Manuscript accepted on :05-09-2024
Published online on: 24-09-2024
Plagiarism Check: Yes
Reviewed by: Dr. Ramlah Kadir and Dr. Nitish Chaudhari
Second Review by: Dr. Preeti Yaduvanshi Parmar
Final Approval by: Dr. Anton R Keslav
Qubaa Ahmed Elzubair1 , Mohamed Alfaki2
, Mohamed Alfaki2 , Musaab Ahmed3
, Musaab Ahmed3 , Khalid Sukar4,7
, Khalid Sukar4,7 , Sara Mohammed4
, Sara Mohammed4 , Sofiyat Zayyad4
, Sofiyat Zayyad4 , Salma Elnour4
, Salma Elnour4 , Salma Mohamed5 and Abdalraheem Babiker6
, Salma Mohamed5 and Abdalraheem Babiker6 , Asaad Babker4
, Asaad Babker4 , Alaa Abdalhadi8
, Alaa Abdalhadi8 and Marwan Ismail4*
and Marwan Ismail4*
1Department of Histopathology and Cytology, Alemadi Hospital, Doha, Qatar.
2Sidra Medicine, Doha, Qatar.
3Gezira Centre for Gastrointestinal Tract Endoscopy and Laparoscopic Surgery, Gezira, Sudan.
4College of Health Sciences, Gulf Medical University, Ajman, United Arab Emirates
5Department Histopathology and Cytopathology, Wad Medani College of Medical Sciences and Technology, Doha, Qatar.
6Department Histopathology and Cytopathology, Collage of Medical Laboratory Sciences, Wad Madani, Sudan.
7Faculty of medicine, University of Bakht Alruda, Sudan.
8Primary Health Care, Purelab, Purehealth, Fujairah, UAE.
Corresponding Author E-mail: marwan@gmu.ac.ae
DOI : https://dx.doi.org/10.13005/bpj/2995
Abstract
Introduction: Worldwide, breast cancer is the most prevalent cancer among women to be diagnosed, and it is the primary cause of cancer-related mortality, coming in second only to lung cancer. High levels of Ki67, a nuclear marker of cell proliferation, in breast cancer are linked to worse outcomes. Methods and materials: This retrospective cross-sectional laboratory investigation aimed to examine Ki67 expression as a prognostic predictor in invasive ductal carcinoma (IDC) utilizing manual tissue microarrays (MTMAs) technology. The study was done from June 2018 to July 2019 at the Elrahman Health Centre in Khartoum, Sudan, using thirty-five paraffin block samples collected from patients previously diagnosed with invasive ductal carcinoma (IDC). The study population ranged in age from 31 to 71 years. Results: The study found that 94.3% (n=33/35) of the tissues were positive for the Ki67 antigen, while 5.7% (n=2/35) were negative. Age and score correlation is (P=0.047), and a favorable prognosis could be the cause of the two unfavorable results. Conclusion: This study highlights the importance of the Ki67 biomarker as a prognostic indicator in invasive ductal carcinoma (IDC) of the breast. High levels of Ki67 expression (94.3%) were associated with more aggressive tumors and poorer prognostic outcomes. However, there was no significant correlation between Ki67 scores and patient age, indicating age does not influence the prognostic value of Ki67 in this cohort.
Keywords
Breast cancer; Invasive Ductal Carcinoma; Ki-67; Tissue Microarrays
Download this article as:| Copy the following to cite this article: Elzubair O. A, Alfaki M, Ahmed M, Sukar K, Mohammed S, Zayyad S, Elnour S, Mohamed S, Babiker A, Babker A, Abdalhadi A, Ismail M. Evaluation of Ki67 Biomarker as a Prognostic Marker in Breast Invasive Ductal Carcinoma in Khartoum State in Sudan. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Elzubair O. A, Alfaki M, Ahmed M, Sukar K, Mohammed S, Zayyad S, Elnour S, Mohamed S, Babiker A, Babker A, Abdalhadi A, Ismail M. Evaluation of Ki67 Biomarker as a Prognostic Marker in Breast Invasive Ductal Carcinoma in Khartoum State in Sudan. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3XDFrw1 |
Introduction
Breast cancer is the most common malignancy in women worldwide and the primary cause of cancer-related deaths. Breast cancer is becoming an increasingly critical concern in low- and middle-income countries (LMICs), where incidence rates have risen by up to 5% every year 1. Hulka and colleagues defined biological markers as cellular, biochemical, or molecular changes in biological media. They are used for disease prediction, etiology, diagnosis, progression, regression, and treatment outcome, and have expanded to include biological properties 2. Tissue microarrays (TMAs) are created by extracting punches from paraffin-embedded tissue blocks and transferring them to a positionally encoded array. While not used for clinical diagnosis, they offer advantages over traditional histological sections, allowing hundreds of analyses per microarray. 3
The process of multiplying a cell is known as cell proliferation, and it is characterized by the equilibrium between cell divisions and cell loss via differentiation or death. Tumors exhibit increased cell division.4
Ki-67 expression changes during the cell cycle, raising concerns that cycling cells may be misclassified as resting cells. According to the data as a whole, Ki-67 levels are low throughout the G1 and early S phases and progressively rise to a peak during mitosis. Anaphase is the start of a sharp decline in expression 5.
There is a lot of interest in using cell proliferation indicators to help classify cancers, notably malignant lymphoma, as well as possible indications of therapeutic responsiveness to chemotherapeutic drugs. Two types of antibodies can be utilized successfully on paraffin sections: Ki67 (MIB1) and PNCA. The G1, S, G2, and M phases of the cell cycle are those in which proliferating cells express a nuclear antigen that is recognized by the monoclonal antibody Ki67. G0 cells do not express Ki67 while they are in the resting phase. An extremely labile epitope that is only reliably found in frozen sections or cytological materials is recognized by the Ki67 antibody 6. Newer antibodies have recently been produced against recombinant portions of the Ki67 molecule. MIB1 is a robust reagent for Ki67 expression assessment in breast cancer, enhancing prognostic capabilities 7. It can be used consistently in formalin-fixed paraffin-embedded tissues after microwave antigen retrieval.
In Sudan, the incidence of breast cancer has been raising to be the most common cancer 8.The objective of this investigation was to ascertain the proliferative index level utilizing the immunohistochemical marker Ki67 in connection with the histological grade of breast cancer. Additionally, we sought to investigate the correlation between the level of Ki67 marker expression and the histological grade of breast cancer, as well as the relationship between proliferative marker Ki67 intensity and patient age and IHC score.
Materials and Methods
This is a retrospective cross-sectional laboratory investigation conducted from June 2018 to July 2019 at the Alrahma Medical Laboratory in Khartoum, Sudan. In 2018, Breast cancer tissues were histologically examined using hematoxylin and eosin-stained slides, then Tissue Microarrays technique (TMAs) and Ki67 monoclonal antibody stained for microscopical examination.
This study included only invasive ductal carcinoma cases with complete information about age, diagnosis, and histological grade. Ethical considerations for Alrahma Medical Laboratory in Khartoum. Data was acquired from a master sheet of patient data, which included age and histological grade, with the type of cancer previously classified as invasive ductal carcinoma (IDC). The procedure involved a surgeon taking a biopsy or breast resection specimen, depositing it in formalin, and analyzing it to select and cut tissue sections.
Two tissue blocks were stained and cut into sections for Hematoxylin and Eosin stain and immunohistochemistry techniques, mounted on glass slides and backed at 60 C for six hours.
Manual Tissue Arrayer
A paraffin block was created by pouring liquid paraffin into a mold, covered with a tissue cassette, and then examined for bubbles or holes. Extra paraffin from plastic cassette was used to identify donor tissue blocks’ regions of interest, determine ideal TMA architecture, and create a TMA block summary. The TMA 1 arrayer’s two punches were aligned on an empty paraffin block, ensuring proper alignment and similar centers in the circular indents they create. Tighten the adjustment nut to the required depth within the Paraffin block, typically 0.5-1 mm above the plastic tissue cassette base.
The process involved creating holes in the array’s first position, adjusting micrometers, releasing tissue punches, discarding paraffin wax, and moving the bigger punch into the sampling position, removing the donor block bridge.
The donor block was manually held on the donor block bridge, and the tissue core region was sampled beneath the sample punch, which was pushed downward. The tissue microarray (TMA) is created by creating a gap of 0.8-1 mm between sample centers. The TMA is then sectioned using an adhesive-side-down tape window, a microtome blade, and a 5-micrometer slice. The tissue is then placed on a microscopic slide and cured using a UV lamp. The slide was placed in TPC SOLVENT for 3 minutes, then a fresh section was cut for H&E staining, Ki67 staining, and immunohistochemistry (IHC) was performed.
Results
Breast cancer is the most prevalent cancer in women worldwide, and it has high Ki67 expression. 35 tissue samples previously diagnosed with aggressive breast ductal carcinoma were used in this retrospective study to evaluate the expression of Ki67 proliferative marker as a prognostic marker in invasive ductal carcinoma using manual tissue microarrays (MTMA). The patients were between the ages of 31 and 71. The age group that was most prevalent was 50-60 years old (31.4%) (11/35), followed by 30- 40 years old patients (10/35) 28.6% (10/35) and 40 to 50 years old patients (10/35) 28.6%, then 60-70 years old (3/35) 8.6%, and finally 70 to 80 years old (1/35) 2.9%, as shown in Figure (1).
The study’s findings revealed that the bulk of samples were grade three (77.1%, 27/35) and grade two (22.9%, 8/35) with no grade one samples Figure (2). The study found that the bulk of IHC positive results in the nucleus were 94.3% (33/35) with only two negative results accounting for 5.7% (2/35), see figure (3). The study found that the majority of TMA scores were (+) 34.3% (12/30), followed by (++) 31.4% (11/35), (+++) 25.7% (9/35) and only one sample had (++++) 2.9% (1/35) and two negative findings 5.7% (2/35) , see figure (4). The investigation revealed that there is no link between histological grade and patient age (P-value = 0.683) and a very weak correlation (0.065) (tables 01). The study found a medium correlation between score and patient age (P-value = 0.047. Tables (2) and (3) show a substantial correlation (P-value = 0.47) between patient age and score.
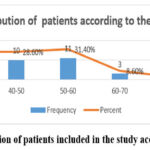 |
Figure 1: Distribution of patients included in the study according to their Age |
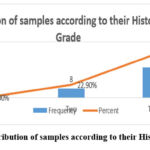 |
Figure 2: Distribution of samples according to their Histological Grade |
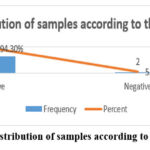 |
Figure 3: Distribution of samples according to their IHC |
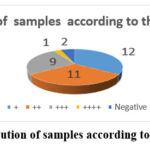 |
Figure 4: Distribution of samples according to their IHC Score. |
|
Chi-Square |
Correlation |
P.value |
|
1.496 |
-.065 |
.683 |
Table 2: The relationship between IHC and patient’s age
|
Chi-Square |
Correlation |
P.value |
|
3.845 |
-.282 |
.279 |
Table 3: The relationship between Score and patient’s age
|
Chi-Square |
Correlation |
P.value |
|
2.994 |
.461 |
.047 |
Table 4: The relationship between Score and TMA
|
Chi-Square |
Correlation |
P. value |
|
35.000 |
.421 |
.000 |
Table 5: The relationship between Grade and Score
|
Chi-Square |
Correlation |
P. value |
|
.488 |
.756 |
.087 |
Discussion
The hallmark of cancer is unchecked proliferation. The most widely used method for measuring tumor growth in breast cancer is the fraction of cells that stain for the nuclear antigen Ki67.
The current study observed that high levels of Ki67 expression corresponded with a prevalent histological grade, with 77.1% of our samples classified as grade III (Figure 2). This finding supports the study conducted by Dowsett and his team, indicating that higher Ki67 levels typically correlate with more aggressive forms of breast cancer and poorer patient outcomes 9. Specifically, Ki67 expression has been shown to be a strong independent prognostic factor in early breast cancer, reinforcing its utility in stratifying patient risk 10.
The current study also found Ki67 positive in 94.3% of specimens (Figure 3), suggesting that proliferative activity is a key characteristic in the majority of IDC cases. Furthermore, our results indicated a significant correlation between Ki67 scores and patient age (P=0.047) (Table 3), hinting at the importance of considering patient demographics when evaluating Ki67 as a prognostic marker. This aspect is particularly relevant given that age is often associated with tumor biology and patient response to treatment 11.
Our study and others have suggested that patients with tumors exhibiting more than 50% proliferation may respond favorably to chemotherapy 12. Conversely, two cases in our study with negative Ki67 results may indicate a better prognosis, potentially representing tumors that are not yet fully proliferative or are in earlier stages of development.
Despite its clinical relevance, the interpretation of Ki67 remains complex due to variability in methodologies used across different laboratories. Scholars, including Alco and his team. 13, have noted that discrepancies in Ki67 measurement could arise from variations in tissue handling, fixation protocols, and antibody specificity. Additionally, there exists an ongoing debate regarding the establishment of an optimal Ki67 cut-off value, which varies significantly among studies and populations 14. Consequently, the need for standardized protocols and guidelines for Ki67 evaluation is paramount to ensure consistent and clinically relevant results across settings.
The integration of Ki67 in multimodal prognostic assessments, alongside established markers such as ER, PR, and HER2, is highly recommended. This combined approach enhances the precision of survival predictions and treatment planning for patients with breast cancer 15 . In our study, the correlation between Ki67 scoring and histological grade (P=0.087, Table 5) indicates that incorporating multiple parameters may offer more robust insights into patient prognosis.
The current study evaluating Ki67 as a prognostic marker in breast invasive ductal carcinoma has several limitations, including its retrospective design and small sample size of only 35 tissue samples, which may affect the generalizability of findings.
Conclusion
The present results indicated the significant role of the Ki67 biomarker as a prognostic indicator in invasive ductal carcinoma (IDC) of the breast. The majority of cases analyzed demonstrated high levels of Ki67 expression (94.3%), which correlate with more aggressive tumor behavior and poorer prognostic outcomes. Importantly, also findings indicate that there is no significant association between Ki67 scores and patient age suggesting that age is not a determining factor in the prognostic impact of Ki67 . Future work should focus on standardization of Ki-67 assessment and specific-cation of its role treatment protocol.
Acknowledgment
The author wishes to express gratitude to Alrahma Medical Laboratory in Khartoum, Sudan, for supporting the research work. Special thanks to the laboratory staff for their assistance and cooperation during the study. The author is also deeply appreciative of the resources and facilities made available, which significantly contributed to the success of this research.
Conflict of Interest
The authors do not have any conflict of interest
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
References
- Francies FZ, Hull R, Khanyile R, Dlamini Z. Breast cancer in low-middle income countries: abnormality in splicing and lack of targeted treatment options. American journal of cancer research. 2020;10(5):1568.
- Hulka BS, Wilcosky T. Biological Markers in Epidemiologic Research. Archives of Environmental Health: An International Journal. 1988;43(2):83-89. doi:https://doi.org/10.1080/00039896.1988.9935831
CrossRef - Nikolaos Pazaitis, Kaiser A. TMA-Mate: An open-source modular toolkit for constructing tissue microarrays of arbitrary layouts. HardwareX. 2023;14:e00419-e00419. doi:https://doi.org/10.1016/j.ohx.2023.e00419
CrossRef - Brown JS, Amend SR, Austin RH, Gatenby RA, Hammarlund EU, Pienta KJ. Updating the definition of cancer. Molecular Cancer Research. 2023;21(11). doi:https://doi.org/10.1158/1541-7786.mcr-23-0411
CrossRef - Ki-67 protein as a tumour proliferation marker. Clinica Chimica Acta. 2019;491:39-45. doi:https://doi.org/10.1016/j.cca.2019.01.011
CrossRef - Đokić S, Gazić B, Grčar Kuzmanov B, Blazina J, Miceska S, Čugura T, Grašič Kuhar C, Jeruc J. Clinical and Analytical Validation of Two Methods for Ki-67 Scoring in Formalin Fixed and Paraffin Embedded Tissue Sections of Early Breast Cancer. Cancers. 2024;16(7):1405-1405. doi:https://doi.org/10.3390/cancers16071405
CrossRef - de Azambuja E, Cardoso F, de Castro G, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12 155 patients. British Journal of Cancer. 2007;96(10):1504-1513. doi:https://doi.org/10.1038/sj.bjc.6603756
CrossRef - M. Abbas A, Shalabi MG, A. Elsiddi S, Eltahir Z, M.A. Babke A, G. Ahmed H. Evaluation of Angiogenesis by Using CD105 and CD34 in Sudanese Breast Cancer Patients. Pakistan Journal of Biological Sciences. 2021;24(11):1144-1151. doi:https://doi.org/10.3923/ pjbs.2021.1144.1151
CrossRef - Dowsett, M., Nielsen, T. O., A’Hern, R., Bartlett, J., Coombes, R. C., & Smith, I. E. (2011). Prognostic value of Ki67 in early breast cancer: a meta-analysis of the literature. Journal of Clinical Oncology, 29(9), 1185-1192. DOI: 10.1200/JCO.2010.28.6962
- Urruticoechea A, Smith IE, Dowsett M. Proliferation Marker Ki-67 in Early Breast Cancer. Journal of Clinical Oncology. 2005;23(28):7212-7220. doi:https://doi.org/10.1200/jco.2005.07.501
CrossRef - Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P, Colleoni M, Denkert C, Piccart-Gebhart M, Regan M, Senn HJ. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Annals of Oncology. 2019;30(10):1541-1557. doi:https://doi.org/10.1093/annonc/mdz235
CrossRef - Zhu X, Chen L, Huang B, Wang Y, Ji L, Wu J, Di G, Liu G, Yu K, Shao Z, Wang Z. The prognostic and predictive potential of Ki-67 in triple-negative breast cancer. Scientific Reports. 2020;10(1). doi:https://doi.org/10.1038/s41598-019-57094-3
CrossRef - Alco GU, Bozdogan A, Selamoglu D, Pilancı KN, Tuzlalı S, Ordu C, Igdem S, Okkan S, Dincer M, Demir G, Ozmen V. Clinical and histopathological factors associated with Ki67 expression in breast cancer patients. Oncology Letters. 2015;9(3):1046-1054. doi:https://doi.org/10.3892/ol.2015.2852
CrossRef - Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Research and Treatment. 2015;153(3):477-491. doi:https://doi.org/10.1007/s10549-015-3559-0
CrossRef - Nielsen TO, Leung SC, Rimm DL, Dodson A, Acs B, Badve S, Denkert C, Ellis MJ, Fineberg S, Flowers M, Kreipe HH. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. JNCI: Journal of the National Cancer Institute. 2020;113(7). doi:https://doi.org/10.1093/jnci/djaa201
CrossRef







