Sadeghi M, Sarrafzadegan N. How different stressors affect quality of life: An application of multilevel latent class analysis on a large sample of industrial employees. Risk Manag Healthc Policy. 2020;Volume 13:1261-70.
CrossRef - Doewes RI, Gangadhar L, Subburaj S. An overview on stress neurobiology: Fundamental concepts and its consequences. Neurosci Inform. 2021;1(3):100011.
CrossRef - Araki M, Fuchikami M, Omura J, Miyagi T, Nagashima N, Okamoto Y, Morinobu S. The role of glucocorticoid receptors in the induction and prevention of hippocampal abnormalities in an animal model of posttraumatic stress disorder. Psychopharmacol. 2020;237(7):2125-37.
CrossRef - Yue N, Huang H, Zhu X, Han Q, Wang Y, Li B, Liu Q, Wu G, Zhang Y, Yu J. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation. 2017;14(1):102.
CrossRef - Surget A, Belzung C. Adult hippocampal neurogenesis shapes adaptation and improves stress response: A mechanistic and integrative perspective. Mol Psychiatry. 2022;27(1):403-21.
CrossRef - Zeng S, Lin X, Wang J, Hu X. Sleep’s short-term memory preservation and long-term affect depotentiation effect in emotional memory consolidation: Behavioral and eeg evidence. Sleep. 2021;44(11):zsab155.
CrossRef - Schulz D. Depression development: From lifestyle changes to motivational deficits. Behav Brain Res. 2020;395:112845.
CrossRef - Vahid-Ansari F, Albert PR. Rewiring of the serotonin system in major depression. Front Psychiatry. 2021;12:802581.
CrossRef - Mbiydzenyuy NE, Hemmings SMJ, Shabangu TW, Qulu L. Exploring the influence of stress on aggressive behavior and sexual function: Role of neuromodulator pathways and epigenetics. Heliyon. 2024;10(5):e27501.
CrossRef - Canet G, Hernandez C, Zussy C, Chevallier N, Desrumaux C, Givalois L. Is ad a stress-related disorder? Focus on the hpa axis and its promising therapeutic targets. Front Aging Neurosci. 2019;11:269.
CrossRef - Spencer RL, Deak T. A users guide to HPA axis research. Physiol Behav. 2017;178:43-65.
CrossRef - Harris BN. Stress hypothesis overload: 131 hypotheses exploring the role of stress in tradeoffs, transitions, and health. Gen Comp Endocrinol. 2020;288:113355.
CrossRef - Zhao J, Shi W, Lu Y, Gao X, Wang A, Zhang S, Du Y, Wang Y, Li L. Alterations of monoamine neurotransmitters, HPA-axis hormones, and inflammation cytokines in reserpine-induced hyperalgesia and depression comorbidity rat model. BMC psychiatry. 2022;22(1):419.
CrossRef - Noushad S, Ahmed S, Ansari B, Mustafa U-H, Saleem Y, Hazrat H. Physiological biomarkers of chronic stress: A systematic review. Int J Health Sci. 2021;15(5):46.
- Yan Z, Rein B. Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: Pathophysiological implications. Mol Psychiatry. 2022;27(1):445-65.
CrossRef - Harsanyi S, Kupcova I, Danisovic L, Klein M. Selected biomarkers of depression: What are the effects of cytokines and inflammation? Int J Mol Sci. 2022;24(1):578.
CrossRef - Porreca F, Navratilova E. Reward, motivation, and emotion of pain and its relief. Pain. 2017;158(1):S43-S9.
CrossRef - Sarapultsev A, Sarapultsev P, Dremencov E, Komelkova M, Tseilikman O, Tseilikman V. Low glucocorticoids in stress-related disorders: The role of inflammation. Stress. 2020;23(6):651-61.
CrossRef - Thakurdesai P. Centella asiatica (Gotu kola) leaves: potential in neuropsychiatric conditions. In: Ghosh D, editor. Nutraceuticals in Brain Health and Beyond. 1st ed. London: Elsevier, Inc; 2021. p. 307-27.
CrossRef - Teerapattarakan N, Benya-Aphikul H, Tansawat R, Wanakhachornkrai O, Tantisira MH, Rodsiri RJP. Neuroprotective effect of a standardized extract of centella asiatica ECa233 in rotenone-induced parkinsonism rats. Phytomedicine. 2018;44:65-73.
CrossRef - Mando Z, Mando H, Afzan A, Shaari K, Hassan Z, Mohamad Taib MNA, Zakaria F. Biomarker triterpenoids of Centella asiatica as potential antidepressant agents: Combining in vivo and in silico studies. Behav Brain Res. 2024;466:114976.
CrossRef - Wanasuntronwong A, Wanakhachornkrai O, Phongphanphanee P, Isa T, Tantisira B, Tantisira MH. Modulation of neuronal activity on intercalated neurons of amygdala might underlie anxiolytic activity of a standardized extract of Centella asiatica ECa233. Evid Based Complement Alternat Med 2018;2018:3853147.
CrossRef - Kalshetty P, Aswar U, Mohan V, Bodhankar SL, Arulmozhi S, Thakurdesai PA. Antidepressant effects of standardized extract of Centella asiatica L in olfactory bulbectomy model. Biomed Aging Pathol. 2012;2(2):48-53.
CrossRef - Bobade V, Bodhankar SL, Aswar U, Mohan V, Thakurdesai PA. Prophylactic effects of asiaticoside based standardized extract of Centella asiatica (L.) Urban leaves in experimental migraine: Involvement of 5HT1A/1B receptors. Chin J Nat Med. 2015;13(4):274-82.
CrossRef - Gupta SK, Kumar S. An overview on intranasal drug delivery system: Recent technique and its contribution in therapeutic management. Curr Res Pharm Sci. 2019;9(2):208.
CrossRef - Jeong S-H, Jang J-H, Lee Y-B. Drug delivery to the brain via the nasal route of administration: Exploration of key targets and major consideration factors. J Pharm Investig. 2023;53(1):119-52.
CrossRef - Bicker J, Fortuna A, Alves G, Falcão A. Nose-to-brain delivery of natural compounds for the treatment of central nervous system disorders. Curr Pharm Des. 2020;26(5):594-619.
CrossRef - Deshpande P, Thakurdesai P, Mohan V. Preclinical safety assessment of standardized extract of Centella asiatica (L.) urban leaves. Toxicol Int. 2015;22:10-20.
CrossRef - Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci Biobehav Rev. 2019;99:101-16.
CrossRef - Goyal RK, Bhise SB, Srinivasan BP, Rao CM, Sen T, Koneri R. Curriculum for pharmacology in pharmacy institutions in india: Opportunities and challenges. Indian J Pharmacol. 2014;46(3):241-5.
CrossRef - CPCSEA. CPCSEA guidelines for laboratory animal facility. Indian J Pharmacol. 2003:257-74.
- Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: Routes of administration and factors to consider. J Am Assoc Lab Anim Sci. 2011;50(5):600-13.
- Khan MS, Patil K, Yeole P, Gaikwad R. Brain targeting studies on buspirone hydrochloride after intranasal administration of mucoadhesive formulation in rats. J Pharm Pharmacol. 2009;61(5):669-75.
CrossRef - Li S, Wang C, Wang W, Dong H, Hou P, Tang Y. Chronic mild stress impairs cognition in mice: From brain homeostasis to behavior. Life Sciences. 2008;82(17-18):934-42.
CrossRef - Gáll Z, Farkas S, Albert Á, Ferencz E, Vancea S, Urkon M, Kolcsár M. Effects of chronic cannabidiol treatment in the rat chronic unpredictable mild stress model of depression. Biomolecules. 2020;10(5):801.
CrossRef - Kedia S, Chattarji S. Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J Neurosci Methods. 2014;233:150-4.
CrossRef - Chandrasekhar BV, Elango P, Maheswari SU, Rajukumar D. A focus on the effect of l-theanine on improving depression and cognition in C57BL/J male mice subjected for chronic stress induced neuroinflammation. Biomed Pharmacol J. 2017;10(2):1015-27.
CrossRef - Wang J, Yuan J, Pang J, Ma J, Han B, Geng Y, Shen L, Wang H, Ma Q, Wang Y, Wang M. Effects of chronic stress on cognition in male samp8 mice. Cell Physiol Biochem. 2016;39(3):1078-86.
CrossRef - Géa LP, Colombo R, da Rosa ED, Antqueviezc B, de Aguiar ÉZ, Hizo GH, Schmidt GB, de Oliveira LF, Stein DJ, Rosa AR. Anhedonic-like behavior correlates with ifnγ serum levels in a two-hit model of depression. Behav Brain Res. 2019;373:112076.
CrossRef - Deng X-Y, Li H-Y, Chen J-J, Li R-P, Qu R, Fu Q, Ma S-P. Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav Brain Res. 2015;291:12-9.
CrossRef - Wang J, Xu S, Chen X, Wang L, Li J, Li G, Zhang B. Antidepressant effect of egcg through the inhibition of hippocampal neuroinflammation in chronic unpredictable mild stress-induced depression rat model. J Funct Foods. 2020;73:104106.
CrossRef - Wei S, Ji XW, Wu CL, Li ZF, Sun P, Wang JQ, Zhao QT, Gao J, Guo YH, Sun SG, Qiao MQ. Resident intruder paradigm-induced aggression relieves depressive-like behaviors in male rats subjected to chronic mild stress. Med Sci Monit. 2014;20:945-52.
CrossRef - Jagadeesan S, Chiroma SM, Mohd Moklas MA, Hidayat Baharuldin MT, Mat Taib CN, Amom Z, Vishnumukkala T, Thomas W, Mahdi O. Centella asiatica L. Urban protects against morphological aberrations induced by chronic unpredictable mild stress in rat’s hippocampus via attenuation of oxidative stress. Egypt J Basic Appl Sci. 2022;9(1):324-39.
- Liu Z, Liu X, Luo S, Chu C, Wu D, Liu R, Wang L, Wang J, Liu X. Extract of sesame cake and sesamol alleviate chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits. J Funct Foods. 2018;42:237-47.
CrossRef - Strekalova T, Liu Y, Kiselev D, Khairuddin S, Chiu JLY, Lam J, Chan Y-S, Pavlov D, Proshin A, Lesch K-P, Anthony DC, Lim LW. Chronic mild stress paradigm as a rat model of depression: Facts, artifacts, and future perspectives. Psychopharmacol. 2022;239(3):663-93.
CrossRef - Sequeira-Cordero A, Salas-Bastos A, Fornaguera J, Brenes JC. Behavioural characterisation of chronic unpredictable stress based on ethologically relevant paradigms in rats. Sci Rep. 2019;9:17403.
CrossRef - Alqurashi GK, Hindi EA, Zayed MA, Abd El-Aziz GS, Alturkistani HA, Ibrahim RF, Al-thepyani MA, Bakhlgi R, Alzahrani NA, Ashraf GM, Alghamdi BS. The impact of chronic unpredictable mild stress-induced depression on spatial, recognition and reference memory tasks in mice: Behavioral and histological study. Behav Sci. 2022;12(6):166.
CrossRef - Petković A, Chaudhury D. Encore: Behavioural animal models of stress, depression and mood disorders. Front Behav Neurosci. 2022;16:931964.
CrossRef - Thakurdesai P, Nimse S, Deshpande P. Characterization and preclinical toxicity assessment of intranasal administration of standardized extract of Centella asiatica (L.) Urban leaves (INDCA-NS) in laboratory rats. Toxicol Int. 2023:391-407.
CrossRef - Jastrzębska-Więsek M, Partyka A, Rychtyk J, Śniecikowska J, Kołaczkowski M, Wesołowska A, Varney MA, Newman-Tancredi A. Activity of serotonin 5-HT1A receptor biased agonists in rat: Anxiolytic and antidepressant-like properties. ACS Chem Neurosci. 2017;9(5):1040-50.
CrossRef - Smith AL, Harmer CJ, Cowen PJ, Murphy SE. The serotonin 1A (5-HT1A) receptor as a pharmacological target in depression. CNS drugs. 2023;37(7):571-85.
CrossRef - Willner P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress. 2017;6:78-93.
CrossRef - de Brouwer G, Fick A, Harvey BH, Wolmarans DW. A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive–compulsive disorder: Mapping the way forward. Cogn Affect Behav Neurosci. 2019;19(1):1-39.
CrossRef - Dixit PV, Sahu R, Mishra DK. Marble-burying behavior test as a murine model of compulsive-like behavior. J Pharmacol Toxicol Methods. 2020;102:106676.
CrossRef - Cleal M, Fontana BD, Ranson DC, McBride SD, Swinny JD, Redhead ES, Parker MO. The free-movement pattern y-maze: A cross-species measure of working memory and executive function. Behav Res Methods. 2021;53(2):536-57.
CrossRef - Lamtai M, Zghari O, Ouakki S, Marmouzi I, Mesfioui A, El Hessni A, Ouichou A. Chronic copper exposure leads to hippocampus oxidative stress and impaired learning and memory in male and female rats. Toxicol Res. 2020;36(4):359-66.
CrossRef - Othman MZ, Hassan Z, Che Has AT. Morris water maze: A versatile and pertinent tool for assessing spatial learning and memory. Exp Anim. 2022;71(3):264-80.
CrossRef - Xin L, Qiong-ying W, Zhao-tian M, Hong-hao S, Xue Y, Xiao-qiao R. Effects of CUMS combined with crs on hippocampal glial cells and synaptic plasticity in depressed mice. J Hainan Med Univ. 2023;29(2):215.
- Markov DD. Sucrose preference test as a measure of anhedonic behavior in a chronic unpredictable mild stress model of depression: Outstanding issues. Brain Sciences. 2022;12(10):1287.
CrossRef - Tang M, Lei J, Sun X, Liu G, Zhao S. Stress-induced anhedonia correlates with lower hippocampal serotonin transporter protein expression. Brain Res. 2013;1513:127-34.
CrossRef - Sharp T, Barnes NM. Central 5-HT receptors and their function; present and future. Neuropharmacol. 2020;177:108155.
CrossRef - Gorlova A, Svirin E, Pavlov D, Cespuglio R, Proshin A, Schroeter CA, Lesch K-P, Strekalova T. Understanding the role of oxidative stress, neuroinflammation and abnormal myelination in excessive aggression associated with depression: Recent input from mechanistic studies. Int J Mol Sci. 2023;24(2):915.
CrossRef - Siddall R. Ethorobotic rats for rodent behavioral research: Design considerations. Front Behav Neurosci. 2023;17:1281494.
CrossRef - Popova NK, Tsybko AS, Naumenko VS. The implication of 5-HT receptor family members in aggression, depression and suicide: Similarity and difference. Int J Mol Sci. 2022;23(15):8814.
CrossRef - da Cunha-Bang S, Knudsen GM. The modulatory role of serotonin on human impulsive aggression. Biol Psychiatry. 2021;90(7):447-57.
CrossRef - Aswar U, Shende H, Aswar M. Buspirone, a 5-HT1A agonist attenuates social isolation-induced behavior deficits in rats: A comparative study with fluoxetine. Behav Pharmacol. 2022;33(5):309-21.
CrossRef - Aggarwal A. Hypothalamo-pituitary-adrenal axis and brain during stress, yoga and meditation. Int J Health Clin Res. 2020;3(9):96-103.
- Harrewijn A, Vidal-Ribas P, Clore-Gronenborn K, Jackson SM, Pisano S, Pine DS, Stringaris A. Associations between brain activity and endogenous and exogenous cortisol–a systematic review. Psychoneuroendocrinol. 2020;120:104775.
CrossRef - Miao Z, Wang Y, Sun Z. The relationships between stress, mental disorders, and epigenetic regulation of bdnf. Int J Mol Sci. 2020;21(4):1375.
CrossRef - Boyle CC, Bower JE, Eisenberger NI, Irwin MR. Stress to inflammation and anhedonia: Mechanistic insights from preclinical and clinical models. Neurosci Biobehav Rev. 2023:105307.
CrossRef - Castrén E, Monteggia LM. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiatry. 2021;90(2):128-36.
CrossRef - Larsen MH, Mikkelsen JD, Hay-Schmidt A, Sandi C. Regulation of brain-derived neurotrophic factor (BDNF) in the chronic unpredictable stress rat model and the effects of chronic antidepressant treatment. J Psychiatr Res. 2010;44(13):808-16.
CrossRef - Naert G, Ixart G, Maurice T, Tapia-Arancibia L, Givalois L. Brain-derived neurotrophic factor and hypothalamic-pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol Cell Neurosci. 2011;46(1):55-66.
CrossRef - Correia AS, Cardoso A, Vale N. Bdnf unveiled: Exploring its role in major depression disorder serotonergic imbalance and associated stress conditions. Pharmaceutics. 2023;15(8):2081.
CrossRef - Meng F, Liu J, Dai J, Wu M, Wang W, Liu C, Zhao D, Wang H, Zhang J, Li M, Li C. Brain-derived neurotrophic factor in 5-HT neurons regulates susceptibility to depression-related behaviors induced by subchronic unpredictable stress. J Psychiatr Res. 2020;126:55-66.
CrossRef - Guerrera CS, Furneri G, Grasso M, Caruso G, Castellano S, Drago F, Di Nuovo S, Caraci F. Antidepressant drugs and physical activity: A possible synergism in the treatment of major depression? Front Psychol. 2020;11:857.
CrossRef - Sbrini G, Brivio P, Fumagalli M, Giavarini F, Caruso D, Racagni G, Dell’Agli M, Sangiovanni E, Calabrese F. Centella asiatica L. Phytosome improves cognitive performance by promoting bdnf expression in rat prefrontal cortex. Nutrients. 2020;12(2):355.
CrossRef - Gofir A, Wibowo S, Hakimi M. Potential neurological applications of Centella asiatica: A brief review. Indones J Pharmcol Ther. 2021;2(3):136-43.
CrossRef - Annisa R, Aida Ratna W, Husnul K, Muljohadi A, editors. Protection of Centella asiatica Extract Through BDNF Expression on Stunting Model Zebrafish Larvae (Danio rerio) by Rotenone Induced. Proceedings of the 1st Paris Van Java International Seminar on Health, Economics, Social Science and Humanities (PVJ-ISHESSH 2020); 2021 2021/03/08: Atlantis Press.
- Sari DCR, Arfian N, Tranggono U, Setyaningsih WA, Romi MM, Noriaki E. Centella asiatica (gotu kola) ethanol extract up-regulates hippocampal brain-derived neurotrophic factor (bdnf), tyrosine kinase b (trkB) and extracellular signal-regulated protein kinase 1/2 (ERK1/2) signaling in chronic electrical stress model in rats. Iran J Basic Med Sci. 2019;22(10):1218-24.







