Nagaraju Bandaru1* , Namanda Shamim2
, Namanda Shamim2 , Siripalli Bhagaya Nagalakshmi3
, Siripalli Bhagaya Nagalakshmi3 , Thumalapalli Sunanda4, Ch.Hanisha5
, Thumalapalli Sunanda4, Ch.Hanisha5 , Makarand Suresh Gambhire1
, Makarand Suresh Gambhire1 , Prashik B. Dudhe1
, Prashik B. Dudhe1 , Yalla Kranthi6
, Yalla Kranthi6 , Perli.Kranti Kumar1
, Perli.Kranti Kumar1 and PNS Gowravi7
and PNS Gowravi7
1School of Pharmaceutical Sciences (SOPS), Sandip University, Nasik, Maharashtra, India.
2Department of Pharmacology, College of Pharmacy, Koneru Lakshmaiah Education Foundation, Vaddesweram, AP, India.
3Department of Pharmacology, Lydia College of Pharmacy,Ethakota, Ravulapalem, AP, India.
4Department of Pharmacology, Nalla Narasimha Reddy Group of Institutions, Peerzadiguda, Hyderabad, Telangana, India.
5Department of Pharmaceutics, School of Pharmacy, Mohanbabu University, Tirupathi, AP, India.
6Department of Pharmaceutical Analysis, Shri Vishnu College of Pharmacy, Vishnupur, Bhimavaram, AP, India.
7Andhra University College of Pharmaceutical Sciences, Andhra University South Campus, Chinna Waltair, AP, India.
Corresponding Author E-mail: bnrajupharma@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2971
Abstract
Aim: To evaluate the Neuroprotective activity of Biophytum reinwardtii Platinum nanoparticles Methods: Biophytum reinwerdtii platinum nanoparticles were subjected to evaluation of the neuroprotection activity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced zebra fishes. Experimental fishes are divided into 5 groups, each containing 8 fishes. Group I is considered a normal group; Group II is a toxic group means treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine 225 mg/kg, i.p. for 5 days; Group III, IV, and V are treatment groups means treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (225 mg/kg) with 0.3 µmol, 0.4µmol, and 0.5 µmol of Biophytum reinwardtii Platinum nanoparticles respectively for 5 days. Results: In the in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine group, locomotor activity and complex I activity are decreased, Malondialdehyde levels increased, antioxidants, and catecholamines levels decreased, whereas Biophytum reinwardtii Platinum nanoparticles treated fishes exhibit significant locomotor and increased levels of antioxidants and catecholamines. Conclusion: These results suggest that Biophytum reinwardtii Platinum nanoparticles. Showed significant neuroprotection activity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine -induced Parkinson’s zebra fishes.
Keywords
Antioxidants; Locomotor Activities; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; Nanoparticles; Parkinson’s; Platinum
Download this article as:| Copy the following to cite this article: Bandaru N, Shamim N, Nagalakshmi S. B, Sunanda T, Hanisha C, Gambhire M. S, Dudhe P. B, Kranthi Y, Kumar P. K, Gowravi P. N. S. Preparation of Platinum Nanoparticles of Biophytum reinwardtii and Evaluation of Neuroprotective Activity of MPTP-induced Parkinson’s Disease in Zebra Fish. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Bandaru N, Shamim N, Nagalakshmi S. B, Sunanda T, Hanisha C, Gambhire M. S, Dudhe P. B, Kranthi Y, Kumar P. K, Gowravi P. N. S. Preparation of Platinum Nanoparticles of Biophytum reinwardtii and Evaluation of Neuroprotective Activity of MPTP-induced Parkinson’s Disease in Zebra Fish. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/4ejhHo7 |
Introduction
Parkinsonism is a neurodegenerative disease that mainly affects the dopamine neurons inside the nigrostriatal part of the brain. It is mainly characterized by motor symptoms like tremors, bradykinesia, muscle rigidity, and instability in posture 1. MPTP acts as a neurotoxin for the induction of Parkinson’s disease in brain glial cells. MPTP is converted into MPP+, by using dopamine-transported MPP+ enters dopaminergic neurons, where it interferes with normal cellular signalling pathways, and gene regulation, and causes neuronal death by inhibiting mitochondrial function by blocking respiratory chain complex I activity and increases the free radicals like superoxide anion, hydroxyl radicals, and peroxide radicals 2-4. Organic nanoparticles lower the concentrations of reactive oxygen and nitrogen species by their free radical scavenging activity 5, 6.As per research studies, platinum nanoparticles have reduced the production of reactive oxygen species, free radicals and increased the 1complex I activity in mitochondria 7. Based on these studies, there is a possibility for the usage of platinum nanoparticles for the treatment of Parkinson’s 8, 9. It is needed to produce platinum nanoparticles by using eco-friendly and non-polluting methods because, chemical methods has some drawbacks like consumption of high energy and it produces some side effects 10. So, to overcome this problem, authors used green Nano technological procedures, and it is a new era in nanoparticle production 11. This method uses biological microorganisms or plant extracts. Compared to other biological processes, using plants for the synthesis of nanoparticles has several advantages 12, 13.
Biophytum reinwardtii belongs to the family Oxalidaceae, a small family of flowering plants consisting mainly of herbs, shrubs, and small trees. The family is most famous for the genus Oxalis, which is known for its clover-like appearance. Biophytum is a lesser-known genus, but it holds importance in ethnomedicine, particularly in tropical regions. Biophytum reinwardtii is distributed primarily in tropical and subtropical regions of Asia and Africa.14-16 It is commonly found in India, Sri Lanka, Myanmar, Indonesia, and some parts of tropical Africa. The plant thrives in humid environments, often growing in open forests, grasslands, and even along roadsides. It prefers sandy or loamy soils and is commonly seen in areas with moderate to heavy rainfall 17-18.
The plant has adapted well to the warm, humid climates typical of tropical and subtropical regions. Its wide distribution across various countries suggests its ability to tolerate varying environmental conditions. In India, particularly in the states of Kerala, Tamil Nadu, and Karnataka, Biophytum reinwardtii is frequently seen in traditional medicine practices 19-20.
Biophytum reinwardtii is widely used in folk medicine for its anti-inflammatory, wound-healing, and antimicrobial properties. It is applied as a paste on wounds and cuts to promote healing and prevent infection. The plant’s decoction is consumed to alleviate respiratory ailments like asthma and bronchitis, as well as to manage fever and malaria. Additionally, it is believed to have hypoglycemic properties for diabetes management and diuretic effects to treat urinary tract infections 21.
This research used a one-step green synthesis methodology to produce platinum nanoparticles using an aqueous extract of Biophytum reinwerdtii (BR). As it is used in treating the Parkinson’s disease for its flavonoid compounds, these compounds boost the blood flow to the brain, enhance mood, cognition, overall neuronal cells health, and have other effects like antioxidant, anxiolytic, anti-stress, and anti-inflammatory 22.
Recent research shows that BR extract has significant neuroprotective activity by inhibiting the glutamate-induced neurotoxicity in HT22 hippocampal cells by modifying the activity of redox-regulated proteins including NF-kappa, Sirt1, EPK1/2 & p66Shc 23. Based on the above research, the authors used Biophytum reinwerdtii extract for the preparation of phytoplatinum nanoparticles. In the current research, Biophytum reinwerdtii platinum nanoparticles were prepared and evaluated the neuroprotective activity in the MPTP-induced PD model of zebra fish.
Materials and Methods
Materials required
All required chemicals like Platinum nanoparticles, MPTP, NADH, DTNB, EDTA, Perchloric acid, and octane sulfonic acid was procured from Southern Scientific Corporation, Chennai. Biophytum reinwardtii was collected from Rampachowdavaram forest in East Godavari and approved by Dr. Prasanna Kumari of the Department of Botany, D.N.R College, and Bhimavaram. The Department of Pharmacology, Shri Vishnu College of Pharmacy, and Bhimavaram maintained a sample voucher. Dried Whole plant was used for synthesis of platinum nanoparticles.
Synthesis of Biophytum reinwardtii-Stabilized Platinum Nanoparticles (BR-PtNPs)
Platinum nanoparticles were prepared using a generic approach, with a few minor alterations. 50 g of Biophytum reinwardtii (Slender Tree Plant) leaves were taken in a 250 mL beaker containing 100 mL of distilled water for Biophytum reinwardtii leaves extract (BRE), which was then kept at 80°C for two hours before being decanted. A 0.45 µ & 0.2 µ Millipore membrane filter was used to filter the solution respectively. In an Erlenmeyer flask at room temperature, 40 mL of a 1 mM solution was mixed with 10 mL of an extract derived from Biophytum reinwardtii leaves, resulting in the formation of platinum nanoparticles. The mixture’s transformation of pale yellow to dark brown indicates the presence of platinum nanoparticles. The concentration of 6 × 10−4 M metal nanoparticles was present in the solution. The authors found more stable and no visible changes after storing it in a closed container 24.
Characterization
The methods previously described 25 were used to characterise the produced nanoparticles. UV absorbance measurements were made to determine the stability and identification of the BR-PtNPs. The retention of nanoparticles in all the mixes was confirmed at absorbance at 330-380nm. The size of nanoparticle was determined by using JEOL TEM SCAN 2000EX at 80KeV. In this method, sample suspension was placed on standard carbon coated grids and dried for 30 minutes by using an IR lamp. Using Bruker Tensor 27 FTIR spectrometer, analysis of nanoparticles is done at 2000-400 cm-1 spectral range. Energy dispersive analysis of nanoparticles is done by JEOL EDX JSM-5610 LV.
Oxidation of NADH by BR-PtNPs
Chemical transformation in BR-PtNPs via NADH oxidation is investigated with UV-VIS surface plasmon resonance at 200-800nm. During the experiment, 100 𝜇m NADH was subjected to a 2-hour incubation period with 50 𝜇g/mL of BR-PtNPs dissolved in water at room temperature. Subsequent to incubation, it underwent centrifugation, and the resulting mixture is dispersed using an equal water volume. This entire procedure iterated 8-10 times for comprehensive analysis 26.
Evaluation of Neuroprotective Activity on MPTP-induced Parkinson’s Disease in Zebra Fish
Animals
The adult type Zebra fish were procured from a local aquarium shop in Vijayawada. Fishes were kept at 12:12 hrs light and dark cycle. Fishes were acclimated for at least 2 weeks before experiment 27.This protocol was approved by Shri Vishnu College of Pharmacy, Institutional Animals Ethics Committee (IAEC). IAEC NO: 439/PO/01/a/CPCSEA.
Experimental Groups
In this research, experimental fishes are divided into 5 groups, each containing 8 fishes.
Group I is considered as Normal group; Group II is toxic group, means treated with MPTP 225 mg/kg, i.p. for 5 days; Group III, IV, and V are treatment groups means treated with MPTP (225 mg/Kg) with 0.3 µmol, 0.4 µmol, and 0.5µmol of BR-PtNPs, respectively for 5 days. After 5 days, fishes were subjected to evaluation of behavioural parameters. At the end, all fishes were sacrificed. The brain was isolated and various antioxidants, complex I activity, and catecholamine levels were estimated-28.
Loco motor Activity Assessment
The fish movements were evaluated as per protocol of Xia. 2010, including some modifications 28. In this research we took 30 cm*10 cm*15 cm tank and filled it with 3 litres of water. Now the tank was divided into four segments and placed a transparent plastic film on each segment. Now fishes are placed individually in the tank and recorded the movements of fishes like swimming behaviour, distance travelled, means speed for 5 minutes, and also observed the movement of fish from one segment to another for 5 minutes video observation.
Evaluation of antioxidants levels and Complex I activity in fish brain samples
After behavioural assessment, supernatants of brain were collected for estimating various antioxidants like lipid peroxidation, glutathione, superoxide dismutase, glutathione peroxidase, catalase levels & complex I activity in crude mitochondrial preparation in zebra fish’s brain using standard protocols 29-30.
Catecholamine Measurements
In the fish brain homogenate, the dopamine along with its metabolites is estimated as per Luo et. al.. One half volume of supernatant liquid is mixed with 0.3 M potassium dihydrogen phosphate, 0.02 M potassium citrate & 0.002 M sodium EDTA and incubated for 1 hour in ice bath. After incubation the mixture was centrifuged at 15000 rpm at 5ºC. Now the supernatant layer was collected and analysed for catecholamine like dopamine and DOPAC estimation by HPLC 31-36.
Results
Preparation of Biophytum reinwerdtii Platinum Nanoparticles (BR-PtNPs).
In this research, the platinum coated nanoparticles of Biophytum reinwerdtii are synthesized with chloroplatinic acid interaction. The synthesised nanoparticles exhibit brown colour due to reduction of platinum ions (as shown in Figure 1).
 |
Figure 1: Synthesized nanoparticles extract with 1Mm PtCl6 before (A) and after (B) reaction. |
Characterization of Biophytum reinwerdtii Platinum Nanoparticles (BR-PtNPs).
By using UV Visible spectroscopy, formation of BR-PtNPs is conformed to a surface plasmon absorption maximum at ∼340 nm (as shown in Figure 2). Spherical nanoparticles of 5-20 nm size were analysed by using TEM analysis (As shown in Figure 3).
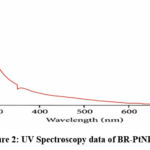 |
Figure 2: UV Spectroscopy data of BR-PtNPs |
 |
Figure 3: TEM image of BR-PtNPs |
The compositional analysis and purity of nanoparticles were assessed by EDX and got a strong Pt signal (as shown in Figure 4). The functional group analysis was performed by using FTIR and got prominent bands at 616, 887, 1015, 1049, 1270, 1389, & 1705 cm-1 and the peaks were allotted to C-N stretching vibrations of aliphatic amines, Phenolic –N stretching of aromatic amines, terminal methyl, C=C groups or aromatic ring, & carboxyl groups respectively (as shown in Figure 5). Based on these results, BR-PtNPs were found to contain alcohols, ketones, carboxylic acids, aldehydes and flavonoids.
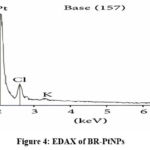 |
Figure 4: EDAX of BR-PtNPs |
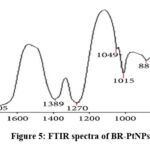 |
Figure 5: FTIR spectra of BR-PtNPs |
The oxidation of NADH by BR-PtNPs was resulted by incubation of 50µg/ml nanoparticles with 100µM NADH for 3 & 6 hrs respectively. The absorbance is maximum at 260nm and minimum at 360nm (as shown in Figure 6). This indicates oxidation NADH by BR-PtNPS.
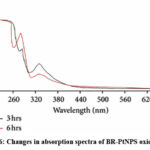 |
Figure 6: Changes in absorption spectra of BR-PtNPS oxidise NADH. |
Effect of Biophytum reinwerdtii Platinum Nanoparticles (BR-PtNPs) on Biochemical Parameters
Administration of MPTP enhances the oxidative stress in zebra fishes leading to increased production of reactive oxygen species. These enhances the production of MDA and decreases the levels of SOD, GSH, GPx, and CAT when compared to normal fishes (as shown in Figure 7) whereas BR-PtNPs (0.5 µmol/kg) treated fishes shows significant decrease in the levels of MDA and increase in the levels of SOD, GSH, GPx and CAT. This clearly indicates that the BR-PtNPs showed protection in MPTP induced Parkinson disease in zebra fishes.
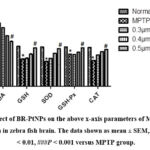 |
Figure 7: Effect of BR-PtNPs on the above x-axis parameters of MTPT induced Parkinsonism in zebra fish brain. The data shown as mean ± SEM,. 𝑛 = 6–8, ∗∗𝑃 < 0.01, ###𝑃 < 0.001 versus MPTP group. |
Effect of Biophytum reinwerdtii Platinum Nanoparticles (BR-PtNPs) on Complex I
The primary site for ROS production is complex I site in mitochondria. In few oxidative stress conditions like Parkinsonism, high levels of ROS generations is observed due to suppression or inhibition of complex I activity. In this current research, more ROS production is observed due to inhibition of complex I activity in MPTP treated fishes (as shown in Figure 8), whereas 0.5 µmol/kg BR-PtNPs treated fishes showed low levels of ROS generation because the nanoparticles enhanced the activity of complex I in mitochondria. This indicates BR-PtNPs showed protection activity in MPTP induced Parkinson disease in zebra fishes.
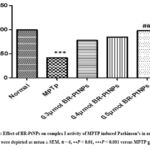 |
Figure 8: Effect of BR-PtNPs on complex I activity of MPTP induced Parkinson’s in zebra fish. Data were depicted as mean ± SEM. 𝑛 = 6, ∗∗𝑃 < 0.01, ∗∗∗𝑃 < 0.001 versus MPTP group. |
Effect of Biophytum reinwerdtii Platinum Nanoparticles (BR-PtNPs) on Catecholamines
The lower levels of dopamine with its metabolites like dihydroxy phenyl acetic acid and homo vanillic acid are observed in the post-mortem brain of patients with Parkinsonism. In our research, the levels of dopamine and its metabolites are significantly decreased in MPTP treated fishes compared to normal fishes which indicates MPTP significantly induced the Parkinson’s condition (as shown in Figure 9) whereas, the levels of dopamine and its metabolites are significantly increased in BR-PtNPs treated fishes. This indicates nanoparticles exhibit neuroprotection against MPTP induced Parkinson’s in fishes.
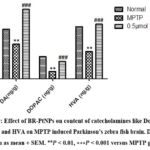 |
Figure 9: Effect of BR-PtNPs on content of catecholamines like Dopamine, DOPAC and HVA on MPTP induced Parkinson’s zebra fish brain. Data were shown as mean + SEM. **𝑃 < 0.01, ∗∗∗𝑃 < 0.001 versus MPTP group. |
Effect of Biophytum reinwerdtii Platinum Nanoparticles (BR-PtNPs) on Locomotor Activity
MPTP administered fishes showed significant reduction in locomotor functions like movement, mean distance per movement, & mean velocity than the control fishes (as shown in Figure 10). This is mainly due to dopamine loss in brain due to MPTP. However, these effects are like loco motor activity reversal in case of fishes treated with BR-PtNPs especially 0.5 µmol/kg. This protective action may be due to reduction in ROS generation in brain.
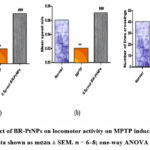 |
Figure 10: Effect of BR-PtNPs on locomotor activity on MPTP induced Parkinson’s zebra fish. Data shown as mean ± SEM. 𝑛 = 6–8; one-way ANOVA test was done |
Discussion
Our novel method of creating Biophytum reinwerdtii phytochemicals coated platinum nanoparticles (BR-PtNPs) is 100% biogenic and involves the direct interaction of chloroplatinic acid with Biophytum reinwerdtii extract in aqueous media. No external synthetic chemicals are used in this process. A deep brown color was seen in the BR-PtNPs colloidal solution, indicating a reduction in platinum ions. (figure 1). The formation of BR-PtNPs was further confirmed by tracing the reaction with UV Visible spectroscopy. The absorption spectrum of the brown platinum collides prepared by biogenic process showed a surface plasmon absorption band with a maximum of ∼340 nm (figure 2). TEM analysis exposes mostly spherical shaped platinum nanoparticles of approximate size of 5–20 nm (Figure 3). Under careful observation, it was evident that the edges of the particles were lighter than the centres, suggesting that some bioorganic compounds such as proteins in Biophytum reinwerdtii extract capped the platinum NPs contributing to excellent robustness against agglomeration.
The compositional analysis through energy dispersive X-ray (EDX) spectrometers illustrated the purity of the platinum, with the spectra showing a strong Pt signal (Figure 4).Prominent bands were observed in the FTIR spectra (figure 5) at 616, 887, 1015, 1049, 1270, 1389, and 1705 cm−1, these peaks are assigned to alcohols C–N stretching vibration of aliphatic amines, phenolic groups, C– N stretching vibration of aromatic amines, germinal methyls, C=C groups or aromatic rings, and carbonyl groups, respectively. These results indicate that phytochemicals of BM leaf extract like flavonoids that have functional groups of amines, alcohols, ketones, aldehydes, and carboxylic acid are robustly coated over the platinum nanoparticles synthesized. Because of the phytochemical coating and the redox chemistry of BR-PtNPs, it is possible that they are biologically active as antioxidants.
To determine if BR-PtNPs can oxidize NADH, 100 𝜇M NADH was incubated with 50 𝜇g/mL BR-PtNPs for 3 h and 6 h, respectively. The absorbance decreased and increased with time at 340 and 260 nm, respectively (figure 6).This observation indicated that BR-PtNPs oxidized. NADH to NAD +. This is because the bands at 340 and 260 nm are from the n-𝜋∗ transition of dihydronicotinamide part and 𝜋∗-𝜋∗ transition of the adenine ring, respectively. This result demonstrates that BR-PtNPs have an activity similar to mitochondrial NADH: Ubiquinone oxidoreductase, which is concurrence with the earlier published results of pectin protected platinum nanoparticles. This suggests that BR-PtNPs are a potential medicinal substance for oxidative stress mediated disease with suppressed mitochondrial complex I, namely, Parkinson’s disease (PD).
Oxidative stress was generated in zebra fish by exposure to MPTP, which is an intracellular free radical-generating compound resulting in corresponding Parkinson symptoms. The administration of a single dose of MPTP (225 mg/kg bwt) resulted in a profound increase in the levels of MDA, diminished activities of antioxidant defence mechanism in charge for scavenging free radicals and maintaining redox homeostasis such as SOD, CAT, GPx, GSH, and complex I were observed in experimental Parkinsonism induced group (MPTP) (Figure 7).The BR-PtNPs concentrations tested were 0.3, 0.4, and 0.5 𝜇mol, respectively. The MDA levels were significantly decreased by 0.5 𝜇mol of BR-PtNPs. This makes clear the inhibitory effect of BR-PtNPs over ROS generation during MPTP-induced oxidative stress.
The activities of antioxidant defense enzymes in charge for scavenging free radicals and maintaining redox homeostasis such as GSH, SOD, catalase, and glutathione peroxidase are diminished during oxidative stress induced by MPTP. In the present study, a statistically significant increase in the levels of GSH, SOD, catalase, and glutathione peroxidase in the MPTP treated zebrafish with 0.5 𝜇mol of BR-PtNPs is being proved. This study demonstrates that BR-PtNPs act as reductive catalyst, by the ability to scavenge ROS, superoxide anion radicals (O2 −), and hydrogen peroxide (H2O2)
The mitochondrial respiratory chain, especially at complexes I, is thought of as a primary site of ROS generation. In some oxidative stress diseases such as Parkinson’s disease, excessive ROS generation is responsible for pathogenesis due to the suppression of complex I. In the current study a significant inhibition of complex I activity was observed in the experimental Parkinsonism-induced group (Figure 8) which was attenuated by the pre-treatment of various concentrations of BR-PtNPs. However, 0.5 𝜇mol of BR-PtNPs demonstrated a noteworthy effect on restoring the complex I activity as well as the levels of GSH, SOD, catalase, and glutathione peroxidase in the MPTP treated zebra fish. This result demonstrates that BR-PtNPs serve dual functions as mitochondrial complex I to lower ROS generation and as SOD/catalase mimetics to scavenge generated excessive ROS.
Post-mortem studies provided evidence for the decrease in the content of dopamine (DA) and its metabolites dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) in the brains of Parkinson’s disease. Our results showed that the DA, DOPAC, and HVA contents in MPTP zebra fish were markedly lower than those of control fish, and BR-PtNPs increased DA, DOPAC, and HVA levels (Figure 9)
In Parkinson’s disease, the most debilitating symptom of the disease is the loss of motor control. Figure 10 shows the results for the locomotion activity. MPTP administration results in a significant reduction in the total movement distance, mean velocity, and mean distance per movement in zebra fish compared to the control animals. This finding points the correlate loss of dopamine due to MPTP neurotoxicity. However, these reductions were significantly improved in BR-PtNPs (0.5 𝜇mol/kg body weight) treatment animals. Our current results suggest that BR-PtNPs may be potentially effective in protecting against ROS mediated disease, by scavenging ROS under pathophysiological conditions.
Conclusion
In this current research, platinum nanoparticles were synthesised by using Biophytum reinwardtii extract. The particles size (5-20 nm) were determined by EDAX and TEM analysis. FTIR studies suggested that nanoparticles stabilise and prevent the agglomeration of particles. Neuroprotection of BR-PtNPs were attained by increasing locomotor functions of fishes, decreasing the levels of free radicals, and increasing the amount of antioxidant, complex I activity, and catecholamine’s levels in fish brain when compared to MPTP treated fishes. Future research is required to explore the significant neuroprotection mechanism in MPTP induced Parkinsonism in fishes.
Liquid Chromatography; EDAX- Energy-dispersive X-ray spectroscopy.
Acknowledgment
All authors are thankful to Management of School of Pharmaceutical sciences; Nasik and Shri Vishnu College of Pharmacy, Bhimavaram for provide facilities to conduct this research work
Conflict of Interest
The authors do not have any conflict of interest
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Data Availability Statement
This statement does not apply to this article.
Ethical Statement
This protocol was approved by Shri Vishnu College of Pharmacy, Institutional Animals Ethics Committee (IAEC). IAEC NO: 439/PO/01/a/CPCSEA.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
References
- Ricciardi, L., Apps, M. & Little, S. Uncovering the neurophysiology of mood, motivation and behavioural symptoms in Parkinson’s disease through intracranial recordings. npj Parkinsons Dis.2023; 9:120-136
CrossRef - Ramírez-Carreto RJ, Zaldívar-Machorro VJ, Pérez-Ramírez DJ, Rodríguez-López BE, Meza C, García E, Santamaría A, Chavarría A. Oral Administration of Silybin Protects Against MPTP-Induced Neurotoxicity by Reducing Pro-inflammatory Cytokines and Preserving BDNF Levels in Mice. Mol Neurobiol. 2023;60(12):6774-6788.
CrossRef - Omar NA, Kumar J, Teoh SL. Parkinson’s disease model in zebra fish using intraperitoneal MPTP injection. Frontiers in Neuroscience. 2023; 25(17):1236049.
CrossRef - Mustapha M, Taib CN. MPTP-induced mouse model of Parkinson’s disease: A promising direction for therapeutic strategies. Bosnian journal of basic medical sciences. 2021;21(4):422.
CrossRef - Quispe, Cristina. “Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases.” Oxidative medicine and cellular longevity vol. 2021; 15:3149223.
CrossRef - Burns J, Buck AC, D’ Souza S, Dube A, Bardien S. Nanophytomedicines as Therapeutic Agents for Parkinson’s Disease. ACS Omega. 2023 ;8(45):42045-42061.
CrossRef - Jagaran K, Singh M. Lipid Nanoparticles: Promising Treatment Approach for Parkinson’s Disease. Int J Mol Sci. 2022 19;23(16):9361.
CrossRef - Jan H, Gul R, Andleeb A, Ullah S, Shah M, Khanum M, Ullah I, Hano C, Abbasi BH. A detailed review on biosynthesis of platinum nanoparticles (PtNPs), their potential antimicrobial and biomedical applications. Journal of Saudi Chemical Society. 2021;25(8):101297.
CrossRef - Fahmy SA, Preis E, Bakowsky U, Azzazy HME. Platinum Nanoparticles: Green Synthesis and Biomedical Applications. Molecules. 2020;25(21):4981
CrossRef - Mikhailova EO. Green Synthesis of Platinum Nanoparticles for Biomedical Applications. J Funct Biomater. 2022;13(4):260.
CrossRef - Jeyapaul U, Kala M. J, Bosco A. J, Piruthiviraj P, Easuraja M. An Eco-friendly Approach for Synthesis of Platinum Nanoparticles using Leaf Extracts of Jatropa Gossypifolia and Jatropa Glandulifera and its Antibacterial Activity. Orient J Chem 2018;34(2)
CrossRef - Malode, U., Patil, Y.S., Selokar, Y.N. Sustainable approaches for the synthesis of biogenic platinum nanoparticles. Bull Natl Res Cent 2023;47:130.
CrossRef - Ali NH, Mohammed AM. Biosynthesis and characterization of platinum nanoparticles using Iraqi Zahidi dates and evaluation of their biological applications. Biotechnology Reports. 2021;30:e00635.
CrossRef - Renju Krishna V. A study on field level variability and diversity in Biophytum Reinwardtii (zucc.) Klotzsch. Var. Reinwardtii. International journal of agriculture sciences.2018;10(13):6508-6509.
- Nagaraju Bandaru, Y.Kranthi, K.Prasad , K.Chandrasekhar. Evaluation of anti cataractogenic activity of Biophytum Reinwardtii on olanzapine induced cataract on isolated goat lens. J. Pharm. Sci. & Res. 2019;11(6):2121-2126
- Fisseha, Nebeyi. “Anticonvulsant Activity of Hydro Alcoholic Extract and Solvent Fractions of Biophytum umbraculum Welw. Syn (Oxalidaceae) Root in Mice.”Journal of experimental pharmacology.2022;14:291-299.
CrossRef - Luqman, E. M., Hestianah, E. P., Widjiati, W., Kuncorojakti, S., & Hendrawan, V. F. Beneficial effects of kebar grass (Biophytum petersianum klotzsch) ethanol extract to increase motor reflex and spatial memory in mice offspring (Mus musculus) from lactating mothers exposed to carbofuran. Research in pharmaceutical sciences, 2022; 17(3), 324–333
CrossRef - L. S. Varghese, P. S. Sreekkutty, G. Purushothaman, D. G. Muricken and E. George. A study on the in-vitro antifungal, larvicidal and antioxidantactivities of root and shoot of Biophytum Sensitivum (linn.) Dc. IJPSR, 2018; 9(10): 4248-4255.
- Pawar AT, Vyawahare NS. Protective effect of standardized extract of Biophytum Sensitivum against calcium oxalate Urolithiasis in rats. Bulletin of Faculty of Pharmacy, Cairo University. 2015;53(2):161-72.
CrossRef - Kavitha M, Jeyaraj S, Muthukumar P, Jeevagan AJ. Green synthesis of silver nanoparticles using Biophytum sensitivum extract and its electrocatalytic activity towards dioxygen reduction. Materials Today: Proceedings. 2021 1;47:1767-72.
CrossRef - Malterud KE. Ethnopharmacology, chemistry and biological properties of four Malian medicinal plants. Plants. 2017; 21;6(1):11.
CrossRef - Liu L, Jing Y, Guo A, Li X, Li Q, Liu W, Zhang X. Biosynthesis of platinum nanoparticles with Cordyceps flower extract: characterization, antioxidant activity and antibacterial activity. Nanomaterials. 2022;12(11):1904.
CrossRef - Faisal, S., Tariq, M.H., Abdullah . Bio synthesis, comprehensive characterization, and multifaceted therapeutic applications of BSA-Resveratrol coated platinum nanoparticles. Sci Rep. 2024;14:7875.
CrossRef - Eltaweil AS, Fawzy M, Hosny M, Abd El-Monaem EM, Tamer TM, Omer AM. Green synthesis of platinum nanoparticles using Atriplex halimus leaves for potential antimicrobial, antioxidant, and catalytic applications. Arabian Journal of Chemistry. 2022;15(1):103517.
CrossRef - Aruna K and Kavitha K. Synthesis and Characterization of Platinum Nanoparticles (Pt-NPs) from Centella Asiatica L Leaf Extract. Annals of R.S.C.B. 2021; 25(5):16-22
- Jeyaraj M, Gurunathan S, Qasim M, Kang MH, Kim JH. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials (Basel).;9(12):1719.
CrossRef - Nagaraju Bandaru, A. Narayana Rao,Alekhya ketha,N. Srilaxmi4,Yasho Deepika, Nalini Panatula,Marshet Getachew Argaw. Protective activity of ferulic acid on rotenone-induced Neurodegeneration in Zebra-fish model. Journal of Survey in Fisheries Sciences.2023;10(2S):2386-2397
- Li X, Gao D, Paudel YN, Li X, Zheng M, Liu G, Ma Y, Chu L, He F, Jin M. Anti-Parkinson’s disease activity of sanghuangprous vaninii extracts in the MPTP-induced zebrafish model. ACS Chemical Neuroscience. 2022;13(3):330-9.
CrossRef - Bashirzade AA, Cheresiz SV, Belova AS, Drobkov AV, Korotaeva AD, Azizi-Arani S, Azimirad A, Odle E, Gild EY, Ardashov OV, Volcho KP. MPTP-treated zebrafish recapitulate ‘late-stage’Parkinson’s-like cognitive decline. Toxics. 2022 ;10(2):69.
CrossRef - Pensado-López A, Fernández-Rey J, Reimunde P, Crecente-Campo J, Sánchez L, Torres Andón F. Zebrafish Models for the Safety and Therapeutic Testing of Nanoparticles with a Focus on Macrophages. Nanomaterials (Basel). 2021 ;11(7):1784.
CrossRef - Chia K, Klingseisen A, Sieger D, Priller J. Zebrafish as a model organism for neurodegenerative disease. Front Mol Neurosci. 2022;15:940484.
CrossRef - Eduard Dumitrescu, Aaditya Deshpande, Kenneth N. Wallace, Silvana Andreescu. Time-Dependent Monitoring of Dopamine in the Brain of Live Embryonic Zebrafish Using Electrochemically Pretreated Carbon Fiber Microelectrodes. ACS Measurement Science Au.2022;2:261-270.
CrossRef - d’Amora M, Schmidt TJN, Konstantinidou S, Raffa V, De Angelis F, Tantussi F. Effects of Metal Oxide Nanoparticles in Zebrafish. Oxid Med Cell Longev. 2022 ;2022:3313016
CrossRef - Nellore, Jayshree, Pauline, Cynthia, Amarnath, Kanchana, Bacopa monnieri Phytochemicals Mediated Synthesis of Platinum Nanoparticles and Its Neurorescue Effect on 1-Methyl 4-Phenyl 1,2,3,6 Tetrahydropyridine-Induced Experimental Parkinsonism in Zebrafish, Journal of Neurodegenerative Diseases, 2013;8: 972391
CrossRef - Qiang L, Arabeyyat ZH, Xin Q, Paunov VN, Dale IJF, Lloyd Mills RI, Rotchell JM, Cheng J. Silver Nanoparticles in Zebrafish (Danio rerio) Embryos: Uptake, Growth and Molecular Responses. Int J Mol Sci. 2020 ;21(5):1876.
CrossRef - Haque E, Ward AC. Zebra fish as a model to evaluate nanoparticle toxicity. Nanomaterials. 2018;8(7):561.
CrossRef








