Manuscript accepted on :22-08-2024
Published online on: 12-09-2024
Plagiarism Check: Yes
Reviewed by: Dr. Sohayla Mohamed
Second Review by: Dr. Randa Salah Goma
Final Approval by: Dr. Prabhishek Singh
Alaa H. Sayed1 , Amira S. Ahmed1*
, Amira S. Ahmed1* , Mahmoud Hozayn2
, Mahmoud Hozayn2 , Ola A. M. Mohawed1
, Ola A. M. Mohawed1 , Hanaa H. Ahmed1,3
, Hanaa H. Ahmed1,3 and Rehab S. Abohashem1,3
and Rehab S. Abohashem1,3
1Hormones Department, Medical Research and Clinical Studies Institute, National Research Centre, Dokki, Giza, Egypt
2Field Crop Research Department, Agricultural and Biological Research Institute, National Research Centre, Dokki, Giza, Egypt
3Stem Cell Lab, Center of Excellence for Advanced Sciences, National Research Centre, Dokki, Giza, Egypt
Corresponding Author E-mail: dr.amira2007@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2966
Abstract
DM is a collection of metabolic disorders brought on by abnormalities in secretion, action, or combination of both of insulin. Nowadays, many efforts are made to change lifestyles to get a moderate outcome with the fewest possible side effects and reduce complications. Although magnetized water (MW) has been promoted since 1930s, it has not received wide approbation since its effectiveness is still in question; however, the therapeutic potential of MW on the body has been reported. This study investigated the impact of MW supplementation on glucose, insulin, antioxidant status, inflammatory condition, DNA fragmentation and gene expression associated with the metabolism of glucose in STZ-induced diabetes in rats. Adult female Wistar rats (6 groups) were used in this study: G1: Control group+ tap water (TW); G2: Control group+ MW; G3: Diabetic group+ TW; G4: Diabetic group+ MW; G5: Diabetic group+ metformin (Met)+ TW; G6: Diabetic group+ Met+ MW. Additionally, lowering serum glucose and raising insulin level, MW consumption repaired DNA damage, enhanced antioxidant status, reduced inflammatory response, and upregulated genes linked to glucose metabolism. Furthermore, as shown by the histological analysis of pancreatic tissue sections, supplementation with MW could reverse the detrimental effects of STZ on the pancreas. This study offers novel insights into how MW consumption can help reduce T2DM by reducing hyperglycemia, restoring the equilibrium between antioxidants and oxidants, reducing inflammatory responses, and altering genes involved in glucose metabolism. Therefore, MW may be used as an adjuvant in T2DM management.
Keywords
Diabetes mellitus; DNA damage; Inflammation; Magnetized water; Metformin; Oxidative stress
Download this article as:| Copy the following to cite this article: Sayed A. H, Ahmed A. S, Hozayn M, Mohawed O. A. M, Ahmed H. H, Abohashem R. S. A comparison of the Anti-diabetic Potential of Magnetized Water, Metformin, and Their Combination in A Rat Model of Type II Diabetes. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Sayed A. H, Ahmed A. S, Hozayn M, Mohawed O. A. M, Ahmed H. H, Abohashem R. S. A comparison of the Anti-diabetic Potential of Magnetized Water, Metformin, and Their Combination in A Rat Model of Type II Diabetes. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/47nM1LD |
Introduction
Diabetes mellitus (DM) is a long-term, complex illness with a wide range of possible side effects. Treatment for the condition can only postpone and extend its latter stages. By 2030, diabetes is expected to rank as the seventh most common cause of death, according to the World Health Organization1. Furthermore, T2DM (non-insulin-dependent DM) is a multifaceted type with lifestyle habits, genetics, and acquired health status affecting the disease progress. Newly validated models of T2DM pathogenesis suggest that nutritional loading causes an increase in blood insulin level, prompting insulin resistance till beta cells failure, challenging the conventional belief that insulin resistance leads to an increase in blood insulin level in T2DM development2. The International Diabetic Federation (IDF) predicts that around 537 million people would be diabetic. It is estimated that by 2045, there will be 783 million people (12.2%) worldwide, up from 643 million (11.3%) in 20303,4. In Egypt, there were an estimated 10.9 million diabetics; by 2030 and 2045, that number is expected to rise to 13 million and 20 million, respectively. Because of this, Egypt is expected to rank ninth among countries with the highest prevalence of DM by 2045, up from its present ranking of tenth4,5.
The two main causes of T2DM are impaired both pancreatic beta-cells secretion of insulin and tissue sensitivity to insulin6. The risk factors comprise multifaceted metabolic, genetic, and environmental factors contributing to its occurrence. Numerous epidemiological researches reveal that T2DM can be prevented by improving physical activity, obesity, and unhealthy food7. Oxidative stress in hyperglycemic conditions is attributed to an imbalance between the generation of reactive oxygen species (ROS) & the cellular antioxidant system that leads to diabetes development. Production of ROS occurs in the endoplasmic reticulum, phagocytic cell, and peroxisomes, whereby the electron transport chain (ETC) of mitochondria plays a crucial function. Increased generation of ROS has the potential to directly alter the structure and function of proteins, lipids, and nucleic acids. Additionally, it alters many intracellular signaling pathways that result in beta-cell dysfunction and insulin resistance8. In addition to causing other hazardous side effects, hyperglycemia also starts the β cell apoptotic process9. Both Type 1 and 2 DM are metabolic conditions with diverse mechanisms, but with a considerable insulin producing beta-cells death10. In diabetes, pancreatic beta-cell death is caused by caspase-mediated apoptosis, which is triggered by inflammatory cytokines11. Therefore, it is believed to be essential to treat diabetes by reducing blood glucose, oxidative stress, and inflammatory responses.
Many therapeutic approaches have been used to treat this condition, including the use of oral hypoglycemic drugs. The two pharmacotherapies now available are insulin & oral hypoglycemic drugs. These drugs either increase the amount of insulin secreted by the pancreas or decrease gluconeogenesis and increase the absorption of glucose, hence lowering the level of plasma glucose. However, these drugs don’t keep the blood glucose levels in balance for a long time, and they have side effects including hypoglycemia, kidney disease, GIT problems, hepatotoxicity, increased cardiac risk, and insulinoma12.
The biguanide antihyperglycemic drug metformin is the most often prescribed treatment for T2DM. Until now, T2DM patients are counseled to use it as their first option13. It is recommended due to its superior serum glucose-diminishing ability, comparatively less adverse effects, low risk of hypoglycemia & minimal weight increase14. The majority of individuals inevitably need combo medication to keep their blood sugar levels under control15. Furthermore, because the symptoms of T2DM deteriorate with time in many individuals, combination medications are eventually required as the initial monotherapy’s efficacy declines16.
While there has been active promotion of the creation of healthy functional food to reduce oxidative stress and control type 2 diabetes, it is anticipated that the benefits of functional water—the building block of our body will be greater17. To improve the quality of drinking water, several chemical & physical treatments have been established18. Tap water is converted to magnetized water (MW) by passing it via magnetic tubes. As a result, the qualities of the water become exceedingly active and fertile, enhancing the oxygen ratio, salt velocity, and amino acid dissolution19. The pH of magnetized water shifts from acidic to alkaline, in contrast to tap water. Moreover, the salinity and weight of magnetized water rise20.
It has been discovered that magnetized water improves blood picture and sexual hormones, as well as blood circulation, oxygenation, nutrition transportation, enzyme activation, and the removal of internal toxins produced via metabolism19. Additionally, MW exhibits strong antioxidant activity and a high degree of rabid diffusion into tissues21,22. In STZ-induced diabetic rats, MW treatment diminishes blood levels of glucose and improves the lipid profile & antioxidant status of the lung, spleen & heart17. Furthermore, MW consumption may be helpful in preventing T2DM blood parameter abnormalities23. Therefore, the present attempt aimed at exploring the antidiabetic impact of magnetized water in a rat model of T2DM and appraising the probable mechanism triggering this effect.
Materials and Methods
Chemicals
The following supplies were acquired: glucose solution (10% w/v), phosphate buffered saline (PBS) with pH 7.4, citrate buffer with pH 4.5, nicotinamide (NA) & streptozotocin (STZ) from biodiagnostic (USA). The 500 mg tablets of metformin hydrochloride were purchased from MINAPHARM Pharmaceuticals Company (10th of Ramadan City, Egypt). The remaining compounds were of the purest grade possible.
Preparation of magnetized water (MW)
Magnetized water was obtained by letting tap water pass through a static magnetic unit constructed by National Research Centre, 33 El-Behouth St., 12622 Dokki, Giza, Egypt with a magnetic intensity of 1500 Gauss24. A hand-held Gauss meter (Hirst Magnetic Instruments, Ltd, UK) with a transverse probe Brand (model Gm07, accuracy ±0.01%) was used to determine and ensure the continuous exposure of the magnetic field dose. The uniform field was vertical to the low speed water flow (0.34/min). An electrical pump kept this speed constant25. The MW was created every day because its effectiveness is limited to 24 hours.
Animals
From the Animal Care Unit of the National Research Centre in Giza, Egypt, the 60 adult female Wistar rats were procured and ranged in weight from 130 to 150 g. To keep the animals survive, we used female rats which are less sensitive to streptozotocin (STZ) and also we used nicotinamide (NA) injection with STZ. The rats were kept in five rats per plastic cage, in an environmentally controlled room with a temperature (of 25±1◦ C), a light (12-hour) and a dark (12-hour) cycle & limitless access to standard rodent food & tap water. The animals were given ten days to acclimate to this environment before the experiment began. The protocol of the experiment was constructed in agreement with the National Institute of Health guidelines and with the approval of the Institutional Ethical Committee for Medical Research, National Research Centre, Egypt (Approval No. 014110622).
Experimental protocol
Following the accommodation period, 20 rats were split into two groups at random (10 rats/group); Group 1: Control+ TW group; which consisted of healthy rats that were intraperitoneally (i.p.) injected with single dose of 0.1 M-citrate buffer (pH 4.5) and allowed to consume tap water (TW) for 4 weeks freely; Group 2: Control+ MW group that were intraperitoneally (i.p.) injected with single dose of 0.1 M-citrate buffer (pH 4.5) and freely allowed to drink MW (1500 gauss) for 4 weeks.The other 40 rats were used for induction of T2DM via sequential injection of nicotinamide (NA) and streptozotocin (STZ). Rats that had fasted overnight were administered the intraperitoneal (i.p.) injection of NA (110 mg/kg) fifteen minutes before the intraperitoneal (i.p.) injection of a freshly prepared STZ solution (25 mg/kg) in 0.1 M-citrate buffer (pH 4.5) 26. After 6 hrs of STZ injection, they were supplied with unlimited access to glucose solution (10% w/v) for the following 24 hrs. From the tail vein, a blood sample was obtained 72 hrs following the STZ injection, and a blood glucose monitoring device (One Touch® UltraMini®, Life Scan, Inc., Milpitas, CA, USA) was used to determine the fasting blood glucose (FBG) level. Rats with levels of glucose above 200 mg/dl were assumed diabetic rats & were assigned into four groups (10 rats in each group); Group 3: Diabetic+ TW group; in which the diabetic rats were freely allowed to drink TW for 4 weeks; Group 4: Diabetic+ MW group; in which the diabetic rats were freely allowed to drink MW (1500 gauss) for 4 weeks; Group 5: Diabetic+ TW+ Met group; in which diabetic rats were freely allowed to drink TW for 4 weeks with simultaneous administration of metformin (200 mg/kg/day) dissolved in distilled water27 by oral gavage; Group 6: Diabetic+ MW+ Met group; in which diabetic rats were freely-allowed to drink MW (1500 gauss) for 4 weeks with simultaneous metformin administration (200 mg/kg/day) by oral gavage.
Sampling
After food deprivation (one night) at the end of the experimental period, the blood samples were drawn from the tail vein. Each sample was separated into two samples. The 1st blood sample was left to clot for 30 minutes at room temperature in order to separate sera. Centrifugation for 15 minutes at 1800 xg and 4°C was used to isolate the clear sera samples, then kept at -20°C till biochemical analyses. For the comet assay, the 2nd blood sample was taken as a whole. Following the collection of blood samples, the rats were euthanized by cervical dislocation and each rat’s pancreas was immediately dissected, quickly washed using isotonic saline. After that, it was divided into 3 parts; (a) 1st part was kept in −80°C for additional RNA and molecular analyses; (b) In formalin saline 10%, 2nd part was fixed for additional immunohistochemical and histopathological examination; (c) In ice cold PBS (pH 7.4), 3rd part was homogenized 28 for further biochemical analysis.
Biochemical Estimations
Estimation of Serum Glucose
Using a colorimetric assay kit purchased from Biodiagnostic CO., Egypt, the serum level of glucose was determined spectrophotometrically according to the operating directions.
Estimation of Serum Insulin
Serum level of insulin (INS) was quantified by ELISA using the rats’ kit provided by MyBioSource, USA in compliance with the manufacturer’s directions.
Estimation of pro-inflammatory and anti-oxidant biomarkers in pancreatic homogenate
By ELISA, pancreatic interleukin-1beta (IL-1β) & tumor necrosis factor- alpha (TNF-α) were analyzedusing kits procured from Cloud-Clone, USA following the manufacturer’s manuals. Reduced glutathione (GSH) and glutathione peroxidase (GSH-Px) were estimated calorimetrically using kits procured from Biodiagnostic CO., Egypt following the manufacturer’s manual.
Estimation of glucose metabolism-related genes by real-Time Polymerase Chain Reaction (qPCR)
Using the RNeasy Mini Kit from Norgen (Canada), total RNA was extracted from pancreatic tissues. By using reverse transcription kit (Norgen, Canada), total RNA (1 μg) was reversely transcribed. The qPCR was executed in compliance with the directions of manufacturer by HERA SYBR Green PCR kit (Willowfort, UK). For qPCR, specific primers of glucagon-like peptide-1 (GLP-1), Transcription factor 7-like 2 (TCF7L2) and housekeeping gene; GAPDH were assigned.
GLP-1 gene primer sequences were F: 5′ACCTTCACCAGCGACGTAAG 3′ and R: 5′ TCCTTTTACAAGCCAAGCGA 3′29. TCF7L2 primer sequences were F: 5′ CCGCCCGAACCTCTAACAAA 3′ and R: 5′ TCAGTCTGTGACTTGGCGTC 3′ [29]. Primer sequences of GAPDH were F: 5′ CACCCTGTTGCTGTAGCCATATTC 3′ and R: 5′ GACATCAAGAAGGTGGTGAAGCAG 3′30.
Under the following conditions, the qPCR was executed; initial denaturation (for 4 min at 94°C), then 40 cycles (for 15 s at 94°C), annealing step (for 20 s at 60°C), extension (for 20 s at 72°C) & final extension step (for 10 min at 72°C). With a total volume of 20 μl, each reaction consists of 12.5 μl SYBR HERA mix, 0.5 μl each primer, 2 μl cDNA & 4.5 μl nuclease free H2O. The main used equations were; ∆Ct = Ct (gene of interest) – Ct (housekeeping gene), then ∆∆Ct = ∆Ct (treated sample) – ∆Ct (untreated sample). To calculate the relative fold of change, the overall formula was 2–∆∆Ct.
Comet Assay for Measuring DNA Damage
Preparation of Cell
In a Ficoll-Paque density gradient (Pharmacia LKB Biotechnology, Piscataway, NJ, USA), centrifuging the 2nd blood sample from each rat for 30 minutes at 1300 xg was allowed for the isolation of peripheral blood leukocytes. After the centrifugation, leukocytes in the buffy coat were aspirated & washed with PBS three times at pH of 7.4.
Cell Microgels Preparation on Slides
Based on Singh et al.31, the comet assay was conducted with modifications in accordance with Blasiak et al.32. Cell microgels preparation was executed as layers. Using a pre-cleaned microscope slide, 100 μl of normal melting point agarose (0.7%) was applied, and the cover was gently slipped to create the initial layer of gel. At 4°C, the agarose solidified & the coverslip was removed. Agarose (0.5%) with a low melting point was prepared in 100 mmol/L PBS & kept at 37°C. The low melting point agarose was combined with mononuclear cells & 100 μl of the resulting combination was added to 1st gel layer. After that, the slides were covered with a coverslip & set at 4°C to solidify. Following the solidification of the 2nd layer, from the cell microgels the coverslips were removed. After adding a last low-melting agarose layer and allowing it to solidify for ten minutes, the coverslips were taken off. DNA unwinding, gel electrophoresis, cell lysis & DNA staining were carried out. The slides were covered with 100 ml of fresh lysis buffer (at pH 10 & 4°C) for one hour. The buffer consists of NaCl (2.5 mol/L), EDTA (100 mmol/L), sodium hydroxide (1%), Tris (10 mmol/L), Triton X-100 (1%) & Dimethylsulfoxide (10% DMSO). After draining, with DNA unwinding solution (300 mmol/L NaOH, 1 mmol/L EDTA & pH 13) at 4°C for 30 min microgels slides were treated & placed directly into a horizontal gel electrophoresis chamber filled with DNA-unwinding solution. Gels were run with constant current (300 mA) for 30 min at 4°C. Following the electrophoresis, the microgels were neutralized for 10 min at pH 7.5 using 0.4 M Trisma base. To stain the slides, 20 μl solution of 10 μg/ml ethidium bromide was used.
Comet Slides Visualization and Analysis
A fluorescent microscope (Leica Microsystems, CMS GM b H, Wetzlar, Germany, Model DM 2500) was used to analyze the slides at a magnification of 400 × with a barrier filter of 590 nm & an excitation filter of 549 nm. A damaged cell appears as a comet, with a brightly fluorescent head and a tail to one side formed by the DNA having strand breaks which were drawn away. Comet pictures were visually assessed to determine the damage extent. For each case, slides were duplicated. The 50 cells were tested per slide & 100 cells per rat were tested.
Histopathological Procedure
After pancreatic tissues fixation for 24 hrs in formalin saline (10%), they were cleaned with TW and subsequently dehydrated by ethyl alcohol dilutions at 50%, 70%, 90%, and 100%. The samples were then cleaned with xylene & inserted in paraffin bee wax, which was heated to 56°C in a hot air oven for a whole day. By a rotary microtome, sections of 4 micron-thick paraffin bee wax tissue blocks were made. Staining with hematoxylin and eosin (H&E) was used after dewaxing and hydrating. By an optical microscope (Olympus, Japan), the histological changes in the pancreatic tissues were detected33.
Immunohistochemistry (IHC) Staining
On positively charged slides, paraffin sections were mounted by avidinbiotin- peroxidase complex (ABC) method. Mice TNF-α monoclonal antibody (Elabscience, Cat# E-AB-22159, Dil.: 1:100) & mouse IL-1β polyclonal antibody (Novusbio, Cat# NBP1-19775SS, Dil.: 1:100). Incubation for sections from each group with antibodies was carried out and subsequently the required reagents were added for ABC method (Vectastain ABC-HRP kit, Vector laboratories). For antigen/antibody complex detection, marker expression was labeled by peroxidase and coloured by diaminobenzidine (DAB, Sigma). Instead of the primary or secondary antibodies, non-immune serum was used for negative controls. By Olympus microscope (BX-53, Japan), IHC stained sections were tested.
Statistical Processing
As mean ± SD, the current data were shown. By ANOVA, statistical analyses of the data were executed followed by LSD comparison test to evaluate the difference between the different studied groups. Statistical significance was set at P ≤ 0.05. The SPSS (Version 22.0) was used for all statistical analyses.
Results
MW Drinking Ameliorates High Blood Glucose Levels in Diabetic Rats
The data presented in Fig. 1 revealed the drinking MW effect on serum level of glucose. The glucose level before inducing diabetes was insignificantly different between the control+ TW group & control+ MW group. On the other side, the glucose level was significantly (P ≤ 0.05) higher in the diabetic+ TW group compared with the control+ TW and control+ MW groups. However, after 4 weeks of the different treatments, the glucose level in the diabetic+ MW, diabetic+ TW+ Met and diabetic+ MW+ Met groups was significantly (P ≤ 0.05) lowered compared to the diabetic+ TW group. The decline in glucose level was more pronounced in the diabetic+ MW+ Met group than in other treated groups. It was observed that this decline was more significant (P< 0.05) in the diabetic+ MW+ Met group versus the diabetic+ MW group.
 |
Figure 1: Effect of MW drinking on serum glucose level in diabetic rats. |
MW Drinking Ameliorates Low Levels of Blood Insulin in Diabetic Rats
To determine the beta-cell function recovery, sera levels of insulin were detected at the study end. The serum insulin level was high and tended to slightly increase in the control+ MW group compared to the control+ TW group, but not significantly different (P ≥ 0.05). The serum level of insulin was significantly (P ≤ 0.05) lower in the diabetic+ TW group versus the control+ TW and control+ MW groups. Following 4 weeks of the different treatments, the insulin level in the diabetic+ MW, diabetic+ TW+ Met and diabetic+ MW+ Met groups was significantly (P˂0.05) raised compared to the diabetic+ TW group. It was observed that this increase was more significant (P˂0.05) in the diabetic+ MW+ Met group versus the diabetic+ MW and diabetic+ TW+ Met groups (Fig. 2).
 |
Figure 2: Effect of MW drinking on serum insulin level in diabetic rats. |
MW Drinking Ameliorates Low Levels of Pancreatic GSH, and GSH Px in Diabetic Rats
Herein, the content of GSH and GSH-Px activity in pancreatic tissues were measured in diabetic rats to observe the antioxidant effect of the MW. There was an insignificant difference in the levels of both biomarkers among control+ TW & control+ MW groups.Compared with the control+ TW group, the diabetic+ TW group exhibited a significant drop (P ≤ 0.05) in pancreatic content of GSH, and GSH-Px activity (Fig. 3). On the other side, the diabetic+ MW, diabetic+ TW+ Met and diabetic+ MW+ Met groups revealed a significant elevation (P˂0.05) in their values contrary to diabetic+ TW group. The activity of GSH-Px was significantly more in diabetic+ TW+ Met group than the diabetic+ MW group.The most prominent rise in pancreatic content of GSH and GSH-Px activity was recorded in the diabetic+ MW+ Met group versus the diabetic+ MW group and the diabetic+ TW+ Met group.
 |
Figure 3: Effect of MW drinking on pancreatic GSH/GSH-Px in diabetic rats. |
MW Drinking Ameliorates High Pancreatic IL-1β and TNF-α Levels in Diabetic Rats
Because inflammation is a precarious mediator of pancreatic injury following exposure to STZ, we investigated the effect of drinking MW on inflammation represented by pancreatic content of IL-1beta and TNF-α. Pancreatic IL-1beta and TNF-α were lessened and tended to slightly decrease in the control+ MW group compared to the control + TW one with insignificant (P ≥ 0.05) difference between the two groups. Meanwhile, STZ induced a significant (P ≤ 0.05) elevation in pancreatic content of IL-1β and TNF-α versus control+ TW and control+ MW groups. On the opposite side, the diabetic+ MW, diabetic+ TW+ Met and diabetic+ MW+ Met groups disclosed a significant decline (P ≤ 0.05) in IL-1β and TNF-α levels versus the diabetic+ TW group. It was observed that the drop in their values was more significant (P ≤ 0.05) in the diabetic+ MW+ Met group compared to the diabetic+ MW group and the diabetic+ TW+ Met group (Fig. 4).
 |
Figure 4: Effect of MW drinking on pancreatic inflammatory mediators in diabetic rats. |
MW Drinking up Regulates Gene Expressions of Pancreatic GLP-1, and TCF7L2 in Diabetic Rats
To evaluate the molecular therapeutic effect of drinking MW on T2DM; GLP-1, and TCF7L2gene expression was evaluated in the pancreatic tissues (Fig. 5). The pancreatic GLP-1 and TCF7L2 mRNA were heightened and tended to slightly increase in the control+ MW group contrary to the control+ TW group, but insignificantly different (P≥ 0.05). There was significant (P ≤ 0.05) down-regulation in the expression level of GLP-1, and TCF7L2in the diabetic+ TW group versus control+ TW and control+ MW groups. Noteworthy, the upregulation of TCF7L2 was more significant (P˂0.05) in the diabetic+ MW+ Met group compared with the diabetic+ MW group and diabetic+ TW+ Met group. Moreover, the upregulation of GLP-1 was more significant (P˂0.05) in the diabetic+ TW+ Met group compared to the diabetic+ MW group. Also, it showed more significant upregulation in the diabetic+ MW+ Met versus diabetic group.
 |
Figure 5: Effect of MW drinking on gene expression of GLP-1 and TCF7L2 in diabetic rats. |
MW Drinking Ameliorates DNA Damage in Diabetic Rats
The comet assay results for DNA damage percentage in the studied groups were shown in Fig. 6. DNA damage percentage was minimized and tended to slightly decrease in the control+ MW group versus the control+ TW group with no significant difference (P ≥ 0.05). There was a significant (P ≤ 0.05) DNA damage percentage in the diabetic+ TW group compared to the control+ TW and control+ MW groups. In the diabetic-treated groups, there was significant (P ≤ 0.05) repair in DNA versus the diabetic+ TW group. Noteworthy, DNA repair was more prominent and significant (P ≤ 0.05) in the diabetic+ MW+ Met group compared with the diabetic+ MW group and diabetic+ TW+ Met group.
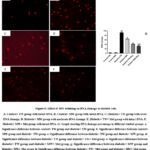 |
Figure 6: Effect of MW drinking on DNA damage in diabetic rats. |
Influence of MW Drinking on Pancreas Histopathological Features in STZ-induced Diabetic Rat Model
The pancreatic histological structure of the control+ TW group showed closely packed pancreatic acini lobules. The acini were formed of pyramidal cells with basal nuclei & apical acidophilic cytoplasm. Langerhans Islets were embedded within the exocrine portions & cells located on the periphery (Fig. 7A). Also, the control+ MW group showed nearly normal Langerhans islets with a normal pale large round to ovoid shape containing cells which embedded in the pancreatic exocrine portion (Fig. 7B). On the opposite hand, diabetic+ TW group showed changes in pathology of pancreatic endocrine and exocrine portion characterized by shrunken Langerhans islets with cell components degeneration and necrosis, vacuolation where its nucleus has a dense basophilic and pyknotic. Some exocrine acini showed focal acinar necrosis & pyknotic nuclei (Fig. 7C). In the diabetic+ MW group, the islets showed moderate improvement with centrally located β-cells; mild pyknotic nuclei & some scattered blood vessels were congested (Fig. 7D). The diabetic+ TW+ Met group showed almost normal islets of Langerhans with centrally located β-cells, few vacuolated cells, mild pyknotic nuclei, some scattered blood vessels were congested and necrosis of some exocrine acini (Fig. 7E). The diabetic+ MW+ Met group demonstrated nearly normal islets of Langerhans with centrally located β-cells, pancreatic exocrine acini appeared nearly normal with some congested blood vessels that were located in between the acini (Fig. 7F).
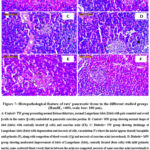 |
Figure 7: Histopathological feature of rats’ pancreatic tissue in the different studied groups (HandE, ×400, scale bar: 100 μm). |
Influence of MW Drinking on Pancreatic Tissue Inflammatory Markers (IL-1β and TNF-α) in STZ-induced Diabetic Rat Model using Immunohistochemical Reactions
Evaluation of immunohistochemical analysis of the diabetic and diabetic-treated groups showed that STZ notably (P˂0.05) enhanced the IL-1β and TNF-α expression in pancreatic tissues when compared with control+ TW & control+ MW groups. Conversably, diabetic+ MW, diabetic+ TW+ Met, and diabetic+ MW+ Met groups significantly (P˂0.05) reduced the expression of pancreatic IL-1β & TNF-α compared to diabetic+ TW group. Noteworthy, the TNF-α and IL-1beta expression in pancreatic tissues declined more significantly (P˂0.05) in the diabetic+ MW+ Met group than in diabetic+ MW and diabetic+ TW+ Met groups (Figs. 8, 9).
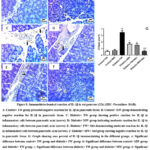 |
Figure 8: Immunohistochemical reaction of IL-1β in rat pancreas (25x) (IHC- Peroxidase -DAB). |
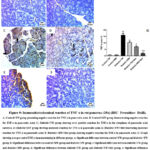 |
Figure 9: Immunohistochemical reaction of TNF-α in rat pancreas (25x) (IHC- Peroxidase -DAB). |
Discussion
Patients with diabetes have higher oxidative stress due to a decline in their antioxidant defense system and reactive oxygen species (ROS) increase. If a substance has high ROS scavenging capability, it has a potential activity against diabetic patients with a high oxidative stress level. Numerous studies have found significant DNA damage in conjunction with elevated blood glucose in individuals with diabetes34,35. In the current study, the effect of magnetized water, metformin, and metformin & magnetized water combination on diabetic rats was investigated. After these therapies were given every day for four weeks, we assessed the various parameters for achieving the study’s objectives.
Herein, the levels of serum glucose in the diabetic groups were found to be significantly higher than those in the control groups, according to the data. This result corroborates the finding of Lee and Kang17 who observed that in STZ-induced diabetes, the blood glucose level is noticeably greater than in the control group. When the experimental animals are given STZ to induce diabetes, the pancreatic β-cells are destroyed, and insulin release is reduced. This leads to improper metabolism and a rise in blood glucose levels. Pancreatic beta cells are specifically destroyed by STZ, however, nicotinamide (NA) lessens the damage that STZ causes, resulting in a condition of partial insulin insufficiency that is comparable to T2DM. Rats with diabetes caused by STZ with NA have substantially milder symptoms than those with diabetes induced by STZ alone; they exhibit moderate hyperglycemia and do not require exogenous insulin to live36.
On the opposite side, the data showed that when compared to diabetic rats, metformin-treated diabetic rats showed considerably lower levels of glucose in their blood. Meng et al.37 also supported our results wherein the STZ-induced T2DM rat model’s blood glucose levels are lowered by metformin. It has been shown to decline blood glucose levels by suppressing gluconeogenesis, which lowers hepatic glucose synthesis, and by increasing peripheral glucose utilization in the liver, muscles, and intestines38,39. MW also lowered blood glucose levels in the current investigation and the obtained results are in agreement with those of Alhammer and his colleagues40. According to those researchers, water that has been magnetically treated dramatically lowers blood glucose levels. This could be explained by the fact that water that has been magnetically treated has more water conductivity, which could improve blood circulation and boost cells’ absorption of glucose17; leading to the decreased blood glucose level.
The gathered data revealed that, compared with the control groups, blood insulin levels in diabetic rats were much lower. These results resemble the Akhtar et al.41 findings who observed that blood insulin levels of rats with diabetes are notably lower than those of the control group.This could be clarified by the fact that following an injection of STZ, the β-cells in the pancreatic islets die42. Serum insulin levels significantly decrease as a result of pancreatic cell death43; as it is responsible for insulin production.
The results of the study made it clear that metformin treatment improved the insulin levels in diabetic rats. Similarly,in the STZ-diabetic rats model, Ibrahim and his colleagues44 observed a noteworthy rise in serum insulin levels subsequent to metformin treatment. Metformin may be a useful treatment for T2DM because it inhibits the manufacture of cholic acid and the enzyme CYP8B1 expression, which is needed for 12α hydroxylated bile acid synthesis 45. There is proof that insulin sensitivity and 12α-hydroxylated bile acid levels—primarily cholic acid—are correlated46. Thus, metformin suppresses the levels of 12-hydroxylated bile acid, increasing insulin sensitivity47. Furthermore, metformin can raise serum insulin levels most likely through improving GLUT-4 expression and function in cells as well as by inducing the release of glucagon-like peptide-1 (GLP-1), both of which are linked to the development of β-cells48. Remarkably, serum insulin levels significantly increased following MW consumption, in contrast to diabetic rats. Our results are consistent with those of Hanafy and his colleagues49, who hypothesized that diabetic rats given MW had improved their insulin, HOMA-IR, and HbA1c levels more than untreated diabetic rats. T2DM-related chronic hyperglycemia, oxidative stress, and dyslipidemia are especially harmful to beta cells. If β-cells function is harmed, this results in an under insulin production50. Nevertheless, MW has stopped this action where it impairs oxidative stress and repairs β-cell function17 and hence restores serum insulin levels.
Comparing diabetic rats with control rats, there was a significant decrease in pancreatic content of GSH and GSH-Px activity. GSH (an intracellular thiol rich tripeptide), which has a crucial role in maintaining the equilibrium of oxidative stress by protecting tissue structures and cells51. The decreased GSH level in the diabetic group could be the result of its increased utilization to scavenge free radicals under oxidative stress52. Additionally, the findings of Obafemi et al.53 showed that in a STZ-induced diabetic rat model, STZ can lower SOD, catalase, and GSH-Px enzyme activity. It is known that oxidative stress and diabetes are linked. Oxidative stress is often characterized by elevated lipid peroxidation (LPO) products together with decreased levels and/or antioxidant enzyme activities including catalase, SOD, and GSH-Px54. This is particularly evident in the pancreatic β-cells, which have a lowered antioxidant capacity55. Finally, chronic hyperglycemia linked with T2DM is a major cause of the intracellular antioxidant system depletion with subsequent development of oxidative stress, which leads to a vast accumulation of intracellular free radicals56.
Herein, comparing the metformin-treated diabetic rats to the untreated ones, there was a significant rise in the pancreatic GSH content and GSH-Px activity. Chukwunonso Obi et al.52 also noted that in rats with alloxan-induced diabetes, the administration of metformin significantly lowers the concentration of malondialdehyde (MDA) and significantly improves the altered activities of the antioxidant enzymes (SOD, CAT), as well as the level of GSH. This may be explained by metformin’s capacity to stimulate AMP-activated protein kinase (AMPK) and increase the expression of its target proteins57; as AMPK preserves redox homeostasis and regulates the expression of antioxidant enzymes58. Similarly, compared with the diabetic rats treated with TW, the treatment with MW markedly raised the pancreatic GSH content and activity of GSH-Px. These findings are consistent with several investigations that show MW lowers LPO levels and raises SOD, catalase, and GSH-Px activity in cardiac tissue59,60. It is commonly known that MW is more electrically conductive and has a higher pH than ordinary drinking water61. According to certain studies, water that has been magnetized has more permeability across cell membranes62, and the magnetic field directly affects intracellular fluid and substances to activate enzymes inside the cells and to speed up the biochemical reactions in the body63. Therefore, it’s possible that consuming MW could cause the body’s antioxidant system to become active17.
The current approach’s results showed that the diabetic rats had significantly higher pancreatic inflammatory markers levels (IL-1β and TNF-α) than the controls. Our results align with the findings of Lee and his colleagues64 who observed that when comparing STZ-induced diabetic rats with normal rats, pro-inflammatory cytokines levels, IL-6 & TNF-α, increased by about 3.4 and 2.9 fold, respectively. It has been reported that the elevated oxidative stress resulting from continuous hyperglycemia in DM stimulates the activation of transcription factor nuclear factor-kappa B (NF-κB) p65. This triggers many pro-inflammatory cytokines, including TNF-α synthesis 65.
Herein, there was a decrease in the pancreatic inflammatory mediators in diabetic rats after metformin treatment compared with untreated diabetic rats. Mirroring our data, the reported data by Han et al.66 informed that in STZ-induced diabetic mice, metformin lowers pancreatic levels of IL-1β &TNF-α. It has been reported that metformin could stimulate the signaling of AMPK/PI3K/Akt, so presenting anti-inflammatory actions through inhibiting NF-κB with subsequent decline in the pro-inflammatory cytokine generation67. Additionally, compared to diabetic rats, the results showed that consuming MW significantly lowered the pro-inflammatory markers levels (IL-1β and TNF-α). It was proposed that the magnetic field modifies hydrogen proton’s magnetic spin in water through magnetic resonance & hydrogen bond becomes deformed & weaker, affecting hydration and protonation of ions, and so, the hydrogen-rich water could scavenge ROS68,69. So, one can hypothesize that by scavenging ROS via MW, inflammation can be reduced as ROS are involved in the initiation, progression, and promotion of the inflammatory responses70.
In the current attempt, STZ injection significantly reduced the expression of the GLP-1 and TCF7L2 genes in the pancreas compared to the control group. These results are parallel to the previously published results by Abdel Aziz and his colleagues29 who stated that when rat pancreatic islets are treated with STZ in vitro, the expression of the GLP-1 and TCF7L2 genes is down-regulated. TCF7L2 has an essential role in the metabolism of glucose by regulating the GLP-1 hormone production that modifies glucose dependent insulin secretion71. TCF7L2 gene expression was positively correlated with the insulin gene expression, which encodes insulin72. It was found that STZ dramatically down-regulates the expression of the insulin gene73. Therefore, by down-regulating insulin gene expression by STZ, TCF7L2 gene expression will be also down-regulated in turn. The transcription factor TCF7L2 performs an important role in the regulation of GLP-1 secretion and gene expression in pancreatic β- cells viaregulation of pro-glucagon expression, the precursor encoding GLP-1 gene74. Thus, TCF7L2 gene expression down-regulation leads to down- regulation of GLP-1 gene expression in turn.
However, metformin and/or MW supplementation up-regulate TCF7L2 and GLP-1 genes expression compared to the untreated diabetic rats. It has been found that oxidative stress induces insulin gene expression down-regulation via the c-Jun N-terminal kinase (JNK) pathway29. Down-regulation of insulin gene expression results in suppression of TCF7L2 gene expression72. Moreover, down-regulation of TCF7L2 gene expression leads to down-regulation of GLP-1 gene expression74. Studies have demonstrated that metformin counteracts this effect by enhancing the altered status of oxidative and nitrosative stress in individuals with T2DM75. In the same way, it has been hypothesized that magnetized water recovers oxidative stress17,21 and thus up-regulates pancreatic TCF7L2 and GLP-1 gene expression as shown in the current work.
The current data demonstrated that the diabetic group’s DNA damage percentage was much greater than that of the control group. This comes in agreement with that observed in Toğay et al. study 76, in which the injection of STZ raised DNA damage in diabetic rats compared to controls. Comet assay is a widely used technique to detect oxidative damage in DNA lymphocyte & oxidative stress degree is associated with comet tail size77. This extremely sensitive assay can identify very small amounts of damage. High blood glucose levels have been shown to enhance DNA breakage and hinder cellular DNA repair in vitro78. Thus, the hyperglycemia that results from diabetes itself increases oxidative stress and ROS production, both of which cause oxidative DNA damage.
On the contrary side, DNA damage percentage was significantly lowered by metformin treatment in diabetic rats versus the untreated one. The current research supports the hypothesis that metformin shields nuclear DNA from oxidative damage79,80. Oxidative stress has the ability to activate p53 and initiate p53-mediated DNA repair. If the oxidative stress intensity is high, p53 would act as a pro-apoptotic factor and facilitate cellular death pathways. However, p53 would have antioxidant activity and diminish ROS accumulation if the oxidative stress intensity is low, so activates the repairing of DNA damage. It is generally accepted that the elevated intracellular stress in T2DM will result in high ROS levels; however metformin would decline ROS levels in which p53 gains antioxidant activity and stimulates cellular survival by hindering DNA damage81. When MW was administered to the group of diabetes, damage of blood lymphocyte DNA was significantly improved compared with the group of diabetes received TW. This result is similar to that observed in the study of Lee and Kang17, in which MW administration reduced lymphocyte DNA damage in the cancer experimental animals’ model induced by diethyl nitrosamine. Oxidative stress is generated during DM and it is involved in pancreatic beta-cell dysfunction. The discovery that MW consumption decreases oxidative stress in diabetic rats, which in turn lessens the damage to lymphocyte DNA, suggests potential applications for both diabetes prevention and treatment17. It is unclear, meanwhile, how magnetized water affects DNA damage within the body.
From a histological perspective, the pancreas from the control rat that received TW was made up of densely packed pancreatic acini lobules. The acini are formed of pyramidal cells with basal nuclei & apical acidophilic cytoplasm. Langerhans Islets were embedded within the exocrine portions & cells located on the periphery. The control+ MW group showed nearly normal pancreatic islets with a normal pale large round to ovoid shape with cells embedded in the pancreatic exocrine portion. However, the histological structure of the pancreas obtained from diabetic rats that received TW showed pathological changes of both pancreatic endocrine and exocrine portions represented by shrunken pancreatic islets with cell components degeneration and necrosis, vacuolation where its nucleus has a dense basophilic & pyknotic is evident. Some exocrine acini revealed focal acinar necrosis and pyknotic nuclei. Additionally, according to Özdek et al.28, STZ causes atrophy in the islet of Langerhans as well as degeneration and necrosis in islet cells. STZ stimulation results in significant production of IL-1beta & TNF-α, which have both been implicated in islet inflammation and beta-cell dysfunction in pancreatic tissues82. The aforementioned pathological damage in the STZ-induced experimental diabetic model may thus be partially attributed to an inflammatory response. The pancreas histological structure of the rats in the diabetic+ MW group showed that the islets appeared moderated improvement comparable to that of the rat in the diabetic group that received TW. The islets of Langerhans appeared more or less normal with centrally located β-cells and mild pyknotic nuclei. Some scattered blood vessels that in between the acini were congested. This may be associated with MW anti-oxidant properties17,22. While; the pancreatic tissue of the diabetic+ TW+ metformin group showed almost normal islets of Langerhans, few vacuolated cells, and mild pyknotic nuclei. Some scattered blood vessels in between the acini were congested and necrosis of some exocrine acini still present. This is consistent with the findings of Han et al.66, who found that metformin treatment in STZ-induced diabetes mice significantly but moderately improves the histological alterations of the pancreatic islets. Because of their low levels of antioxidant enzyme activity, β-cells are particularly vulnerable to oxidative stress and damage83. Thus, it is possible that the elevated oxidative stress in DM is the cause of the degenerative and shrinking alterations in the islets of Langerhans84. It’s interesting to note that metformin’s antioxidant action most likely plays a role in the previously reported histological changes.
The pancreatic tissue histological structure of rats in the diabetic+ Met+ MW group showed nearly normal islets of Langerhans with centrally located β-cells. Pancreatic exocrine acini appear nearly normal with some blood vessels in between the acini congested. The synergistic impact of metformin and MW on the regeneration shown in pancreatic islets could be related to MW’s antioxidant characteristics17,22 and also to the anti-oxidant action of metformin52.
According to this study, MW alone dramatically reduced hyperglycemia and raised insulin synthesis. Additionally, the antioxidant status and inflammatory mediators in pancreatic tissues were improved, particularly with the combination of metformin and MW therapy. Furthermore, the down-regulation of the TCF7L2 and GLP-1 genes in the pancreas and the repair of DNA damage in diabetic rats suggest that insulin manufacture and function are likely to improve when glucose levels decline.
Conclusion
As the current study has shown, the mechanisms underlying the anti-diabetic effects of MW include reduction of inflammation and oxidative stress, along with improvements in insulin secretion and glucose metabolism. The results of the biochemical, molecular genetics, DNA damage, histological, and immunohistochemical analyses of this study also spoke for that the combination of metformin and magnetized water had superior anti-diabetic effectiveness than either one alone. This provides additional support to the idea that combination medication may be more effective than monotherapy in type II diabetes mellitus management. To clarify the precise mechanisms underlying the antidiabetic action of this combination therapy, more research on the anti-diabetic effect of co-administration of metformin and magnetized water should be carried out.
Acknowledgment
The authors thank National Research Centre where the study was done.
Conflicts of Interest
The author(s) declares no conflict of interest.
Funding Source
The author(s) received no financial support for the research, authorship, and/or publication of this article
References
- Su J., Luo Y., Hu S., Tang L., Ouyang S. Advances in research on type 2 diabetes mellitus targets and therapeutic agents. Int J Mol Sci. 2023; 24: 13381.
CrossRef - Janssen J. A. M. J. L. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int J Mol Sci. 2021; 22: 7797.
CrossRef - World Health Organization. Diabetes 2018. Accessed 01 October 2022. https://www.who.int/news-room/fact-sheets/detail/diabetes.
- Magliano D. J., Boyko E. J. IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS [Internet]. 10th ed. Brussels: International Diabetes Federation. 2021; PMID: 35914061.
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B. B., Magliano D. J. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022; 183: 109119.
CrossRef - Galicia-Garcia U., Benito-Vicente A., Jebari S., Larrea-Sebal A., Siddiqi H., Uribe K. B., Martín C. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020; 21: 6275.
CrossRef - Schellenberg E. S., Dryden D. M., Vandermeer B., Ha C., Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: A systematic review and meta-analysis. Ann Intern Med. 2013; 159: 543–551.
CrossRef - Bhatti J. S., Sehrawat A., Mishra J., Sidhu I. S., Navik U., Khullar N., Reddy P. H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic Biol and Med. 2022; 184: 114-134.
CrossRef - Kilanowska A., Ziółkowska A. Apoptosis in type 2 diabetes: Can it be prevented? Hippo pathway prospects. Int J Mol Sci. 2022; 23: 636.
CrossRef - Marrif H. I., Al-Sunousi S. I. Pancreatic β cell mass death. Front Pharmacol. 2016; 7: 172499.
CrossRef - Berchtold L. A., Prause M., Størling J., Mandrup-Poulsen T. Cytokines and pancreatic β-cell apoptosis. Adv Clin Chem. 2016; 75: 99-158.
CrossRef - Deshmukh C. D., Jain A., Nahata B. Diabetes mellitus: a review. Int J Pure Appl Biosci. 2015; 3: 224-230.
- American Diabetes Association. Standards of medical care in diabetes—2020 abridged for primary care providers. Clin Diabetes. 2020; 38: 10–38.
CrossRef - Rhee S. Y., Kim H. J., Ko S. H., Hur K. Y., Kim N. H., Moon M. K., Kim J. H. Monotherapy in patients with type 2 diabetes mellitus. Diabetes Metab J. 2017; 41: 349.
CrossRef - Kahn S. E., Haffner S. M., Heise M. A., Herman W. H., Holman R. R., Jones N. P., Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. New England Journal of Medicine. 2006; 355: 2427-2443.
CrossRef - American Diabetes Association. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes—2021. Diabetes care. 2021; 44: S111-S124.
CrossRef - Lee H. J., Kang M. H. Effect of the magnetized water supplementation on blood glucose, lymphocyte DNA damage, antioxidant status, and lipid profiles in STZ-induced rats. Nutr Res Pract. 2013; 7: 34-42.
CrossRef - Shen X. Increased dielectric constant in the water treated by extremely low frequency electromagnetic field and its possible biological implication. J Phys: Conf Ser. 2011; 329: 012019.
CrossRef - Al-Nuemi S. H., Al-Badry K. I., Atteyh A. J., Al-Sabeea W. S., Ibrahim F. F., Rajab B. A. Effect of magnetic water drinking on testis dimension, scrotal circumference and blood parameters of holstein bulls born in Iraq. Adv Anim Vet Sci. 2015; 3: 413-417.
CrossRef - Ibrahim I. H. Biophysical properties of magnetized distilled water. Egypt. J. Sol. 2006; 29: 1-7.
- Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature medicine. 2007; 13: 688-694.
CrossRef - Nakashima-Kamimura N., Mori T., Ohsawa I., Asoh S., Ohta S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother. Pharmacol. 2009; 64: 753-761.
CrossRef - Kajiyama S., Hasegawa G., Asano M., Hosoda H., Fukui M., Nakamura N., Yoshikawa, T. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res. 2008; 28: 137-143.
CrossRef - El Sherif K. H., Eid A. A., Fouda S. F. Effect of magnetic water on semen quality, blood constituents, antioxidant capacity and immunity of rabbit bucks. J Anim Poult Prod. 2020; 11: 13-19.
CrossRef - Pang X. F., Deng B. The changes of macroscopic features and microscopic structures of water under influence of magnetic field. Physica B Condens Matter. 2008; 403: 3571-3577.
CrossRef - Swilam N., Nawwar M. A., Radwan R. A., Mostafa E. S. Antidiabetic activity and in silico molecular docking of polyphenols from Ammannia baccifera L. subsp. Aegyptiaca (Willd.) Koehne Waste: structure elucidation of undescribed acylated Flavonol Diglucoside. Plants. 2022; 11: 452.
CrossRef - Al Za’abi M. A., Ali B. H., Al Suleimani Y., Adham S. A., Ali H., Manoj P., Nemmar A. The effect of metformin in diabetic and non-diabetic rats with experimentally-induced chronic kidney disease. Biomolecules. 2021; 11: 814.
CrossRef - Özdek U., Yıldırım S., Değer Y. The effect of Diplotaenia turcica root extract in streptozotocin-induced diabetic rats. Turkish J Biochem. 2020; 45: 213-222.
CrossRef - Abdel Aziz M. T., El-Asmar M. F., Rezq A. M., Wassef M. A., Fouad H., Roshdy N. K., Hassouna A. Effects of a novel curcumin derivative on insulin synthesis and secretion in streptozotocin-treated rat pancreatic islets in vitro. Chin Med. 2014; 9: 1-12.
CrossRef - Wu X. H., Liu C. P., Xu K. F., Mao X. D., Zhu J., Jiang J. J., Liu C. Reversal of hyperglycemia in diabetic rats by portal vein transplantation of islet-like cells generated from bone marrow mesenchymal stem cells. World J Gastroenterol. 2007; 13: 3342.
CrossRef - Singh N. P., McCoy M. T., Tice R. R., Schneider E. L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988; 175: 184-191.
CrossRef - Blasiak J., Gloc E., Drzewoski J., Wozniak K., Zadrozny M., Skórski T., Pertynski T. Free radical scavengers can differentially modulate the genotoxicity of amsacrine in normal and cancer cells. Mutat Res Genet Toxicol Environ Mutagen. 2003; 535: 25-34.
CrossRef - Bancroft J. D., Gamble M. Theory and practice of histological techniques. 6th ed. Elsevier Health Sciences. 2008; p. 433-469.
- Collins A. R., Rašlová K., Somorovská M., Petrovská H., Ondrušová A., Vohnout B., Dušinská M. DNA damage in diabetes: correlation with a clinical marker. Free Radic Biol Med. 1998; 25: 373-377.
CrossRef - Hinokio Y., Suzuki S., Hirai M., Chiba M., Hirai A., Toyota T. Oxidative DNA damage in diabetes mellitus: its association with diabetic complications. Diabetologia. 1999; 42: 995-998.
CrossRef - Masiello P., Broca C., Gross R., Roye M., Manteghetti M., Hillaire-Buys D., Ribes G. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998; 47: 224-229.
CrossRef - Meng X. M., Ma X. X., Tian Y. L., Jiang Q., Wang L. L., Shi R., Pang S. G. Metformin improves the glucose and lipid metabolism via influencing the level of serum total bile acids in rats with streptozotocin-induced type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2017; 21: 2232-2237.
- Otto M., Breinholt J., Westergaard N. Metformin inhibits glycogen synthesis and gluconeogenesis in cultured rat hepatocytes. Diabetes Obes Metab. 2003; 5: 189-194.
CrossRef - Mithieux G., Rajas F., Zitoun C. Glucose utilization is suppressed in the gut of insulin-resistant high fat-fed rats and is restored by metformin. Biochem pharmacol. 2006; 72: 198-203.
CrossRef - Alhammer A. H., Sadiq G. T., Yousif S. Effect of magnetized water on several biochemical and physical properties in mice. J Babylon Univ Appl Sci. 2013; 21, 910-916.
- Akhtar M. S., Rafiullah M., Hossain M. A., Ali M. Antidiabetic activity of Cichorium intybus L water extract against streptozotocin-induced diabetic rats. JAS. 2023; 9: 565-571.
CrossRef - Friedewald W. T., Levy R. I., Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18: 499-502.
CrossRef - Auddy B., Ferreira M., Blasina F., Lafon L., Arredondo F., Dajas F., Mukherjee B. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol. 2003; 84: 131-138.
CrossRef - Ibrahim I. Y., Ali F. F., Abdel-Hakeem E. A., Saleh A. R., Sayed Z. Pathophysiological mechanisms of type 2 diabetes mellitus and the effects of metformin treatment in adult male albino rats. MJMR. 2023; 34: 209-214.
- Zaborska K. E., Cummings B. P. Rethinking bile acid metabolism and signaling for type 2 diabetes treatment. Curr Diab Rep. 2018; 18: 1-10.
CrossRef - Haeusler R. A., Pratt-Hyatt M., Welch C. L., Klaassen C. D., Accili D. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 2012; 15: 65-74.
CrossRef - Li M., Hu X., Xu Y., Hu X., Zhang C., Pang S. A possible mechanism of metformin in improving insulin resistance in diabetic rat models. Int J Endocrinol. 2019; 2019: 3248527.
CrossRef - Yendapally R., Sikazwe D., Kim S. S., Ramsinghani S., Fraser‐Spears R., Witte A. P., La‐Viola B. A review of phenformin, metformin, and imeglimin. Drug Dev. Res. 2020; 81: 390-401.
CrossRef - Hanafy M. S., Khedr A. A., Kassab M. M. Antidiabetic effects of magnetized water in normal and alloxan-diabetic rats. JHE – Menofia University. 2020; 30: 553-566.
- Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes. 2015; 6: 456.
CrossRef - Mohamadin A. M., Elberry A. A., Gawad H. S. A., Morsy G. M., Al-Abbasi F. A. Protective effects of simvastatin, an HMGCoA reductase inhibitor, against oxidative damage in experimental diabetic rats. Int J Pharm Tech Res. 2011; 3: 1780-1795.
CrossRef - Chukwunonso Obi B., Chinwuba Okoye T., Okpashi V. E., Nonye Igwe C., Olisah Alumanah E. Comparative study of the antioxidant effects of metformin, glibenclamide, and repaglinide in alloxan-induced diabetic rats. J Diabetes Res. 2016; 2016: 1635361.
CrossRef - Obafemi T. O., Jaiyesimi K. F., Olomola A. A., Olasehinde O. R., Olaoye O. A., Adewumi F. D., Ojo O. A. Combined effect of metformin and gallic acid on inflammation, antioxidant status, endoplasmic reticulum (ER) stress and glucose metabolism in fructose-fed streptozotocin-induced diabetic rats. Toxicol Rep. 2021; 8: 1419-1427.
CrossRef - Bin-Jumah M. N. Antidiabetic effect of monolluma quadrangula is mediated via modulation of glucose metabolizing enzymes, antioxidant defenses, and adiponectin in type 2 diabetic rats. Oxidative medicine and cellular longevity. 2019; 2019: 6290143.
CrossRef - Robertson R. P., Harmon J., Tran P. O., Tanaka Y., Takahashi H. Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003; 52: 581-587.
CrossRef - El Gamal H., Eid A. H., Munusamy S. Renoprotective effects of aldose reductase inhibitor epalrestat against high glucose-induced cellular injury. Biomed Res Int. 2017; 2017: 5903105.
CrossRef - Wei J., Wei Y., Huang M., Wang P., Jia S. Is metformin a possible treatment for diabetic neuropathy? J of Diabetes. 2022; 14: 658-669.
CrossRef - Yun H., Park S., Kim M. J., Yang W. K., Im D. U., Yang K. R., Ha J. AMP‐activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. The FEBS journal. 2014; 281: 4421-4438.
CrossRef - Raymond-Whish S., Mayer L. P., O’Neal T., Martinez A., Sellers M. A., Christian P. J., Marion S. L., Begay C., Ropper C. I., Hoyer P. B., Dyer C. A. Drinking water with uranium below the U. S. EPA water standard causes estrogen receptor-dependent responses in female mice. Environ Health Perspect. 2007; 115: 1711-1716.
CrossRef - Hafizi L., Gholizadeh M., Karimi M., Hosseini G., Mostafavi-Toroghi H., Haddadi M., Meibodi, N. E. Effects of magnetized water on ovary, pre-implantation stage endometrial and fallopian tube epithelial cells in mice. Iran J Reprod Med. 2014; 12: 243.
- Xu Y. B., Sun S. Y. Effect of stable weak magnetic field on Cr (VI) bio-removal in anaerobic SBR system. Biodegradation. 2008; 19: 455-462.
CrossRef - Lednev V. V. Possible mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics. 1991; 12: 71.
CrossRef - Liboff A. R., Cherng S., Jenrow K. A., Bull A. Calmodulin‐dependent cyclic nucleotide phosphodiesterase activity is altered by 20 μT magnetostatic fields. Bioelectromagnetics. 2003; 24: 32-38.
CrossRef - Lee Y., Bae C. S., Ahn T. Chlorogenic acid attenuates pro-inflammatory response in the blood of streptozotocin-induced diabetic rats. Lab Anim Res. 2022; 38: 37.
CrossRef - Mariappan N., Elks C. M., Fink B., Francis J. TNF-induced mitochondrial damage: a link between mitochondrial complex I activity and left ventricular dysfunction. Free Radic Biol Med. 2009; 46: 462-470.
CrossRef - Han X., Tao Y. L., Deng Y. P., Yu J. W., Cai J., Ren G. F., Jiang G. J. Metformin ameliorates insulitis in STZ-induced diabetic mice. PeerJ. 2017; 5: e3155.
CrossRef - Isoda K., Young J. L., Zirlik A., MacFarlane L. A., Tsuboi N., Gerdes N., Libby P. Metformin inhibits proinflammatory responses and nuclear factor-κB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006; 26: 611-617.
CrossRef - Xiao L., Miwa N. Hydrogen-rich water achieves cytoprotection from oxidative stress injury in human gingival fibroblasts in culture or 3D-tissue equivalents, and wound-healing promotion, together with ROS-scavenging and relief from glutathione diminishment. Hum cell. 2017; 30: 72-87.
CrossRef - Guo J., Dong W., Jin L., Wang P., Hou Z., Zhang Y. Hydrogen-rich saline prevents bone loss in diabetic rats induced by streptozotocin. Int Orthop. 2017; 41: 2119-2128.
CrossRef - Chelombitko M. A. Role of reactive oxygen species in inflammation: a minireview. Moscow Univ Biol Sci Bull. 2018; 73: 199-202.
CrossRef - Horikoshi M., Hara K., Ito C., Nagai R., Froguel P., Kadowaki T. A genetic variation of the transcription factor 7-like 2 gene is associated with risk of type 2 diabetes in the Japanese population. Diabetologia. 2007; 50: 747-751.
CrossRef - Lyssenko V., Lupi R., Marchetti P., Del Guerra S., Orho-Melander M., Almgren P., Groop L. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest. 2007; 117: 2155-2163.
CrossRef - Vargas Guerrero B., García López P. M., González Santiago A. E., Domínguez Rosales J. A., Gurrola Díaz C. M. Reduction of lns-1 gene expression and tissue insulin levels in n5-STZ rats. Biol Res. 2013; 46: 281-288.
CrossRef - Yi F., Brubaker P. L., Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by β-catenin and glycogen synthase kinase-3β. J Biol Chem. 2005; 280: 1457-1464.
CrossRef - Chakraborty A., Chowdhury S., Bhattacharyya M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res Clin Pract. 2011; 93: 56-62.
CrossRef - Toğay V. A., Sevimli T. S., Sevimli M., Çelik D. A., Özçelik N. DNA damage in rats with streptozotocin-induced diabetes; protective effect of silibinin. Mutat Res Genet Toxicol Environ Mutagen. 2018; 825: 15-18.
CrossRef - Woods J. A., Young A. J., Gilmore I. T., Morris A., Bilton R. F. Measurement of menadione-mediated DNA damage in human lymphocytes using the comet assay. Free Radic Res. 1997; 26: 113-124.
CrossRef - Kaneto H., Fujii J., Suzuki K., Kasai H., Kawamori R., Kamada T., Taniguchi, N. DNA cleavage induced by glycation of Cu, Zn-superoxide dismutase. J Biochem. 1994; 304: 219-225.
CrossRef - Hou X., Song J., Li X. N., Zhang L., Wang X., Chen L., Shen Y. H. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun. 2010; 396: 199-205.
CrossRef - Piro S., Rabuazzo A. M., Renis M., Purrello F. Effects of metformin on oxidative stress, adenine nucleotides balance, and glucose-induced insulin release impaired by chronic free fatty acids exposure in rat pancreatic islets. J Endocrinol Invest. 2012; 35: 504-510.
- Turacli I. D., Candar T., Yuksel E. B., Kalay S., Oguz A. K. Demirtas S. Potential effects of metformin in DNA BER system based on oxidative status in type 2 diabetes. Biochimie. 2018; 154: 62-68.
CrossRef - Eguchi K., Manabe I., Oishi-Tanaka Y., Ohsugi M., Kono N., Ogata F., Nagai R. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012; 15: 518-533.
CrossRef - Greenberg M. M. Abasic and oxidized abasic site reactivity in DNA: enzyme inhibition, cross-linking, and nucleosome catalyzed reactions. Acc Chem Res. 2014; 47: 646-655.
CrossRef - Usman U. Z., Mainasara A. S., Abbas A. Y., Sadiq M. U. E., Mohamed M. Metformin on Insulin, Glucagon, Oxidative Stress Markers and Pancreatic Tissue Histology in Streptozotocin-Induced Diabetic Rats. Sokoto j med lab sci. 2021; 6: 24-29.
CrossRef







