Made Krisna Adi Jaya1,2 , Fita Rahmawati1
, Fita Rahmawati1 , Nanang Munif Yasin1
, Nanang Munif Yasin1 and Zullies Ikawati1*
and Zullies Ikawati1*
1Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia.
2Department of Pharmacy, Faculty of Maths and Science, Universitas Udayana, Bali, Indonesia
Corresponding Author E-mail: zullies_ikawati@ugm.ac.id
DOI : https://dx.doi.org/10.13005/bpj/3021
Abstract
Background: The management of blood glucose levels in patients with type 2 diabetes mellitus (T2DM) often involves the use of effective diabetes medications, such as insulin and sulfonylurea (SU). Despite the potential, these drugs can potentially lead to hypoglycemia during treatment. Objective: Therefore, this study aims to determine the types of insulin and sulfonylureas that commonly cause hypoglycemia. Methods: Using a case-control study design, hospitalized occurrences of hypoglycemia were assessed while considering factors that influenced its incidence through Odds Ratio (OR) calculations at a confidence interval (CI) level of 95%. Results: The results showed that hypoglycemia occurred more often in patients who used insulin, SU, or both compared to non-users (p<0.05). In addition, a risk of 4.5 (CI95%: 1.580-12.817) times higher was found in patients taking insulin and SU compared to others. Conclusion: Ambulatory T2DM patients who use insulin or SU as DM therapy must be given special attention. Education related to the risk of hypoglycemia, how to use medication, and first aid in emergency conditions must be provided by health workers to outpatients with DM.
Keywords
Diabetes Mellitus; Hypoglycemia; Insulin; Medication Safety; Sulfonylurea
Download this article as:| Copy the following to cite this article: Jaya M. K. A, Rahmawati F, Yasin N. M, Ikawati Z. Evaluation of Insulin and Sulfonylurea Types on Severe Hypoglycemia Event Among Ambulatory Type 2 Diabetes Mellitus Patients. A Case-Control Hospital-Based Study in Bali. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Jaya M. K. A, Rahmawati F, Yasin N. M, Ikawati Z. Evaluation of Insulin and Sulfonylurea Types on Severe Hypoglycemia Event Among Ambulatory Type 2 Diabetes Mellitus Patients. A Case-Control Hospital-Based Study in Bali. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/4hhV6dy |
Introduction
Consuming glucose-lowering drugs can heighten the risk of hypoglycemia in individuals with type 2 diabetes mellitus (T2DM)1. This condition leads to symptoms related to autonomic or neuroglycopenic effects caused by low blood sugar levels (≤ 70 mg/dl)2. Hypoglycemia may elevate mortality rates, induce hospital admittance, and lead to substantial medical expenses. Indonesia allocated a total cost of USD 23 million for treating hypoglycemic incidents incurred by their government during the year 2016, an amount that was exceedingly high3,4.
Previous studies indicate that ambulatory T2DM patients in Indonesia are frequently prescribed insulin and sulfonylurea, which are covered by the Indonesian National Health Insurance (BPJS Kesehatan). These drugs have been shown to effectively regulate blood sugar levels5,6, while also being cost-effective compared to other options7,8. However, hypoglycemia can occur when patients lack knowledge on how their correct use. Once educated on proper administration techniques, including initiation, consumption control, or regulation of dose intake along with monitoring, there is no associated risk. A previous report revealed that many patients ignore the associated risk, which makes hypoglycemia occur 4,9,10.
In Bali Island, Indonesia, no reports have been conducted on how insulin and sulfonylurea frequently cause hypoglycemia in T2DM patients. Therefore, this study aims to determine the types of insulin and sulfonylureas that commonly cause hypoglycemia. The finding is significant for clinicians to assess the benefits and challenges in ambulatory patients who use T2DM treatment.
Materials and Methods
Study Design, Setting, and Sample Size
A case-control design was used, and the procedures were conducted in Bali province at three government hospitals. The hospitals were in Badung district, Denpasar City, and Buleleng Regency. Observation of T2DM patients’ demographic data and medical history was carried out. Furthermore, the presentation of results was based on Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for case-control design11. The case and control groups each contained 100 participants using the minimum sample calculation for the design used. The minimum number of patients who were analyzed was 200 participants.
Study Participants, Data Collection, Measurements, and Variables
The population of this study was all patients who were diagnosed with T2DM in three government hospitals in the province of Bali, Indonesia, with age criteria ≥20 years, and those who carry diabetes medication such as biguanide drugs, sulfonylurea, TZDs, incretin mimetics, alpha-glucosidase inhibitors, SGLT2-inhibitors, or insulin. The sample was part of the population that met the inclusion criteria. Participants were included when their medical record data for the previous year was completed. In addition, the intended medical record consisted of a diagnosed individual with T2DM exhibiting primary identity data, age, gender, profile of DM drugs used, duration of DM, blood glucose profile, BMI, and comorbidities. Patients excluded from this study were those with hypoglycemia, which was not a result of medication, some records were not comprehensively reported, such as illegible, scattered, exchanged, and duplicated data.
The hospital medical database was initially sorted by ICD-10 codes E11 and E16.2 to obtain patients’ record numbers and were taken sequentially according to their database from January 2022 to May 2024 for review. The case-control study commenced with the determination of patients’ results in the form of history, and there was no history of hospitalization hypoglycemia. The operational definition of hospitalized hypoglycemia was a patient who comes with signs and symptoms of hypoglycemia characterized by blood sugar levels <54 mg/dL. Medical personnel treated this condition with 10% Dextrose fluid. This outcome was further observed retrospectively as a medication predictor that had the potential to cause outcomes, specifically in the insulin and sulfonylurea medication groups. T2DM patient’s medication variables were analyzed as exposure were the use of basal-bolus, basal, bolus, mixed, sulfonylurea (glimepiride, glibenclamide, glikazide, gliquidone), and a combination use of insulin and sulfonylurea.
The review process was conducted by eight reviewers, who held open meetings to discuss the results. An evaluation phase was carried out until an agreement was reached when there were differences of opinion. Furthermore, for medical records that underwent the review stage and agreed to be utilized in the analysis, patients were contacted by telephone to obtain approval for their inclusion. The data was finally analyzed to observe when patients had obtained consent by signing the consent form, which was sent and signed digitally.
Potential Sources of Bias
Potential bias was the type of insulin and sulfonylurea used by T2DM patients as well as patients’ compliance with their disease control. This bias was controlled by setting inclusion criteria in the form of patients who only had regular check-ups at the multicenter study site from the previous year. Meanwhile, bias in the type of insulin and sulfonylurea used by patients was overcome by conducting a multivariate analysis to assess the interacting variables of the type of insulin and sulfonylurea in obtaining more detailed results as the strongest predictor of causing hypoglycemia.
Statistical Analysis
Data analysis was conducted descriptively and analytically. Descriptive data showed the demographic characteristics of all participants observed. Analytical data was to assess the risk of hypoglycemia in patients using insulin, sulfonylurea, and combinations of insulin and sulfonylurea in the case and control groups. Data analysis was assisted with the IBM SPSS 21 version. In addition, the primary data were analyzed using chi-square analysis with odds ratio (OR) parameters, and 95% confidence intervals were used in the initial analysis. When data was found with a p-value ≤0.25, the variables continued to be analyzed with multivariate logistic regression. Final statistical analyses were two-tailed, and a p-value of <0.05 was considered statistically significant.
Ethical Consideration
This report was part of a larger study in which the data collection process was conducted from January 2022 to May 2024. The Faculty of Medicine, Udayana University, Bali, ethics commission approved this study with an ethical clearance number 1165/UN.14.2.2.VII.14/LT/2024. Ethical clearance was obtained from the multicenter hospitals, namely Denpasar City Hospital, Badung Regency Hospital, and Buleleng Regency Hospital, with ethical clearance number 052/EA/KEPK.RSBM.DISKES/2024, B/475/UN14.6/PT.01.04/2024, and 019/EC/KEPK-RSB/V/2023 respectively. Consent was obtained from participants using an approved and locally translated digital consent form. Patients were informed about the details of the study, including the general overview, purpose, risks, and benefits. Confidentiality was maintained throughout all stages. This study was conducted following the Declaration of Helsinki.
Results
The Flow of Study Subjects and Data Selection
During the study period, 1231 patients were identified in the database. After the screening process, a total of 260 patients were eligible to be participants (Figure 1). Some medical record data was found to be incomplete or scattered because the multicenter site had not fully implemented digital health records. The medical record data reviewed was paper-based hardcopy data.
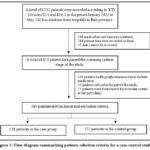 |
Figure 1: Flow diagram summarizing patients selection criteria for a case-control study. |
Some patients were recorded to be suffering from hypoglycemia not due to diabetes medication but due to certain medical conditions such as decompensated cirrhosis, delirium, schizophrenia, non-hemodialysis CKD, complex heart disease, etc. Consequently, it must be excluded from the criteria. A total of 138 patient data in the case group and 122 patient data were successfully included in the analysis until the final stage following the study criteria.
Demographic Characteristics of Study Subjects
The T2DM patients involved in this study were distributed proportionally between men and women. In addition, their age was more distributed in the age group >46 years. Patients who had diabetes for more than five years with typical comorbidities in the form of macrovascular and microvascular complications were more dominant. The detailed demographic characteristics are shown in Table 1.
Table 1: T2DM Patients’ Characteristics
|
No |
Characteristics |
Case Group (Hypoglycemia) (n=138) |
Control Group (Non-Hypoglycemia) (n=122) |
P Value (χ2) |
|
1 |
Gender |
|
0.227 |
|
|
Male (n %) |
70 (50.72) |
71 (58.20) |
||
|
Female (n %) |
68 (49.28) |
51 (41.80) |
||
|
2 |
Ages (Years) |
|
0.036* |
|
|
20-30 (n %) |
2 (1.45) |
3 (2.46) |
||
|
30-45 (n %) |
12 (8.70) |
20 (16.39) |
||
|
46-55 (n %) |
34 (24.64) |
43 (35.25) |
||
|
56-65 (n %) |
46 (33.33) |
30 (24.59) |
||
|
>65 (n %) |
44 (31.88) |
26 (21.31) |
||
|
3 |
BMI (kg/m2) |
|
0.100 |
|
|
Under Weight (n %) |
4 (2.90) |
3 (2.46) |
||
|
Normal (n %) |
44 (31.88) |
52 (42.62) |
||
|
Overweight (n %) |
75 (54.35) |
48 (39.34) |
||
|
Obese (n %) |
15 (10.87) |
19 (15.57) |
||
|
4 |
T2DM Duration (year) |
|
0.001* |
|
|
≥5 (n %) |
118 (85.51) |
75 (61.48) |
||
|
< 5 (n %) |
20 (14.49) |
47 (38.52) |
||
|
5 |
Blood Glucose (HbA1C, fasting, prandial, random) |
0.001* |
||
|
Uncontrolled |
129 (93.48) |
91 (74.59) |
||
|
Controlled |
9 (6.52) |
31 (25.41) |
||
|
6 |
Comorbidity |
|
|
|
|
CKD (n %) |
45 (32.61) |
18 (14.75) |
0.001* |
|
|
Neuropathy DM (n %) |
65 (47.10) |
36 (29.51) |
0.004* |
|
|
Cardiovascular diseases (n %) |
3 (2.17) |
3 (2.46) |
0.879 |
|
|
Retinopathy DM (n %) |
19 (13.77) |
16 (13.11) |
0.878 |
|
|
Diabetic foot (n %) |
26 (18.84) |
22 (18.03) |
0.867 |
|
|
Gastropathy DM (n %) |
84 (60.87) |
69 (56.56) |
0.481 |
|
n: number; T2DM: type 2 diabetes mellitus; DM: diabetes mellitus; CKD: chronic kidney diseases; χ2: Chi-Square analysis; *:statistically significant
Approximately 60% of the total patients involved were consumers of insulin, sulfonylurea, or both in combination. This condition was ideal for exploring more deeply the risk of developing hypoglycemia in the patients and exploring which types of medications were at more risk of causing hypoglycemia. Averagely 85% of the study participants did not have good glucose control, where the average HbA1C level was >7%, average fasting blood >126 mg/dL, random blood, and 2 hours postprandial glucose was above 200 mg/dL.
Evaluation of Insulin and Sulfonylurea Types on Severe Hypoglycemia Event Among Ambulatory Type 2 Diabetes Mellitus Patients
The incidence of hospitalized hypoglycemia in the case and control groups in the variables of patients using insulin, sulfonylurea, a combination of both, and non-users of both was found to be significantly different from bivariate analysis (p<0.05). These results are shown in Table 2. Based on the type of insulin and sulfonylurea, it was found that differences in insulin type significantly influenced the incidence of hypoglycemia in the case group compared to controls when explored. However, no significant difference was observed in the type of sulfonylurea between the case and control groups. For the sulfonylurea type, it was found that the p-value was ≤0.25, so the sulfonylurea-type variable was eligible for inclusion in the multivariate analysis to observe the interaction between variables on the outcome of hypoglycemia.
Table 2: Analysis of hypoglycemia events in patients using insulin, sulfonylurea, and their combination.
|
Variable |
Hypoglycemia Case (n=138) |
Non Hypoglycemia Control (n=122) |
Total |
P Value |
|
Non-insulin and non-SU user |
30 (21.74%) |
54 (44.26%) |
84 (32.31%) |
0.001* |
|
Insulin all types |
66 (47.83%) |
41 (33.61%) |
107 (41.15%) |
|
|
SU all types |
27 (19.57%) |
21 (17.21%) |
48 (18.46%) |
|
|
Insulin and SU as a combination |
15 (10.87%) |
6 (4.92%) |
21 (8.08%) |
|
|
Total |
138 (100%) |
122 (100%) |
260 (100%) |
|
| Non-insulin user |
57 (41.30%) |
75 (61.48%) |
132 (50.77) |
0.001* |
|
Basal bolus insulin |
62 (44.93%) |
22 (18.03%) |
84 (32.31%) |
|
|
Basal insulin |
9 (6.52%) |
14 (11.48%) |
23(8.85%) |
|
| Bolus insulin |
3 (2.17%) |
5 (4.10%) |
8 (3.08%) |
|
|
Mixed insulin |
7 (5.07%) |
6 (4.92%) |
13 (5.00%) |
|
|
Total |
138 (100%) |
122 (100%) |
260 (100%) |
|
|
Non-SU user |
96 (69.57%) |
95 (77.87%) |
191 (73.46%) |
0.161 |
|
Glimepiride |
34 (24.64%) |
21 (17.21%) |
55 (21.15%) |
|
|
Glibenclamide |
4 (2.90%) |
6 (4.92%) |
10 (3.85%) |
|
|
Glikazide |
3 (2.17%) |
0 (0%) |
3 (1.15%) |
|
|
Glikuidone |
1 (0.72%) |
0 (0%) |
1 (0.39%) |
|
|
Total |
138 (100%) |
122 (100%) |
260 (100%) |
P-value ≤0.25 continues multivariate analysis; *: statistically significant; SU: Sulfonylurea; n: number.
Table 3: Stepwise multivariate logistic regression analysis of hypoglycemia events in patients using insulin, sulfonylurea, and their combination.
|
Variable |
OR (CI95%) |
P Value |
|
Non-insulin and non-SU user |
1 Reference |
0.001* |
|
Insulin all types |
2.898 (1.602-5.240)* |
|
|
SU all types |
2.314 (1.122-4.774)* |
0.023* |
|
Insulin and SU as a combination |
4.500 (1.580-12.817)* |
0.005* |
P-value <0.05 is statistically significant; *: statistically significant; OR: odd ratio; CI: confident interval; SU: Sulfonylurea.
Table 3 presented the multivariate analysis, showing that patients using insulin tended to be at risk of experiencing hypoglycemia 2.9 times greater than the control group (CI95%: 1.6-5.2). Meanwhile, sulfonylurea (SU) users had a 2.3 times risk (CI95%: 1.1-4.8) of experiencing hypoglycemia compared to the control group. When insulin and sulfonylurea were combined, the results increased the risk of hypoglycemia events up to 4.5 times (CI95%: 1.6-12.8) compared to the control group.
Multivariate analysis still observed the types of insulin and sulfonylurea that were found to cause the most hypoglycemia hospitalizations. In this study, it was found that patients who used basal-bolus insulin had a risk of experiencing hypoglycemia 4.3 times (CI95%: 2.2-7.7) compared to the control group. Meanwhile, the use of glimepiride sulfonylurea was found to have 2.2 times (CI95%: 1.1-4.1) higher risk of causing hypoglycemia compared to the control group, as shown in Table 4. An interesting result was found in this original study, which includes the use of basal insulin, bolus insulin, or mixed insulin alone, which did not have a significant effect on the incidence of hypoglycemia in the case or control groups. However, sulfonylurea types Glibenclamide, Glikazide, and Gluquidone were found to be insignificant causes of hypoglycemia.
Table 4: Multivariate analysis of all types of insulin and sulfonylurea caused the highest incidence of hypoglycemia in T2DM patients.
|
Variable |
P Value |
OR (CI95%) |
Variable |
P Value |
OR (CI95%) |
|
Model 1 |
|
|
Model 4 |
|
|
|
Glimepiride |
0.019* |
2.231 (1.142-4.358)* |
Glimepiride |
0.013* |
2.279 (1.193-4.353)* |
|
Glibenclamide |
0.640 |
0.719 (0.181-2.853) |
Basal Bolus Insulin |
0.001* |
4.329 (2.373-7.896)* |
|
Glikazide |
0.999 |
0.639 (0.001-20.277) |
Mixed Insulin |
0.316 |
1.802 (0.570-5.693) |
|
Glikuidone |
1.000 |
0.286 (0.001-21.773) |
Model 5 |
||
| Basal Bolus Insulin | 0.001* |
4.292 (2.286-8.057)* |
Glimepiride |
0.018* | 2.168 (1.140-4.123)* |
|
Basal Insulin |
0.936 |
0.001* |
4.256 (2.363-7.665)* |
||
|
Bolus Insulin |
0.979 |
||||
|
Mixed Insulin |
0.339 |
1.765 (0.551-5.659) |
|||
|
Model 2 |
|
|
|||
|
Glimepiride |
0.018* |
2.228 (1.149-4.317)* |
|||
|
Glibenclamide |
0.638 |
0.719 (0.182-2.846)* |
|||
| Basal Bolus Insulin | 0.001* |
4.285 (2.305-7.968)* |
|
||
|
Basal Insulin |
0.933 |
0.960 (0.369-2.495) |
|||
|
Mixed Insulin |
0.338 |
1.763 (0.552-5.623) |
|||
Model 3 |
|||||
| Glimepiride | 0.015* |
2.239 (1.168-4.293)* |
|
||
|
Glibenclamide |
0.634 |
0.717 (0.181-2.830) |
|||
|
Basal Bolus Insulin |
0.001* |
4.313 (2.364-7.871)* |
|||
|
Mixed Insulin |
0.330 |
1.773 (0.561-5.609) |
P-value <0.05 is statistically significant; *: statistically significant; OR: odd ratio; CI: confident interval.
Discussion
Hypoglycemia was often found in T2DM patients when discharged with diabetes medication, which insulin and sulfonylurea caused. A Cochrane systematic study found that hypoglycemic events were 2.0 to 2.6 events per participant taking insulin alone compared with 2.2 to 6.1 events per participant for patients taking insulin and sulfonylurea8. Another study also reported that the overall incidence of hypoglycemia (defined as hospitalization) was more frequent in the elderly, with an OR for hypoglycemia of 4.7 with sulfonylurea and insulin compared with 4.2 for insulin and 3.9 for sulfonylurea9,16. These results were consistent with several of these studies and need to be highlighted as there was a tendency for the incidence of hypoglycemia to be more often found when using basal-bolus insulin and glimepiride as a kind of sulfonylurea. The risk increased two times more when basal-bolus insulin was combined with glimepiride than when combined alone.
The risk of hypoglycemia in insulin users was due to the non-fixed dose of basal-bolus insulin. Patients who adjusted the basal-bolus insulin dose according to nutritional intake were at risk of developing hypoglycemia. Those who fail to understand this dose adjustment are at high risk of hospitalized hypoglycemia. Patients who consume oral antidiabetes (OAD), specifically the sulfonylurea group, must know the most appropriate time to use the drug to avoid the risk of hypoglycemia16-19. The results recommended selecting a combination of basal-bolus insulin with glimepiride as the last choice for blood sugar control in T2DM patients.
In developed countries, there has been a shift in the use of diabetes medication to direct incretin mimetic agents (GLP-1) and indirect agents such as DPP4 inhibitors2,19. SGLT2-I is also widely reported to provide good glycemic control for DM patients. These drugs are considered effective in controlling blood sugar, minimizing hypoglycemia side effects, minimizing weight gain, and promoting weight loss2. The use of these agents is still limited due to cost and access constraints, especially in developing countries2,19. Developing countries such as Indonesia still rely on insulin and SU as blood sugar controllers for patients because they are cost-effective, covered by national health insurance, and have easy access to remote areas12. The findings of this original research were expected to provide a detailed picture of the incidence of hypoglycemia in the use of these agents, along with the types of insulin and SU with the highest incidence of severe hypoglycemia. Basically, ambulatory T2DM patients with insulin and SU have a high risk of hypoglycemia event. So, they are highly recommended to obtain comprehensive information regarding medication use from start, take, add, review, and stop medication when outpatient with insulin, sulfonylurea, or a combination of insulin and sulfonylurea. The role of health workers was essential to ensure the control of this condition. Health workers, caregivers, and patients should pay more attention to how to use drugs, review drug use, and provide information to carry out self-monitoring of blood glucose as well as how to get first aid when an emergency occurs13-15.
This study had several limitations, such as the case-control design and exposure parameters, which were analyzed based on the three available hospital medical records. Therefore, it shared the essential limitations of a hospital-based study. The primary outcome data was analyzed based on the current therapy patients were undergoing. It was possible that patients previously used other types and conditions of treatment. Patients might have been diagnosed with T2DM and prescribed medication at Primary care or other hospitals before the index date, hence, patients were not limited to new users.
Conclusion
In conclusion, T2DM patients who used insulin and sulfonylurea were at risk of developing hypoglycemia while undergoing outpatient therapy compared to control. Furthermore, basal-bolus insulin and OAD sulfonylurea-type glimepiride were discovered to be the types of treatment with the highest and most significant incidence of hospitalized hypoglycemia in T2DM patients.
Acknowledgment
The authors are grateful to the lecturers and staff in the PhD program, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia, for the support in the implementation of this study. The authors are also grateful to Publication Division Pharmacy of Universitas Gadjah Mada and Pharmacy of Universitas Udayana for providing a lot of manuscript writing.
Conflict of Interest
The author(s) do not have any conflict of interest
Funding Source
This study was funded by the Indonesia Center for Education Financial Services (Puslapdik RI), Center for Higher Education Funding (BPPT), and Indonesia Endowment Funds for Education (LPDP) Number 00438/BPPT/BPI.06/9/2023.
Data Availability
This statement does not apply to this article.
Ethics Statement
The Faculty of Medicine, Udayana University, Bali, ethics commission approved this study with an ethical clearance number 1165/UN.14.2.2.VII.14/LT/2024. Ethical clearance was obtained from the multicenter hospitals, namely Denpasar City Hospital, Badung Regency Hospital, and Buleleng Regency Hospital with ethical clearance number 052/EA/KEPK.RSBM.DISKES/2024, B/475/UN14.6/PT.01.04/2024, and 019/EC/KEPK-RSB/V/2023 respectively.
Informed Consent Statement
Consent was obtained from participants using an approved and locally translated digital consent form. Patients were informed about the details of the study, including the general overview, purpose, risks, and benefits, also confidentiality was maintained through all stages. This study was conducted following the Declaration of Helsinki.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
Zullies Ikawati was the research leader and drafter who prepared the manuscript. Made Krisna Adi Jaya contributed to collecting and processing data and writing the manuscript. Fita Rahmawati and Nananag Munif Yasin contributed to designing the data analysis and developing reporting standards for this research.
References
- Nakhleh A., Shehadeh N. Hypoglycemia in diabetes: An update on pathophysiology, treatment, and prevention. World J Diabetes. 2021;12(12):2036–2049.
CrossRef - ADA. Diagnosis and Classification of Diabetes Mellitus. Diabet Care. 2014;37(1):81-90.
CrossRef - Quilliam B.J., Simeone J.C., Ozbay A.B. Risk Factors for Hypoglycemia-Related Hospitalization in Patients With Type 2 Diabetes: A Nested Case–Control Study. Clin. Ther. 2011;33(11):1781–1791.
CrossRef - Ikeda Y., Kubo T., Oda E. Incidence rate and patient characteristics of severe hypoglycemia in treated type 2 diabetes mellitus patients in Japan: Retrospective Diagnosis Procedure Combination database analysis. J. Diabetes Investig. 2018;9(4):925–936.
CrossRef - Pratiwi C., Rumende M., Kshanti I.A.M. Risk Factors for Inpatient Hypoglycemia in a Tertiary Care Hospital in Indonesia. J ASEAN Fed Endocr Soc. 2022;37(2):28–33.
CrossRef - Yunir E., Nugraha A.R.A., Rosana M. Risk factors of severe hypoglycemia among patients with type 2 diabetes mellitus in outpatient clinic of tertiary hospital in Indonesia. Sci. Rep. 2023;13(1):16259.
CrossRef - Min J.Y., Griffin M.R., Hung A.M. Comparative Effectiveness of Insulin versus Combination Sulfonylurea and Insulin: a Cohort Study of Veterans with Type 2 Diabetes. J. Gen. Intern. Med. 2016;31(6):638–646.
CrossRef - Vos R.C, Avendonk M.J, Jansen H. Insulin monotherapy compared with the addition of oral glucose-lowering agents to insulin for people with type 2 diabetes already on insulin therapy and inadequate glycaemic control. Cochrane Database Syst Rev. 2016;18(9):CD006992.
CrossRef - Fu H., Xie W., Curtis B. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30(9):1787–1793.
CrossRef - Mogensen U.M., Andersson C., Fosbøl E.L. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58(1):50–58.
CrossRef - Von Elm E., Altman D.G., Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern. Med. 2007;147(8):573–577.
CrossRef - Laelasari E., Sauriasari R., Banun, A. Cost-Effectiveness Analysis of Insulin, Sulfonylurea, and Sulfonylurea–Metformin in Type 2 Diabetes Mellitus. Asian J Pharm Clin Res. 2017;10(5):50–53.
CrossRef - Sola D., Rossi L., Schianca G.P. State of the art paper Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;4(1):840–848.
CrossRef - American Diabetes Association. Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers. Clin Diabet. 2019;37(1):11–34.
CrossRef - Laires P., Kurtyka K., Witt E.A. Factors associated with physicians’ decision to discontinue or down-titrate sulfonylureas for type 2 diabetes patients. Expert Rev Pharm Out 2019;19(1):71–79.
CrossRef - Filion K.B., Douros A., Azoulay L. Sulfonylureas as initial treatment for type 2 diabetes and the risk of adverse cardiovascular events: A population-based cohort study. Br. J. Clin. Pharmacol. 2019;85(10):2378–2389.
CrossRef - Hemmingsen B., Christensen L.L., Wetterslev J. Comparison of metformin and insulin versus insulin alone for type 2 diabetes: systematic review of randomised clinical trials with meta-analyses and trial sequential analyses. BMJ. 2012;344(1):1771.
CrossRef - Moon J.S., Ha K.S., Yoon J.S. The effect of glargine versus glimepiride on pancreatic β-cell function in patients with type 2 diabetes uncontrolled on metformin monotherapy: open-label, randomized, controlled study. Acta Diabetol. 2014;51(2):277–285.
CrossRef - Gebrie D., Manyazewal T., Ejigu A.D. Metformin-Insulin versus Metformin-Sulfonylurea Combination Therapies in Type 2 Diabetes: A Comparative Study of Glycemic Control and Risk of Cardiovascular Diseases in Addis Ababa, Ethiopia. Diabetes Metab Syndr Obes. 2021;14(1):3345–3359.
CrossRef








