Oluwafunbi Christianah Adeleye* and Ida Masana Risenga
and Ida Masana Risenga
Department of Animal, Plants and Environmental Science, Faculty of Science, University of the Witwatersrand, Johannesburg, South Africa.
Corresponding Author Email: 2415662@students.wits.ac.za
DOI : https://dx.doi.org/10.13005/bpj/3032
Abstract
The study focused on Portulacaria afra, a plant native to South Africa which is renowned for its traditional medicinal applications against oral and skin infections. This study aimed to explore, the phytochemical composition, antimicrobial, and antioxidant capabilities of the leaves, stems, and roots under concurrent extreme temperatures (hot and cold) and water deficit conditions to further validate its medicinal significance. To achieve this, the study subjected Portulacaria afra to various temperatures ranging from 0/5ºC to 35/45ºC combined with water deficit and evaluated its antimicrobial and antioxidant properties. Phytochemical compounds were extracted from different solvent extracts of P. afra and analysed. Antioxidant activity was assessed using metal chelating activity, hydrogen peroxide scavenging, and 2,2-diphenylpicrylhydrazyl (DPPH) free radical assays, while antimicrobial efficacy was determined using the agar-well diffusion method against three microbial strains: Staphylococcus aureus, Escherichia coli, and Streptomyces griseus. Results revealed an enhanced presence of 13 phytochemical groups in Portulacaria afra extracts compared to controls, with peak accumulation of phenolic and flavonoid compounds observed under extreme hot temperatures (35/45ºC) with water deficit. Extracts from these conditions exhibited superior inhibitory effects against Escherichia coli. Moreover, antioxidant activity peaked at hot temperatures (30/40ºC) for 2,2-diphenylpicrylhydrazyl (DPPH) and metal chelating assays, while the most effective hydrogen peroxide scavenging activity was observed under hot temperatures (35/45ºC) with water deficit. Antioxidant activity peaked at mid-range hot temperatures (30/40ºC) for DPPH and metal chelating, while the best hydrogen peroxide scavenging activity was observed during concurrent extreme hot temperatures (35/45ºC) with water deficit. The findings suggest that extreme temperature variations combined with water deficit significantly impact the biological activities of Portulacaria afra, highlighting the plant’s adaptability and resilience while retaining its therapeutic potential.
Keywords
Antioxidant Activity assays; Extreme temperatures; Phytochemical screening; Portulacaria afra; Water deficit; Whole plant
Download this article as:| Copy the following to cite this article: Adeleye O. C, Risenga I. M. Effect of Concurrent Extreme Temperatures and Water Deficit on the Phytochemistry, Antimicrobial and Antioxidant Activities of Portulacaria Afra Jacq Using Four Extraction Solvents. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Adeleye O. C, Risenga I. M. Effect of Concurrent Extreme Temperatures and Water Deficit on the Phytochemistry, Antimicrobial and Antioxidant Activities of Portulacaria Afra Jacq Using Four Extraction Solvents. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3YwmnR9 |
Introduction
Abiotic factors encompass a spectrum of challenging environmental elements that significantly influence ecological systems. These factors include, but are not limited to, temperature variations, drought occurrences, salinity levels, heavy metal concentrations, water stress, cold temperature exposure, anoxic conditions, high-intensity light exposure, and imbalances in nutrient availability1.
The impact of abiotic factors, notably temperature fluctuations and water deficits, presents a significant impediment to the reproductive processes of medicinal plants. This interference consequently disrupts biosynthetic pathways, which in turn affects secondary metabolites accumulation and subsequent alterations in phytopharmacological activities2.
Elevated temperatures induce heat stress, while reduced temperatures result in cold stress. The substantial impact of high temperatures extends to the composition of phytocompounds3. Elevated temperatures typically correlate with heightened biosynthesis of secondary metabolites in plants, thereby enhancing the production of secondary metabolites across various plant species4. Conversely, high temperatures inducing stress were observed to result in reduced levels of secondary metabolites in plants5.
Water deficit results in a decrease of water absorption and potentials within plants, consequently negatively impacting various physiological processes and altering the biosynthesis of secondary metabolites6. Earlier research has demonstrated the profound impact of water deficit or drought stress on the composition of secondary metabolites in herbal plants, leading to increased accumulation. An instance is the increased proportion of flavonoids and phenolics observed in plants as a response to the stress-induced by water deficit7.
In adverse environmental conditions, plants exhibit resilience against diverse abiotic stresses through the activation of adaptive mechanisms8. These mechanisms encompass the synthesis of chemicals facilitating the assembly of proteins and cellular structures, osmotic adjustments, maintenance of general homeostasis, and modulation of the antioxidant system. Additionally, this response potentially leads to heightened production of secondary metabolites such as phenolics1.
According to recent climate change models, it is more likely than previously thought that plants will experience novel or rigorous concurrent abiotic stresses in the years to come9.
Earlier research findings have reported that plant responses to stresses caused by combined antagonistic abiotic factors, are phenomenal compared to when exposed to single ones9. The long-term effects of these abiotic factors may significantly affect the quality and biochemical productivity of medicinal plants such as P. afra by disrupting the phytopharmacological activities, metabolic, developmental, and physiological processes9. These have in turn contributed to the need to study abiotic stress combinations within medicinal plant species.
Portulacaria afra is a succulent evergreen plant indigenous to South Africa, Kenya, and Mozambique. The taxonomic classification of Portulacaria afra falls within the family Portulacaceae or alternatively recently categorized under Didiereaceae. According to ethnobotanical knowledge, and a few studies, P. afra leaves and leaf juice is renowned for its curative properties against skin diseases and oral infections10 andphytochemical studies on P. afra tissue extracts revealed a strong presence of various phytochemicals11. Also, it was previously demonstrated that P. afra extracts exhibited high levels of therapeutic properties in all plant parts, with a strong presence of coumarins known for anti-cancer properties in the methanolic leaf and stem extract12.Basson13, also reported how P. afra leaves showed significant increase in phytochemicals and biological activities after exposure to elevated CO2. The lack of comprehensive scientific documentation regarding the impact of combined environmental factors on P. afra is a notable limitation. This prompted the need to investigate on the impact of concurrent extreme temperatures (hot and cold) with water deficit on the phytochemistry, antimicrobial and antioxidant activities in P. afra.
 |
Figure 1: Portulacaria afra leaves, stems, and roots |
Materials and Methods
Materials
In accordance with standard laboratory protocols, analytical-grade chemicals and solvents were used throughout the experimental procedures. The specific substances, namely gallic acid, Folin-Ciocalteau reagent, methanol, n-hexane, ethyl acetate, Mueller-Hinton agar, Baird Parker agar, dimethyl sulfoxide (DMSO), 2,2-diphenylpicrylhydrazyl (DPPH), and iron (II) chloride, were procured from Sigma-Aldrich, USA, bearing the corresponding CAS numbers1162-658.
In this study, the walk-in convirons (Conviron, Winnipeg, Canada; model No-PGW40) with specifications meticulously detailed bearing serial N0-170028 and manufactured in China (280033R01) were used. It operates at 230/400 Volts, 50 Hz frequency, and a three-phase system. The total input amperage is 29.2, with the compressor drawing 65.5 Amps, while designed to function under high pressure at 400 PSI and low pressure at 200 PSI. Control, lighting, heater, fan, and drier systems require 2.0, 9.4, 4.3, 2.6 HP, and 10.9 Amps respectively. Additionally, specific details regarding receptacle, glycol pump, motor, and auxiliary amperages were included. The system’s minimum circuit ampacity (MCA) is 31.2 Amps, with a maximum overcurrent protection (MOP) set at 32 Amps, and a robust short circuit withstand capacity of 5000 Amps RMS.
Plant Material collection
In July 2021, cuttings of Portulacaria afra were cultivated at the University of the Witwatersrand in Johannesburg, South Africa (26.1899° S, 28.0319° E) for propagation. The plant specimen was verified by a botanist and a voucher specimen (IRM 001) was placed in the institute’s herbarium centre.
Green cuttings with non-lignified stems were carefully chosen from a pathogen-free parent plant of P. afra. To hold the stem cuttings for roots, 225 pots measuring 2 to 2.5 litres each were filled with professional cutting mix soil from Culterrean Potting.
The plants underwent a three-month acclimatization period and root system development in the Oppenheimer Life Sciences greenhouse facilities within the premises of University of the Witwatersrand in Johannesburg, South Africa (26.1899° S, 28.0319° E). The plant growth was observed and sustained by administering 500 ml of water per potted plant every 48 hours due to their low water requirement.
Table 1: Experimental Design
|
|
Control |
Treatment A |
Treatment B |
Treatment C |
Treatment D |
|
Water deficit |
500ml of water every |
No water |
No water |
No water |
No water |
|
Temperatures |
25/27 |
0/5 |
10/15 |
30/40 |
35/45 |
|
Episodic harvesting frequency (hrs) |
Once off |
48, 96, 144 |
48, 96, 144 |
48, 96, 144 |
48, 96, 144 |
Sample size per harvest: n= 15/harvest
The conviron simulations settings used for the treatments were in accordance with the South African Department of Environmental Affairs predictions14, IPCC15, and Mbokodo16 records on the variations in the current and anticipated climatic conditions. The treatments were selected in terms of temperature ranges and the span of heat and cold waves. Control temperature ranges were based on records from the South African Weather Service. The different temperature settings used were (25/27ºC), (0/5ºC), (10/15ºC), (30/40ºC), and (35/45ºC). Each plant was placed in the controlled climate simulation chamber (Conviron chambers, PGW40) with the high and low temperatures set concurrently with water deficit while other parameters such as CO2 (400 ppm), humidity (60%), light (160 nits/level 1) pH, salinity, were kept at ambient.The lightning was programmed to operate for a duration of 12 hours daily, spanning from 6 am to 6 pm17.
Five plants were harvested episodically 3 times every 48hrs for up to 6 days: (48hr, 96hr, 144hr) (Table 1). Samples were subjected to oven drying at 40°C for a period of 4 days post-harvest.This procedure was repeated three times for each of the treatments. Where, n is [15 plants per harvest period-(48hrs, 96hrs, 144hrs) =45 plants per treatment]; n=45 for control (25°), n=45 for (0/5°C), n=45 (10/15°C), n=45 (30/40°C), n=45 (35/45°C) with a total sample size of 225.
Sample Preparation and Extraction
The leaves, stems, and roots were systematically gathered and subjected to a sequential process: initial harvesting, followed by thorough washing using deionized water, and subsequent drying in a hot air dryer maintained at 40°C for a duration of four days. Post-drying, the plant materials were finely ground into a crude powder using an electric grinder. This powdered form was then enclosed in foil and secured in an airtight container. Subsequently, it was stored in a dark cupboard at room temperature (32ºC) until required for subsequent testing procedures18.
Preparation of Extracts
The plant extract of the leaves, stems and roots were produced through sequential extraction using: 80% methanol, n-hexane, ethyl acetate, and distilled water, boiled at 60°C. Each extraction involved placing 3g of powdered plant material into individual glass bottles with cover, with the addition of 30 ml of the respective solvents19. The methanol, n-hexane, ethyl acetate extracts were placed on a shaker, the water extract underwent preparation using a sonicator at a specific temperature (60°C) for a duration of 45 min. The shaking of all extracts lasted 48 hrs, except for the water extracts. The water extracts underwent centrifugation aimed at reducing their viscosity, followed by filtration through filter paper into vials. The residual supernatant was removed, and subsequently, the vials were enveloped in foil and refrigerated at 4ºC until the commencement of the tests.
Preliminary Phytochemical Screening Tests
The chemical constituents within the leaves, stems, and roots extracts underwent evaluation using standard colour test methods. A qualitative analysis was conducted in order to determine the presence or absence of quinones, coumarins, phenols, carbohydrates, terpenoids, flavonoids, glycosides, steroids, saponins, amino acids, phytosteroids, and volatile oils, through visual observation for alterations in colour or precipitation with the adoption of the procedure described by Roghini and Vijayalakshmi20. The observation for each test was then documented.
Evaluation of Total Phenolics and Total Flavonoids Contents
Folin-Ciocalteu reagent assay was used for the approximation of the total phenolics and flavonoids content in Portulacaria afra extracts using the procedure outlined by Teffo21. Standard solutions of gallic acid at varying concentrations were used to construct a calibration curve. The concentrations of total phenolic content within the extracts were extrapolated from this curve, using the equation: y=0.9287x+0.0658, r2=0.9952. Denoting the findings as gallic acid equivalents per gram (mg GAE/g) of the extract’s dry weight.
Similarly, the total flavonoid contents of the plant parts were assessed using a quercetin-based standard curve, using the equation: y=0.2388x-0.0019, r2 =0.9997 with the results expressed as quercetin per gram (mg RE/g) of dry weight.
Antibacterial Activity Assay
The antibacterial inhibitory activity of Portulacaria afra extracts was assessed by following the agar-well diffusion procedure described by Teffo22 with a few modifications. The bacterial strains were obtained from Thermo-Fisher’s laboratory specialists in Johannesburg (Pty) Ltd, South Africa, and the Mueller-Hinton (MH) agar was purchased from Sigma Aldrich Ltd. Briefly, Petri dishes were first sterilized and autoclaved prior to the addition of Mueller-Hinton agar and Baird Parker agar. Once the agar solidified, sterile cotton swabs were used to uniformly inoculate the surfaces of the agar with specific test microorganisms. Baird Parker agar plates were inoculated with Gram+ Staphylococcus aureus (ATCC 25923), whereas Mueller-Hinton agar plates were inoculated with Gram- Escherichia coli (ATCC 25922), and Gram+ Streptomyces griseus and were incubated at 37ºC for 24hours.
Six wells were created in the inoculated media with successful growth of pathogens, using a 6 mm sterile borer. Subsequently, 100 μl of plant extracts were introduced into five of these wells, while the sixth well served as the negative control (dimethyl sulfoxide (DMSO). The plates were then covered and refrigerated for 30 minutes to allow for diffusion before being transferred to an incubator set at 37°C for 24 hours. Following the incubation period, the diameters of the zones of inhibition were measured in millimetres using a ruler. The presence of an inhibitory zone indicated positive antibacterial activity. The experiment was conducted in triplicate, and the data were analysed using Microsoft Excel to express the results as mean ± standard deviation. P < 0.05 was used to determine the statistical significance of the findings.
In vitro Antioxidant Activity Assessment
The assessment of antioxidant potential was conducted using the metal chelating activity assay, hydrogen peroxide scavenging (H₂O₂), and the 2,2-diphenylpicrylhydrazyl (DPPH) free radical assay for the respective plant parts as described below:
DPPH Scavenging Activity Assay
A 2, 2 diphenyl picryhydrazyl (DPPH) free radical assay described by Basson23 was used with some modifications. In the determination of the scavenging potential of DPPH, 50 mg of DPPH was dissolved in 100 ml of 80% methanol to create a stock solution. From this stock solution, a working solution was derived at a ratio of 1:5 with 80% methanol. Each extract had 5 concentrations, ranging from 10 to 50 μL, and was mixed with 700 μL of the working solution which was then adjusted to a final volume of 1 ml with 80% methanol to create a reaction mixture. A control solution was prepared using the working solution and 80% methanol, while a blank was generated using solely 80% methanol. These mixtures were then incubated in darkness for 45 minutes, after which the absorbance was measured at a wavelength of 517 nm using a spectrophotometer. The inhibition (%) was expressed with the following equation:
DPPH Scavenging Effect (%) = [(Absorbance of sample – Absorbance of blank)/Absorbance of control] x 100
Metal Chelating Activity
The metal chelating antioxidant assay was carried out by following the procedure of Adusei24 with few modifications.2 ml of extracts was combined with 0.25 ml of a 250 mM iron (II) chloride solution with an addition of 0.25 ml of ferrozine. The resulting mixture was vigorously shaken and left at room temperature (32ºC) for a duration of 10 minutes. Following this, the absorbance was quantified at a wavelength of 562 nm. Methanol was used as the blank, while a control group (without extracts) was used. The percentage of metal chelation was determined with the formula below:
Metal chelation (%) = {(A0 – A1)/ A0} × 100
A0 represents the absorbance of the control while A1 stands for the absorbance of the test samples.
Hydrogen Peroxide Scavenging (H₂O₂) Assay
The hydrogen peroxide scavenging activity was determined by adapting the methods of Bouabid25. 1 ml of extracts was combined with 3.4 ml of phosphate buffer (50 mM, pH 7.4). Following this, 600 μL of 40 mM hydrogen peroxide (30%) was introduced into the solution. The resulting mixture was left at room temperature (32ºC) for a duration of 40 minutes. Subsequently, the absorbance of the solution was determined using a spectrophotometer at a wavelength of 230 nm. The hydrogen peroxide scavenging activity was quantified using the provided formula:
Scavenged H₂O₂ (%) = [(Absorbance of control – Absorbance of sample)/Absorbance of control] x 1
Statistical Analysis
The concentration values were plotted against the % inhibition values in Microsoft Excel to derive a trend line equation used for calculating the IC50. A one-way analysis of variance (ANOVA) was used to determine the significant differences. All experiments were conducted thrice, and data were presented as mean ± standard error (SE) for n = 3.
P < 0.05 was used to determine the statistical significance of the findings and Tukey HSD post hoc tests in R Studio was used to determine where the significance lies. The significant differences (P < 0.05) were indicated by different letters (a, b, c, d) which were compared with the control within the same group.
Results and Discussion
Qualitative Screening of Phytochemicals
The aqueous (60°C), methanol, n-hexane, and ethyl acetate extracts of P. afra subjected to concurrent extreme hot and cold temperatures and water deficit were screened for their phytochemical constituents. For the qualitative phytochemical screening, the leaves, stems, and roots extracts were evaluated for the presence of saponins, flavonoids, glycosides, quinones, phenols, terpenoids, steroids, phytosteroids, volatile oil, carbohydrate, amino acids and coumarins. In general, phytochemicals in the plant parts varied according to the different solvents mentioned above. Increasing trends were noticed in the phytochemical presence under concurrent cold & hot temperatures with water deficit condition as shown in the heat map (Tables 2 and 3). Table 2 shows the fluctuations in the presence of phytochemical contents of the leaves, stems, and roots of P. afra, when plant parts were subjected to concurrent cold & hot temperatures (10/15°C and 30/40°C) with water deficit conditions. Table 3 summarizes the results of the preliminary phytochemical screening under concurrent cold & hot temperatures (0/5°C and 35/45°C) with water deficit conditions.
 |
Table 2: Heat map for the phytochemical tests of Portulacaria afra extracts exposed to temperatures (10/15°C and 30/40°C) and water deficit |
Temp ºC, temperature; C, control samples; 48, 48hr treatment; 96, 96hr treatment; 144, 144hr treatment; W, hot water extract (60ºC) extract; M, methanolic extract; H, n-hexane extract; E, ethyl acetate extracts.
 |
Table 3: Heat map for the phytochemical tests of Portulacaria afra extracts exposed to temperatures (0/5°C and 35/45°C) and water deficit |
Temp ºC, temperature; C, control samples; 48, 48hr treatment; 96, 96hr treatment; 144, 144hr treatment; W, hot water extract (60ºC) extract; M, methanolic extract; H, n-hexane extract; E, ethyl acetate extracts.
Quantitative analysis of phytochemical
Quantitative results for the total phenolics (TPC) and total flavonoid content (TFC) in Figure 2 – 9, show the variations in the phytoconstituents across all the plant extracts, under concurrent cold and hot temperatures [(10/15ºC), (30/40ºC)] and [(0/5ºC), (35/45ºC)] with water deficit condition. The highest TPC and TFC in the plant extracts in comparison with the control samples were found in cold temperatures (10/15ºC) with water deficit settings (Figures 2 and 3 respectively). The above were recorded in the aqueous stem extracts after a 144-hour treatment period and aqueous leaf extracts after a 48-hour treatment period (9921.55±8.91mg GAE/g and 7529.09±3.12 mg QE/g; Figures 2 and 3respectively) with control samples values for TPC (1820.67±25.08 mg GAE/g) and TFC values (592.70±49.99 mg QE/g) in the aqueous stem and leaf extracts respectively. ANOVA results showed a significant difference (P < 0.05) in P. afra, Tukey test in R Studio revealed where the significance lies among plant parts.
 |
Figure 2: Total Phenolic content in Portulacaria afra leaves (A), stems (B) and roots (C) extracts using four solvents under 10/15ºC and water deficit. |
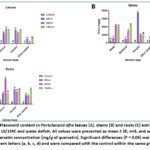 |
Figure 3: Total Flavonoid content in Portulacaria afra leaves (A), stems (B) and roots (C) extracts using four solvents under 10/15ºC and water deficit. |
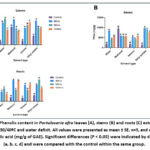 |
Figure 4: Total Phenolic content in Portulacaria afra leaves (A), stems (B) and roots (C) extracts using four solvents under 30/40ºC and water deficit. |
 |
Figure 5: Total Flavonoid content in Portulacaria afra leaves (A), stems (B) and roots (C) extracts using four solvents under 30/40ºC and water deficit. |
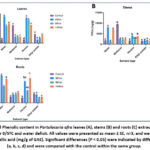 |
Figure 6: Total Phenolic content in Portulacaria afra leaves (A), stems (B) and roots (C) extracts using four solvents under 0/5ºC and water deficit. |
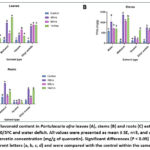 |
Figure 7: Total Flavonoid content in Portulacaria afra leaves (A), stems (B) and roots (C) extracts using four solvents under 0/5ºC and water deficit. |
 |
Figure 8: Total Phenolic content in Portulacaria afra leaves (A), stems (B) and roots (C) extracts using four solvents under 35/45ºC and water deficit. |
 |
Figure 9: Total Flavonoid content in Portulacaria afra leaves (A), stems (B) and roots (C) extracts using four solvents under 35/45ºC and water deficit. |
Antibacterial activity assay
Observed variation in the inhibitory effect of Portulacaria
afra extracts against the tested microorganisms are presented in Table 4 –
7. The highest level of inhibitory effect of 21mm
against gram-negative Escherichia coli
in comparison with the control samples was observed in the methanolic root
extracts after a 48hr-treatment period, upon exposure to combined extreme hot
temperatures (35/45ºC) and water deficit, (Table 7). This was also followed by
a considerable effect of 14mm against Escherichia
coli in the methanolic stem extracts (Table 7). Furthermore, the inhibitory
activities observed in the ethyl acetate extracts were intermediate, ranging
from 9-12mm, with an increasing trend when compared with the control samples
(Table 7). No inhibitory activity was observed against Escherichia coli across all the plant
extracts exposed to hot temperatures (30/40ºC), however, intermediate
inhibitory activities ranged from 11-13mm against gram-positive Streptomyces griseus and Staphylococcus aureus (Table 6). Under the cold temperatures
settings (10/15ºC) with water deficit
condition (Table 4), a slight inhibitory
effect of 11mm was observed only in the n-hexane
and ethyl acetate root extracts against gram-positive Streptomyces griseus and
Staphylococcus aureus after a 48hr and 96-hour treatment period
respectively, with a decline in activity when compared with the control
samples. However, there were no
inhibitory activities observed across all the plant extracts exposed to cold
temperatures (0/5ºC) with water deficit condition (Table 5). From these
findings, the examined plant extracts exposed to temperatures [(30/40ºC) and
(35/45ºC)] with water deficit condition showed better antimicrobial activity
against all the tested microorganisms. ANOVA results showed a significant
difference (P < 0.05) in P. afra extracts. The findings indicate that
the antimicrobial activity of P. afra extracts is subject to the plant
part, temperature and water deficit.
Table 4: Antibacterial activity of Portulacaria afra extracts against Escherichia coli, Staphylococcus aureus, and Streptomyces griseus under 10/15°C with water deficit
|
|
Zone of inhibition (millimetres) of extracts Treatment period (hours) |
||||||||||||||||
|
Microorganisms |
Methanol |
Hexane |
Water (60ºC) |
Ethyl Acetate |
|||||||||||||
|
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
||
|
Leaves
|
Escherichia coli |
0 |
0 |
0 |
0 |
16± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Staphylococcus aureus |
0 |
0 |
0 |
0 |
18± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
14± |
0 |
0 |
0 |
|
|
Streptomyces griseus |
0 |
0 |
0 |
0 |
18± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Stems
|
Escherichia coli |
0 |
0 |
0 |
0 |
13± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Staphylococcus aureus |
0 |
0 |
0 |
0 |
13± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Streptomyces |
0 |
0 |
0 |
0 |
13± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Roots
|
Escherichia coli |
0 |
0 |
0 |
0 |
15± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Staphylococcus aureus |
0 |
0 |
0 |
0 |
17± |
0 |
0 |
0 |
24± |
0 |
0 |
0 |
0 |
0 |
11± |
0 |
|
|
Streptomyces griseus |
0 |
0 |
0 |
0 |
18± |
11± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
C, Control sample extract; 48, 48hr-treatment extract; 96, 96hr-treatment extract; 144, 144hr-treatment extracts. Values are presented as mean ± Standard Deviation, n=3
Table 5: Antibacterial activity of Portulacaria afra extracts against Escherichia coli, Staphylococcus aureus, and Streptomyces griseus under 0/5°C with water deficit
|
|
Zone of inhibition (millimetres) of extracts Treatment period (hours) |
||||||||||||||||
|
Microorganisms |
Methanol |
Hexane |
Water (60ºC) |
Ethyl Acetate |
|||||||||||||
|
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
||
|
Leaves
|
Escherichia coli |
0 |
0 |
0 |
0 |
16± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Staphylococcus aureus |
0 |
0 |
0 |
0 |
18± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
14± |
0 |
0 |
0 |
|
|
Streptomyces griseus |
0 |
0 |
0 |
0 |
18± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Stems
|
Escherichia coli |
0 |
0 |
0 |
0 |
13± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Staphylococcus aureus |
0 |
0 |
0 |
0 |
13± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Streptomyces |
0 |
0 |
0 |
0 |
13± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Roots
|
Escherichia coli |
0 |
0 |
0 |
0 |
15± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Staphylococcus aureus |
0 |
0 |
0 |
0 |
17± |
0 |
0 |
0 |
24± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Streptomyces griseus |
0 |
0 |
0 |
0 |
18± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
C, Control sample extract; 48, 48hr-treatment extract; 96, 96hr-treatment extract; 144, 144hr-treatment extracts. Values are presented as mean ± Standard Deviation, n=3.
Table 6: Antibacterial activity of Portulacaria afra extracts against Escherichia coli, Staphylococcus aureus, and Streptomyces griseus under 30/40°C with water deficit
|
|
Zone of inhibition (millimetres) of extracts Treatment period (hours) |
||||||||||||||||
|
Microorganisms |
Methanol |
Hexane |
Water |
Ethyl Acetate |
|||||||||||||
|
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
||
|
Leaves
|
Escherichia |
0 |
0 |
0 |
0 |
16 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Staphylococcus |
0 |
0 |
0 |
0 |
18 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
14 |
0 |
0 |
0 |
|
|
Streptomyces |
0 |
0 |
0 |
0 |
18 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Stems
|
Escherichia |
0 |
0 |
0 |
0 |
13 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Staphylococcus |
0 |
0 |
0 |
11 |
13 |
0 |
12± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Streptomyces |
0 |
0 |
0 |
0 |
13 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
11 |
0 |
|
|
Roots
|
Escherichia |
0 |
0 |
0 |
0 |
15 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Staphylococcus |
0 |
0 |
0 |
0 |
17 |
0 |
0 |
0 |
24 |
13 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Streptomyces |
0 |
0 |
0 |
0 |
18 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
C, Control sample extract; 48, 48hr-treatment extract; 96, 96hr-treatment extract; 144, 144hr-treatment extracts. Values are presented as mean ± Standard Deviation, n=3.
Table 7:Antibacterial activity of Portulacaria afra extracts against Escherichia coli, Staphylococcus aureus, and Streptomyces griseus under 35/45°C with water deficit
|
|
Zone of inhibition (millimetres) of extracts Treatment period (hours) |
||||||||||||||||
|
Microorganisms |
Methanol |
Hexane |
Water (60ºC) |
Ethyl Acetate |
|||||||||||||
|
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
C |
48 |
96 |
144 |
||
|
Leaves
|
Escherichia coli |
0 |
0 |
0 |
10 |
16 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
10± |
11± |
|
Staphylococcus aureus |
0 |
0 |
0 |
0 |
18 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
14± |
0 |
0 |
0 |
|
|
Streptomyces griseus |
0 |
0 |
0 |
0 |
18 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Stems
|
Escherichia coli |
0 |
14± |
0 |
0 |
13 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
12± |
12± |
|
Staphylococcus aureus |
0 |
0 |
0 |
0 |
13 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Streptomyces griseus |
0 |
0 |
0 |
0 |
13 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Roots
|
Escherichia coli |
0 |
21± |
0 |
0 |
15± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
9± |
11± |
10± |
|
Staphylococcus aureus |
0 |
0 |
0 |
0 |
17± |
0 |
0 |
0 |
24± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Streptomyces griseus |
0 |
0 |
0 |
18± |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
||
C, Control sample extract; 48, 48hr-treatment extract; 96, 96hr-treatment extract; 144, 144hr-treatment extracts. Values are presented as mean ± Standard Deviation, n=3
Antioxidant Activity
The in vitro antioxidant potential of all the extracts was determined by a 2, 2 diphenylpicryhydrazyl (DPPH) free radical assay, hydrogen peroxide scavenging (H₂O₂), and metal chelating activity assay, where IC50 value is the amount of extract required to decrease the absorbance of antioxidant radicals by 50%. Lower IC50 values indicate stronger antioxidant scavenging ability of the extracts. Figure 10 – 21 graphically show the various changes observed in the antioxidant activities of Portulacaria afra extracts upon exposure to hot and cold temperatures and water deficit, when compared with the control samples. Under the cold temperatures settings [ (10/15ºC) and (0/5ºC)] and water deficit (Figure 10 –15), a significant increasing trend of antioxidant capacity was observed across all plant parts when compared to the control samples. Similarly, under hot temperatures [ (30/40ºC) and (35/45ºC)] with water deficit conditions (Figure 16 – 21), an increase in antioxidant activity was observed in some of the extracts in comparison with the control samples. The highest DPPH and metal-chelating antioxidant activity was observed in the ethyl acetate root extracts after a 96-hour treatment period and the methanolic leaf extracts after a 144-hour treatment period (0.26±0.065 mg/ml; Figure 16) and (0.40±0.078 mg/ml respectively; Figure 17), under concurrent hot temperatures settings (30/40ºC) with water deficit. However, n-hexane stem extracts under concurrent hot temperatures settings (35/45ºC) with water deficit conditions showed the strongest hydrogen peroxide scavenging activity (0.14±0.048 mg/ml; Figure 21) after a144-hour treatment period.
Data available here reveal excellent
antioxidant activity of all plant parts. The
methanolic leaf extracts showed the strongest antioxidant activity against
ferric ion radical, ethyl acetate root extracts also showed a very strong
scavenging ability against DPPH radical, while n-hexane stems extract
scavenged hydrogen peroxide radical much better, indicating the strongest
antioxidant activity of Portulacaria afra across the three different
assays. All IC50 values were less than 1 mg/ml with extracts
showing significant differences (P < 0.05).
 |
Figure 10: Graphical representation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) antioxidant activity in Portulacaria afra plant parts under 10/15°C and water deficit. |
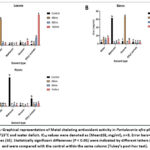 |
Figure 11: Graphical representation of Metal chelating antioxidant activity in Portulacaria afra plant parts under 10/15°C and water deficit. |
 |
Figure 12: Graphical representation of Hydrogen peroxide antioxidant activity in Portulacaria afra plant parts under 10/15°C and water deficit. |
 |
Figure 13: Graphical representation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) antioxidant activity in Portulacaria afra plant parts under 0/5°C and water deficit. |
 |
Figure 14: Graphical representation of Metal chelating antioxidant activity in Portulacaria afra plant parts under 0/5°C and water deficit. |
 |
Figure 15: Graphical representation of Hydrogen peroxide antioxidant activity in Portulacaria afra plant parts under 0/5°C and water deficit. |
 |
Figure 16: Graphical representation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) antioxidant activity in Portulacaria afra plant parts under 30/40°C and water deficit. |
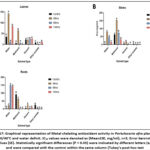 |
Figure 17: Graphical representation of Metal chelating antioxidant activity in Portulacaria afra plant parts under 30/40°C and water deficit. |
 |
Figure 18: Graphical representation of Hydrogen peroxide antioxidant activity in Portulacaria afra plant parts under 30/40°C and water deficit. |
 |
Figure 19: Graphical representation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) antioxidant activity in Portulacaria afra plant parts under 35/45°C and water deficit. |
 |
Figure 20: Graphical representation of Metal chelating antioxidant activity in Portulacaria afra plant parts under 35/45°C and water deficit. |
 |
Figure 21: Graphical representation of Hydrogen peroxide antioxidant activity in Portulacaria afra plant parts under 35/45°C and water deficit. |
Components such as root, stem, leaf, flower, fruits, and seed are categorised according to the synthesis, accumulation, and distribution patterns of phytochemicals26. In specific organs, tissues, and cells, distinct regulatory pathways and transport routes facilitate the synthesis of diverse secondary metabolites across various parts of medicinal plants. This phenomenon arises due to the complexity and diversity inherent in these secondary metabolites26.
Extraction solvents are important in the extraction of potential antioxidant compounds, and are determined by variations in chemical characteristics, polarities, and solubility of these compounds27. Hence, the solvents options for extraction of phytochemicals in this study were chosen based on the polarity of the solute, for better accuracy in dissolving the solute27. The presence of phytochemicals in the leaves, stems, and roots extracts of P. afra exposed to various treatments, when compared with the control, showed that the methanol extracts, across all the parts, in both hot & cold conditions showed the most presence of phytochemicals, followed by the aqueous extracts. This is in accordance with earlier and current research as they are mostly recommended for phytochemical extractions28, due to their high polarity index of 5.1 and 10.2 respectively27, which also validates the use of hot water extraction in traditional medicine.
Previous findings have documented that methanol and water target sugars, amino acids, and glycosides during phytochemical extraction. On the other hand, alkaloids, aglycones and glycosides are commonly extracted with ethyl acetate while n-hexane extracts waxes, fats, fixed oils and volatile29.
Tannins are complex polyphenolic biomolecules with abundant hydroxyls and carboxyl groups, they form strong complexes with macromolecules. They are known for anti-inflammatory and curative properties, used in treating piles, burns, and venereal diseases, and serve as insecticides and regulators of plant growth30. The strong presence of tannins observed across all solvents in the leaf extracts could be responsible for anti-inflammatory and curative properties of P. afra, thus validating its suitability and potency for the treatment of piles, burns and some venereal diseases.
In the pharmaceutical industry, volatile oils are commonly utilized flavouring agents and are known particularly for their antibacterial and carminative attributesTop of Form31. Interestingly, in this study, n-hexane, and ethyl acetate extracts across all plant parts exposed to hot (35/45ºC) & cold (0/5ºC) (Table 3) temperatures with water deficit showed the highest presence of volatile oil concentration, when compared to the other solvents. The concentrations ranged from low to high, the observed outcome is not completely in accordance with earlier research, indicating that warmer seasons or hotter temperatures present better concentrations of volatile oil and essential oils32. In contrast to the above reports, studies have shown how low temperature enhances the production of several groups of volatile terpenoids in essential oil glands on the leaf surface33. Similar observation was reported for the increase in monoterpenes in sage leaves (Salvia officinalis L.) and peppermint essential oil (Mentha piperita L.) mostly under low temperatures33. The increase observed in the volatile oil concentration across the different temperature settings in this study, could suggest the response of this plant species to the physical and chemical stress impacted simultaneously by the combined effect of both extreme temperatures and water deficit than with individual stress. Thus, resulting to the production of terpenoids, which are bioactive compounds that confer several biological activities32. Conversely, volatile oils were produced in low to high quantity in all plant parts under the temperatures [hot (30/40ºC); & cold (10/15ºC)] (Table 2), while methanolic stem extracts detected a strong presence of volatile oils in both treatments.
Phenols are the most prevalent class of phytoconstituents that perform protective roles by shielding plants from pathogens and antagonistic abiotic factors34. Methanolic extracts showed a high presence of phenols in all plant parts under the temperatures [hot (30/40ºC); & cold (10/15ºC)] (Table 2), with water deficit when compared with the control samples. Meanwhile, the concentrations of terpenoids and steroids, also showed an increase in comparison with the control sample extracts across all plant parts under both temperatures with water deficit.
Coumarins present in all plant parts of Portulacaria afra, which is commonly used in cancer treatment, renal cell carcinoma and blood diseases has caught the attention of many researchers for its photochemotherapy and therapeutic application in cancer35. Results as shown in the heat map (Table 3) suggest that the production of coumarins is best under hot (35/45ºC) & cold (0/5ºC) temperatures with water deficit. The methanolic and aqueous extracts of all plant parts, showed a significant increase in comparison to the control in both hot (35/45ºC) and cold (0/5ºC) temperatures with water deficit (Table 3). Plant carbohydrates are important biochemical indicators of temperature acclimation and cold tolerance development33. Therefore, the carbohydrate concentration across all plant extracts, compared to the control samples showed an increasing trend only in the methanolic extracts. This observation shifted from absent to considerable amounts in both hot (35/45ºC) & cold (0/5ºC) temperatures with water deficit (Table 3). While in the methanolic stem extracts exposed to temperatures [hot (30/40ºC); & cold (10/15ºC)] temperatures with water deficit (Table 2), an increasing pattern was also noted.
Studies have shown that water stress may amplify the accumulation of phytochemicals36, contrary to the belief of the negative impact of severe water deficit. This has been extensively researched to establish its effects on the secondary metabolite profile, frequently leading to increasing output in most cases, which indicates higher quality37. The qualitative phytochemical data from this study, however, is attributed to the simultaneous impact of both extreme hot and cold temperatures with water deficit stress.
A broad class of polyphenols with the chemical structure benzoyl-pyrone are called flavonoids. They are prevalent everywhere in plants, produced by the phenylpropanoid pathway and perform a wide range of pharmacological actions38. It is believed that the organoleptic properties of fresh fruits, fruit juices, and wine are also significantly influenced by phenolic chemicals, including tannins and flavonoids. Therefore, their potential impact on human health will primarily depend on the environmental variables that regulate their amounts and quality of accumulation in plants.
It was observed that hot temperatures (35/45ºC) with water deficit increase the accumulation of total phenolics and total flavonoids in Portulacaria afra (Figures 8 and 9), in comparison with cold temperatures (0/5ºC) and water deficit. Al-Huqail1 reported similar higher accumulation of total phenolics content (TPC) in plants under stress, especially in basil plants (Ocimum basilicum L.) treated with high temperature, as well as an increase in total phenolics content (TPC) and total flavonoids content (TFC) concentrations in response to water stress and a highly significant increase in flavonoids under high climate temperature condition. The increase in the contents of the chemical compounds analysed, agrees with data in the literatures indicating that this is a response to the generation of reactive oxygen species. Reactive oxygen species (ROS) are produced by plants in response to environmental stress factors such as shade, excessive salt levels, extreme temperatures, drought, or water deficit, which when present in excess can lead to oxidative stress. Moreover, a vast variety of substances, such as arylpyrones and styrylpyrones, stibenes, tannins, coumarins, flavonoids, lignins, and lignans, are included in the category of phenolics and they are produced in response to abiotic stress7. Consequently, they protect the plants and increase their tolerance against various abiotic and biotic stresses7. Phenolic compounds, due to their redox attributes, act as antioxidants, effectively neutralizing singlet oxygen, displaying notable pharmacological significance. They have demonstrated antioxidative, anticarcinogenic, antibacterial, and anti-inflammatory effects in previous findings1, which supports the traditional therapeutic use of P. afra. Additionally, exogenous factors are responsible for the biosynthesis and accumulation of phenolic compounds while endogenous factors, developmental stage, and tissue differentiation39 are responsible for the site of synthesis in plants. As a result, variation in the climatic, biotic, and environmental factors experienced during seasons can be used to explain the observed differences across seasons. Hence, the content of phenolic compounds and their temperature-dependent fluctuations as seen in this study, suggest that there is a link between their distinct functions in each unique plant parts, and the stimuli causing their synthesis.
Lately, there has been a significant rise in antibiotic resistance, creating a growing therapeutic challenge. Using medicinal plant materials is one way to lessen antibiotic resistance40. Plants create a wide range of chemicals to defend themselves against different diseases. Plant extracts with targets locations different from those used by antibiotics are anticipated to be effective against microorganisms with drug resistance41.
The antimicrobial activity results of P. afra extracts showed that the antibacterial activity of the extracts is subject to temperature with water deficit (Table 4 – 6). The inhibitory activity observed in the methanolic root and stem extracts (Table 7) against Escherichia coli, are similar to the report of previous research demonstrating maximum antibacterial activity against Escherichia coli by the leaves of Amaranthus spinosus L. during the summer month May-June42. The antibacterial activity observed could be correlated with the maximum building up of bioactive compounds in the methanolic stem and root of P. afra as a response to the concurrent effect of hot temperatures (35/45ºC) with water deficit. Conversely, the other extracts across the plant parts were not active against Staphylococcus aureus and Streptomyces griseus. A similar trend was observed in Harpephyllum caffrum bark extracts where the highest inhibitory activity was detected with plant material collected during summer (December) with increasing antibacterial activity against gram-negative bacteria whereas it declined against gram-positive bacteria43. Meanwhile in hot temperatures (30/40ºC) with water deficit (Table 6), no positive antibacterial activity was observed against Escherichia coli across all the plant extracts. However, intermediate inhibitory activities, ranging from 11-13mm were observed against gram-positive Streptomyces griseus and Staphylococcus aureus. Here, the different fractions of the extracts appeared to be more effective against the gram-positive microorganisms. These results can be explained by studies that have proven that gram-negative bacteria have an outer membrane permeability barrier, which makes them more resistant to antimicrobial treatments than gram-positive bacteria44. This might also explain the inhibitory effect seen against S. aureus and S. griseus to the extracts and non-effectiveness of the tested fractions against E. coli.
The slight inhibitory effect of 11mm observed only in the n-hexane and ethyl acetate root extracts against gram-positive Streptomyces griseus and Staphylococcus aureus under cold temperatures (10/15ºC) with water deficit (Table 4), is partly in line with findings from Ncube45, where the best antibacterial activity was observed in winter season from dichloromethane bulb extracts against S. aureus. The observed variation in efficacy to inhibit microorganism growth may be as a function of the distinct cell wall layers characteristic of each microorganism46. The outcomes of this study might be linked to various factors, including the chemical composition of distinct solvents, temperature variations, specific plant parts used, microbial proliferation, and the extent of exposure of the test microorganisms46.
Antioxidants inhibit and delay oxidation process, by transferring electrons to free radicals to limit fluctuations and to prevent further reaction47. The antioxidant activity and medicinal power of plants are significantly dependent on the secondary metabolites present in them, which in turn results to a reduction of oxidative stress by absorbing and neutralising free radicals48. Depending on the amount of antioxidant molecules present in plants, which are greatly influenced by their growth environment, plants have various antioxidant properties49.
Natural antioxidants attract attention in research, and food production, for their therapeutic properties and defence against oxidative stress-induced diseases50. In fact, they are most beneficial for use since they are mostly free of negative side effects in comparison to the synthetic ones49.
Antioxidant potential of Portulacaria afra leaves, stems, and roots extracts were investigated through a 2, 2 diphenylpicryhydrazyl (DPPH) free radical assay, hydrogen peroxide scavenging (H₂O₂) and metal chelating activity assay.
The variations observed in Figure 10 – 21 in this study could potentially be because of the dissimilarities in the concentration or potency of active compounds in each part of P. afra under these stress conditions51. In addition, different antioxidant enzymes have temperature sensitivity, and they can only function at specific temperatures52. Their actions also vary according to the crop varieties, their growth phases, and the growing season’s tolerance or susceptibility to temperature53. Moreover, the availability of various precursors that the plant needs to produce the active components can vary depending on the temperature and time of the year54.
Thus, the results from this study are similar to Basson13, where accumulation of phytochemicals and biological activities in P. afra were found to either remain constant or increased after exposure to elevated CO2. Consequently, these results suggest that the responses in the biological activities in the plant parts under concurrent hot and cold temperatures with water deficit condition serve as an adaptive and protective mechanism of P. afra against the concurrent stressors exposed to in this study.
Conclusion
In conclusion, the phytochemical content, and biological activities in Portulacaria afra were significantly influenced by extreme temperature simulations and water deficit. The observed variability in plant responses provided significant insights into the diverse reactions of various plant parts to distinct environmental stimuli and extraction solvents. These include the modulation of secondary metabolites, alterations in antibacterial properties, and adjustments in antioxidant activities, highlighting fluctuations in response to the combined abiotic factors. It is evident that abiotic factors in combination may influence the biological activities of P. afra, which shows the adaptation and resilience of the plant reflected in its ability to still provide therapeutic treatment against targeted diseases. Consequently, optimum temperatures and water deficit conditions are recommended for peak concentrations of phytochemicals and biological activities in P. afra. Although the results provide valuable insights, one limitation of this study is the use of one plant species, which may affect the generalizability of the findings for combined temperature and water deficit stress on plants. This is because plant responses are species dependent. More diverse plant species would be necessary to further confirm the trends observed both in this study and previous research in order to strengthen the overall validity of the conclusions. Further studies are still recommended for the characterization of the bioactive compounds in these extracts for in vivo studies.
Acknowledgements
Authors are Grateful to South Africa’s National Research Foundation (NRF) for supporting this work. Special appreciation to Michael Tobin, Gontse and Mokgadi for assistance with Laboratory related work.
Funding source
This work was supported by South Africa’s National Research Foundation (NRF) under grant number:TTK201129577193
Conflict of interest
The author(s) do not have any conflict of interest.
Data Availability Statement-
This statement does not apply to this article
Ethics Statement-
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required
Clinical Trial Registration-
This research does not involve any clinical trials
References
- Al-Huqail A, El-Dakak R. M, Sanad M. N, Badr R. H, Ibrahim M. M, Soliman D, Khan F. Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in sweet basil (Ocimum basilicum L.) leafy vegetable. Scientifica. 2020;2020(1):3808909.
CrossRef - Teffo T. K, Dukhan S, Ramalepe P, Risenga I. Possible implications of climate change on the medicinal properties of Bulbine species with a particular focus on Bulbine abyssinica, Bulbine frutescens and Bulbine natalensis in South Africa. Journal of Pharmacognosy and Phytochemistry. 2021;10(5):49-56.
CrossRef - Van Der Walt A. J, Fitchett J. M. Extreme temperature events (ETEs) in South Africa: a review. South African Geographical Journal. 2022;104(1):70-88.
CrossRef - Li Y, Kong D, Fu Y, Sussman M. R, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant physiology and biochemistry. 2020;148:80-89.
CrossRef - Gupta A, Singh P. P, Singh P, Singh K, Singh A. V, Singh S. K, Kumar A. Medicinal plants under climate change: impacts on pharmaceutical properties of plants. In Climate change and agricultural ecosystems. 2019;1 (pp. 181-209). Woodhead Publishing.
CrossRef - Onyekachi O. G, Boniface O. O, Gemlack N. F, Nicholas N. The effect of climate change on abiotic plant stress: a review. Abiotic and biotic stress in plants: A Review [Internet]. IntechOpen; 2019.
- Albergaria E. T, Oliveira A. F, Albuquerque U. P. The effect of water deficit stress on the composition of phenolic compounds in medicinal plants. South African Journal of Botany. 2020;131:12-17.
CrossRef - Ramasar R, Naidoo Y, Dewir Y. H, El-Banna A. N. Seasonal change in phytochemical composition and biological activities of Carissa macrocarpa (Eckl.) A. DC. leaf extract. Horticulturae. 2022;8(9):780.
CrossRef - Suzuki N, Rivero R. M, Shulaev V, Blumwald E, Mittler R. Abiotic and biotic stress combinations. New Phytologist. 2014;203(1):32-43.
CrossRef - De Wet H, Nciki S, van Vuuren S. F. Medicinal plants used for the treatment of various skin disorders by a rural community in northern Maputaland, South Africa. Journal of Ethnobiology and Ethnomedicine. 2013;9:1-10.
CrossRef - Tabassum S, Ahmad S, Rehman Khan K. U, Tabassum F, Khursheed A, Zaman Q. U, Bukhari N. A, Alfagham A, Hatamleh A. A, Chen Y. Phytochemical profiling, antioxidant, anti-inflammatory, thrombolytic, hemolytic activity in vitro and in silico potential of Portulacaria afra. Molecules. 2022;27(8):2377.
CrossRef - Adeleye O. C, Risenga I. M. Screening of phytochemical profile and biological activities in the leaves, stems and roots of South African Portulacaria afra using four extraction solvents. Biomedical and Pharmacology Journal. 2022;15(3):1561-1572.
CrossRef - Basson D. C, van Vuuren S, Risenga I. M. The effect of elevated carbon dioxide on the medicinal properties of Portulacaria afra. South African Journal Science. 2024; 120:1-2.
CrossRef - South African Department of Environmental Affairs. South Africa’s draft National Climate Change Adaptation Strategy [document on the Internet]. c2019 [cited 2019 Jun 05]. Available from: https://www. google. com/url?a=t&source=web&rct=j&url=https:// www. environment. gov.za/sites/ default/files/legislations/session2 _draftnational_adaptationstrategy. pdf&ved=2ahUKEwio3aXQitfiAh UvVRUIHdYkA9UQFjADegQIBhA B&usg=AOvVaw0tV53-1w9QW-kNo19rQeva
- IPCC 2018 “Global Warming of 1.5°C: Summary for Policymakers,” in Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Eds.
CrossRef - Mbokodo I, Bopape M. J, Chikoore H, Engelbrecht F, Nethengwe N. Heatwaves in the future warmer climate of South Africa. Atmosphere. 2020;11(7):712.
- SAWS 2020 Weather questions. Retrieved July 23, 2020, from https://www.weathersa.co.za/home/weatherques [Google Scholar]
- Labar R, Sarkar I, Sen A, Bhattacharya M. Effect of solvent with varying polarities on phytochemical extraction from mature tea leaves and its evaluation using biochemical, antimicrobial, and in-silico approaches. International Research Journal of Pharmacy. 2019;10(8):59-67.
CrossRef - Pakade V, Cukrowska E, Chimuka L. Comparison of antioxidant activity of Moringa oleifera and selected vegetables in South Africa. South African Journal of Science. 2013;109(3):1-5.
CrossRef - Roghini R, Vijayalakshmi K. Phytochemical screening, quantitative analysis of flavonoids and minerals in ethanolic extract of Citrus paradisi. International Journal of Pharmaceutical Sciences and Research. 2018;9(11):4859-4864.
- Teffo T. K, Dukhan S, Ramalepe P, Risenga I. Phytochemical analysis and biological activities of various parts of Bulbine natalensis (Baker): A comparative study. Journal of Herbmed Pharmacology. 2024;13(1):52-60.
CrossRef - Teffo T. K, Dukhan S, Ramalepe P, Risenga I. Comparative phytochemical analysis, antioxidant and antibacterial activities in the leaves, underground stems and roots of Bulbine abyssinica. Biomedical and Pharmacology Journal. 2022;15(3):1323-1335.
CrossRef - Basson D. C, Teffo T. K, Risenga I. M. A phytochemical screening, antioxidant and antibacterial activity analysis in the leaves, stems and roots of Portulacaria afra. Journal of Herbmed Pharmacology. 2022;12(1):109-117.
CrossRef - Adusei S, Otchere J. K, Oteng P, Mensah R. Q, Tei-Mensah E. Phytochemical analysis, antioxidant and metal chelating capacity of Tetrapleura tetraptera. Heliyon. 2019;5(11).
CrossRef - Bouabid K, Lamchouri F, Toufik H, Faouzi M. E. Phytochemical investigation, in vitro and in vivo antioxidant properties of aqueous and organic extracts of toxic plant: Atractylis gummifera L. Journal of Ethnopharmacology. 2020;253:112640.
CrossRef - Belkheir A. K, Gaid M, Liu B, Hänsch R, Beerhues L. Benzophenone synthase and chalcone synthase accumulate in the mesophyll of Hypericum perforatum leaves at different developmental stages. Frontiers in Plant Science. 2016;7:921.
CrossRef - Abubakar A. R, Haque M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. Journal of Pharmacy and Bioallied Sciences. 2020;12(1):1-10.
CrossRef - Nawaz H, Shad M. A, Rehman N, Andaleeb H, Ullah N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Brazilian Journal of Pharmaceutical Sciences. 2020;56:e17129.
CrossRef - Roopashree K. M, Naik D. Advanced method of secondary metabolite extraction and quality analysis. Journal of Pharmacognosy and Phytochemistry. 2019;8(3):1829-1842.
- Das A. K, Islam M. N, Faruk M. O, Ashaduzzaman M, Dungani R. Review on tannins: Extraction processes, applications and possibilities. South African Journal of Botany. 2020;135:58-70.
CrossRef - Irshad M, Subhani M. A, Ali S, Hussain A. Biological importance of essential oils. Essential Oils-Oils of Nature. 2020; 1:37-40.
CrossRef - Soni U, Brar S, Gauttam V. K. Effect of seasonal variation on secondary metabolites of medicinal plants. International journal of pharmaceutical sciences research. 2015;6(9):3654-3662.
- Olennikov D. N, Chirikova N. K, Kashchenko N. I, Gornostai T. Y, Selyutina I.Y, Zilfikarov I. N. Effect of low temperature cultivation on the phytochemical profile and bioactivity of Arctic plants: A case of Dracocephalum palmatum. International journal of molecular sciences. 2017;18(12):2579.
CrossRef - Takó M, Kerekes E. B, Zambrano C, Kotogán A, Papp T, Krisch J, Vágvölgyi C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants. 2020;9(2):165.
CrossRef - Lončar M, Jakovljević M, Šubarić D, Pavlić M, Buzjak Služek V, Cindrić I, Molnar M. Coumarins in food and methods of their determination. Foods. 2020;9(5):645.
CrossRef - Yeshi K, Crayn D, Ritmejerytė E, Wangchuk P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules. 2022;27(1):313.
CrossRef - Mahajan M, Kuiry R, Pal P. K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. Journal of Applied Research on Medicinal and Aromatic Plants. 2020;18:100255.
CrossRef - Agidew M. G. Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bulletin of the National Research Centre. 2022;46(1):87.
CrossRef - Chowdhary V, Alooparampil S, Pandya R. V, Tank J. G. Physiological function of phenolic compounds in plant defense system. In Phenolic Compounds; Badria, FA, Ed.; IntechOpen: London, UK. 2016; Volume 11, p. 13. ISBN 0000957720.
- Álvarez-Martínez F. J, Barrajón-Catalán E, Micol V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines. 2020;8(10):405.
CrossRef - Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites. 2019;9(11):258.
CrossRef - Mitra P. K. In-vitro antibacterial activity of leaves of Amaranthus spinosus L.: Seasonal variation. World Journal of Pharmaceutical Sciences. 2014:1702-1706.
- Buwa L. V, Van Staden J. Effects of collection time on the antimicrobial activities of Harpephyllum caffrum bark. South African Journal of Botany. 2007;73(2):242-247.
CrossRef - Zouari-Bouassida K, Trigui M, Makni S, Jlaiel L, Tounsi S. Seasonal variation in essential oils composition and the biological and pharmaceutical protective effects of Mentha longifolia leaves grown in Tunisia. BioMed research international. 2018;2018(1):7856517.
CrossRef - Ncube B, Finnie J. F, Van Staden J. Seasonal variation in antimicrobial and phytochemical properties of frequently used medicinal bulbous plants from South Africa. South African Journal of Botany. 2011;77(2):387-396.
CrossRef - Cengiz M, Baytar O, Şahin Ö, Kutlu H. M, Ayhanci A, Vejselova Sezer C, Gür B. Biogenic Synthesized Bare and Boron-Doped Copper Oxide Nanoparticles from Thymbra spicat ssp. spicata: In Silico and In Vitro Studies. Journal of Cluster Science. 2024;35(1):265-284.
CrossRef - Pisoschi A. M, Pop A, Iordache F, Stanca L, Predoi G, Serban A. I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. European Journal of Medicinal Chemistry. 2021;209:112891.
CrossRef - Kim H, Seo K. S, Yun K. W. Antioxidant activity and flavonoid estimation in Rosa multiflora and Rosa wichuraiana fruits and flowers. Biomedical and Pharmacology Journal. 2022;15(2):747-755.
CrossRef - Akbari B, Baghaei‐Yazdi N, Bahmaie M, Mahdavi Abhari F. The role of plant‐derived natural antioxidants in reduction of oxidative stress. BioFactors. 2022;48(3):611-633.
CrossRef - Idamokoro E. M, Afolayan A. J. In vitro Evaluation of the Phytochemical and Antioxidant Properties of Bulbine abyssinica Extracts. International Journal of Agriculture and Biology. 2020; 24(6):1781-1787
- Toscano S, Trivellini A, Cocetta G, Bulgari R, Francini A, Romano D, Ferrante A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Frontiers in plant science. 2019;10:1212.
CrossRef - Estrada-Cárdenas P, Cruz-Moreno D. G, González-Ruiz R, Peregrino-Uriarte A. B, Leyva-Carrillo L, Camacho-Jiménez L, Quintero-Reyes I, Yepiz-Plascencia G. Combined hypoxia and high temperature affect differentially the response of antioxidant enzymes, glutathione and hydrogen peroxide in the white shrimp Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2021;254:110909.
CrossRef - Ramazan S, Qazi H. A, Dar Z. A, John R. Low temperature elicits differential biochemical and antioxidant responses in maize (Zea mays) genotypes with different susceptibility to low temperature stress. Physiology and Molecular Biology of Plants. 2021;(6):1395-1412.
CrossRef - Hasanuzzaman M, Nahar K, Alam M. M, Roychowdhury R, Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. International journal of molecular sciences. 2013;14(5):9643-9684.
CrossRef








