Manuscript accepted on :25-07-2024
Published online on: 11-09-2024
Plagiarism Check: Yes
Reviewed by: Dr. Nicolas Padilla and Dr. Audrey Jacob
Second Review by: Dr. Ahmed Salah
Final Approval by: Dr. Prabhishek Singh
Prasad Arvind Thakurdesai1* , Pooja Abhay Bhalerao2
, Pooja Abhay Bhalerao2 and Urmila Manoj Aswar2
and Urmila Manoj Aswar2
1Department of Scientific Affairs, Indus Biotech Limited, Pune, India
2Department of Pharmacology, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, India
Corresponding Author E-Mail:prasad@indusbiotech.com
DOI : https://dx.doi.org/10.13005/bpj/2955
Abstract
The present study aimed to determine the efficacy of intranasal administration of a standardized extract of Gotu kola, i.e., Centella asiatica (L.) Urban (INDCA-NS) with marker triterpenoids for the prevention of nitroglycerine- (NTG)-induced recurrent migraine in rats. Adult rats of both sexes in a group of 12 were administered intraperitoneal NTG (10 mg/kg) on alternate days (D1 to D9) and once daily intranasal solutions of either vehicle (saline, 50 µL/rat/day), sumatriptan (80 µL/rat/day of 12 mg/ml) as positive control, or INDCA-NS (10, 30, or 100 µg/rat/day) for 21 days. Behavioral and biochemical parameters related to concurrent migraine pain (facial expressions on the grimace scale, thermal hyperalgesia, mechanical allodynia, and plasma and brain levels of pituitary adenylate cyclase-activating polypeptide and nitric oxide), and stress (photophobia and cortisol levels in the brain and serum) were measured. The intranasal administration of INDCA-NS prevented NTG-induced migraine-like pain, photophobia, and stress in a dose-dependent manner. At the same time, sumatriptan alleviated pain and anxiety but not photophobia. In conclusion, the intranasal administration of INDCA-NS showed prophylactic efficacy against recurrent NTG-induced migraine pain in rats.
Keywords
Anti-nociception; Centella asiatica (L.) Urban leaves; Gotu kola; Recurrent migraine
Download this article as:| Copy the following to cite this article: Thakurdesai P. A, Bhalerao P. A, Aswar U. M. Intranasal Administration of Standardized Extract of Gotu Kola Leaves Against Nitroglycerine-Induced Recurrent Migraine-Like Pain in Rats. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Thakurdesai P. A, Bhalerao P. A, Aswar U. M. Intranasal Administration of Standardized Extract of Gotu Kola Leaves Against Nitroglycerine-Induced Recurrent Migraine-Like Pain in Rats. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/4d1ahEz |
Introduction
Migraine is a persistent and debilitating neurological condition marked by intense headache pain, as well as other symptoms such as nausea, vomiting, and sensitivity to light 1. Chronic migraine is a highly disabling condition characterized by headaches for 15 days or more per month compared to 2. Migraine is the most disabling neurological condition3, affecting approximately 12% of the global population 4. They are a major cause of significant disability and reduced quality of life 5.
Migraine is often accompanied by photophobia, phonophobia, and gastrointestinal distress such as nausea and vomiting6. These disorders not only have a significant personal impact by decreasing the quality of life but also impose a financial burden7, 8. Chronic migraine is a progressive disorder 9 that requires prophylactic treatment to reduce attack frequency, severity, and duration, increase responsiveness to acute migraine therapy, and improve the overall quality of life 9, 10.
Despite significant advancements in conventional therapies, multipronged attacks on neurological (pain and inflammation) and psychological (stress and neurotransmitter imbalances) aspects are required for effective migraine prophylaxis. Intranasal administration of sumatriptan (SUMA) has recently been explored as a prophylaxis against migraine-like headaches, but limitations in formulation and bioavailability have been reported 11. In this context, natural plant-based products with multipronged actions are promising and safer alternatives for migraine prevention.
Gotu kola leaves are a natural source of ingredients reported to have prophylactic efficacy against experimental migraine. Traditionally, the whole plant or leaves of Gotu kola (Centella asiatica (L.) Urban i.e. C. asiatica) have been known as a brain tonic12. The broad pharmacological activity profile was attributed to pentacyclic triterpenoid glycosides, which are secondary metabolites of the leaves 13, with The most promising compounds being centelloids (asiaticoside, madecassoside, centelloside, brahmoside, brahminoside, thankuniside, and sceffoleoside) and their aglycone acids (asiatic, madecassic, brahmic, and centellic acids), which are known for their efficacy against neurological and psychological cognition 14. The efficacy of “triterpenoid-based standardized extract of C. asiatica leaves” (INDCA) against chronic mild stress 15, social isolation stress-induced suicidal behavior 16, post-ictal depression 17, olfactory bulbectomy-induced depression and anxiety 18 with 5-HT1A and 5-HT1B receptor involvement 19.
The nasal route of administration is an attractive alternative to oral anti-migraine medications because it avoids disadvantages, such as limited effectiveness, slow onset, and poor bioavailability in the brain 20, 21. The trigeminal nerve innervates the nasal mucosa and connects the brainstem, where migraine pain originates 22, 23. In addition, intranasal SUMA acts on the trigeminal pathway to provide faster pain relief24.
Intranasal instillation of INDCA nasal solution (INDCA-NS) shown to alleviate acute pain induced by a single injection of nitroglycerin (NTG) 19. However, a single administration of NTG in animals does not mimic the pulsative behavioral symptoms of human migraine, namely episodic pain 25 and photophobia 26, as stated in the “International Classification of Headache Disorders diagnostic criteria” 27. Therefore, the present study evaluated the effects of INDCA-NS on behavioral and biochemical changes induced by repeated doses of NTG in rats, a clinically relevant animal model of recurrent migraine.
Materials and Methods
Animals
Sprague-Dawley rats (Male and female, weighing 150-200 grams) were purchased from Crystal Biological Solutions (Pune, India). The “Guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals” was followed 28. The rats were housed in polypropylene cages and maintained in a controlled environment as recommended by the CPCSEA 29. The rats were provided unrestricted access to drinking water and feed pellets supplied by Nutrivet Life Sciences (Pune, India). The protocol was approved by “Institutional Animal Ethics Committee” of study center with approval number: CPCSEA/PCL/32/2018, Dated 20-2-2019.
All observations were conducted between 9:00 AM and 4:00 PM. Each rat was used once during the experiment. A blinded observer (unaware of the administered treatment) performed all the observations. The rats were acclimated to the laboratory conditions (room temperature 25 ± 2 °C and relative humidity of 45-55% under a 12h light:12h dark cycle) before being subjected to the testing protocol.
Drugs and chemicals
NTG was procured as an injection from Samarth Life Sciences Pvt. Ltd (Mumbai, India) in ampule form (concentration 5 mg/ml) and utilized as a stock solution. The stock solution of NTG (5 mg/ml) was diluted with saline to prepare a fresh solution to administer a daily intraperitoneal (i.p.) dose of 10 mg/kg based on the body weight of each rat on alternate days from D1 to D9 to induce chronic migraine pain in rats, as reported previously 25. SUMA succinate was a gift from Lupin Pharmaceuticals (Pune, India). SUMA solutions (12 mg/ml) were freshly prepared in saline for intraperitoneal administration in rats in the volume of 0.96 µg/rat/day (80 µl/rat/day as 40 µl per rat, two times a day) to match the dose of 0.3 mg/kg as suggested earlier 30, 31.
Cortisol (Catalog no: CSB-E05112r, Cusabio, Houston, TX, USA) and “pituitary adenylate cyclase-activating polypeptide 38” (catalog number: E-EL-R1435, PACAP-38, Elab Bioscience, Houston, TX, USA) estimation in rat tissue samples was performed by Enzyme-linked immunosorbent assay (ELISA) kits, that are purchased from a local distributor (GK BioScience, Pune, India).
The test substance, INDCA-NS, was provided by Indus Biotech Limited (Pune, India) as an INDCA powder. A stock solution (1 mg/ml, 1000 μg/ml) of INDCA-NS was prepared and suitably diluted (3 and 10 times dilution). The final dose per rat was 2.5, 7.5, or 10 μg /nostril, twice daily, that is, 10, 30, and 100 μg per rat per day. Each dose was intranasally administered 30 min before NTG in a volume of 25 μl/nostril, which is a safe intranasal volume in rats 32.
Grouping and Treatment Schedule
The study was performed on 72 rats and randomized into six groups (G1–G6) of 12 rats each (six males and six females). G1 served as a vehicle control (VC) and received intranasal treatment with 25 µL of saline twice daily (50 µL/rat/day) for 21 days. G2 (NTG-C) received intraperitoneal administration of NTG (10 mg/kg) every alternate day from D1 to D9 of the study and intranasal administration of saline (25 µL/nostril twice a day, 50 µL/rat/day) for the remaining 21 days of the study (21 days). Groups G3–G6 received intraperitoneal administration of NTG (10 mg/kg) every alternate day from D1 to D9, along with other treatments as follows: In G3, the positive control (SUMA) received daily intraperitoneal treatment with SUMA succinate (80 µL/rat/day, i.e., 40 µL/rat, twice daily). Groups G4–G6 received intranasal INDCA-NS at doses of 10, 30, and 100 µg/rat/day (2.5, 7.5, and 25 µg/nostril/twice a day).
Facial Response to Pain
The pain was quantified by examining and scoring the facial features of rats from 15 to 30 min (15-min duration) of NTG administration using “rat grimace scale” (RGS) 33, 34 by an observer blinded to the treatment. During the test, rats were kept in stable cages to reduce stress. Four facial features such as Orbital tightening (visible wrinkles around the eyes, closing of the eyelid, narrowing of orbital area), Nose/cheek (and sunken look), ear changes (curl inwards and angled ‘pointed’ shape, increased space between the ears), and whisker changes (stiffened, angle along the face, ‘clump’ together, lose ‘downward’ curve) scored on the scale: zero (not present), one (moderate) and two (obvious) 33, 34. The total score for all features was calculated as the total grimace score.
Mechanical allodynia using Von Frey Filament Test
Mechanical allodynia was measured using a von Frey esthesiometer (ALMEMO 2390-5, IITC Life Science, USA), following a previously reported procedure 25, 35. Briefly, on every alternate day of the study, each rat was acclimated for 30 min in a von Frey aesthesiometer before testing for mechanical allodynia. Mechanical allodynia was measured by applying pressure to the mid-plantar region of the hind paw through a von Frey filament probe in ascending order until the reaction of the rats (paw withdrawal) was recorded on the display and paw withdrawal latency (PWL expressed in grams). The PWLs of both paws were added and analyzed.
Thermal hyperalgesia using Tail-flick Test
Thermal analgesia was measured by pain latency using a tail-flick analgesiometer (UGO Basile, Italy), as reported previously 31. Every alternate day of the study, the tail was placed on a hot wire, and 90 min after NTG administration, pain literacy (time taken by the rat to withdraw its tail) was recorded with a cut-off time of 15 seconds to prevent potential burn injuries.
Photophobia using Light-dark Box
Photophobia was evaluated using a conditioned place apparatus (VJ Instruments, Karanja, India) as previously reported 26. The apparatus consisted of two chambers: one light (white wall) and one dark (black wall) chamber. Every other day, the rat was positioned in the corner of the white chamber, with its back turned towards the experimenter. To prevent latent learning, the rats were allowed to investigate the white and dark chambers for 10 min during the habituation process. The behavior of the rats was monitored for 20 min using an automated video tracking system (MazeMaster, VJ Instruments, Karanja India) to measure “time spent in the light chamber,” “total transitions between the chambers” and “ ratio of time spent in the light to dark chamber” were calculated and analyzed.
Determination of Biochemical Markers in Plasma, Brain, and Serum Samples
Rats were euthanized on the last day of the study (day 21). Blood samples (four ml) were collected from the hearts. One milliliter of blood was drawn and allowed to clot, subsequently yielding the serum. Two milliliters of blood were mixed with an anticoagulant, ethylenediaminetetraacetic acid, and centrifuged (Remi Diagnostics, Mumbai, India) at 15,000 rpm for 15 min at 4 °C. Brain samples were collected, weighed, and homogenized in phosphate buffer solution. All biological samples (serum, plasma, and brain) were stored at -20 °C till the biochemical estimations were performed. To ensure the integrity of the samples, the blood and brain samples collected for NO measurements were preserved with addition of PBS, N-ethylmaleimide (NEM), and EDTA, and were subsequently homogenized in the same solution as previously suggested36.
The concentrations of PACAP and cortisol were estimated using ELISA kits according to the manufacturer’s instructions. The concentration of NO was determined by measuring the total nitrite concentration using Griess reaction, and absorbance was measured at 546 nm using a spectrophotometer (Shimadzu, Kyoto, Japan) 37. A plot of absorbance versus log (concentration) was plotted. The results were expressed as concentration per milliliter (plasma, serum, and brain homogenate), and per g (content/brain weight) was calculated.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical analysis was performed using GraphPad Prism for Windows (V. 8.0, GraphPad, San Diego, USA). A plot of facial response to pain (total grimace score), mechanical allodynia (PWL on von Fray), thermal hyperalgesia (PWL in tail-flick test), or photophobia (time spent in the light chamber) with “day of treatment” was plotted, “area under the curve” (AUC) was calculated and analyzed using one-way ANOVA followed by Dunnett’s test for between the groups comparison. Data on the number of transitions, the ratio of time spent between light and dark chambers, and biochemical markers (PACAP, cortisol, and NO) were analyzed using one-way ANOVA and Dunnett’s test for comparison between the groups. Differences were considered statistically significant at P < 0.05.
Results
The results obtained from pain-related responses, such as the total grimace score, tail-flick latencies, and von Fray latency, are presented in Figure 1. The effects on NTG-induced photophobia in terms of time spent in the light chamber, total transitions between chambers, and time obtained from the light-dark paradigm are presented in Figure 2. The effects of NTG-induced pain-related biomarkers, namely brain PACAP, plasma PACAP, brain NO, and serum NO, are shown in Figure 3. The effects of NTG-induced stress-related biomarkers, namely brain cortisol and serum cortisol, are presented in Figure 4.
Effects on Facial Response to Pain
One-way ANOVA of the facial response to pain (total Grimace score) data revealed a highly significant treatment effect [F(5.66) = 60.36, P < 0.001]. Dunnett’s multiple comparisons test showed NTG-C group has 700% (P < 0.001) higher Grimace score than VC. SUMA-treated rats exhibited a 48% (P < 0,.001) lower score, whereas the INDCA-NS-treated groups showed significantly (P < 0.001) and dose-dependent (50%, 72%, 78%) lower scores compared with NTG-C (Figure 1A).
Effects on Mechanical allodynia
One-way ANOVA of the mechanical allodynia data in terms of the AUC of the tail-flick latency revealed a significant treatment effect [F(5, 66) = 10.78, P < 0.001]. The 37% decrease (P < 0.05) in NTG-C group than VC. The SUMA and INDCA-NS (10) groups did not show statistically significant effects, whereas the INDCA-NS (30) and INDCA-NS (100) groups showed 72% and 119% increases (P < 0.01 and P < 0.001, respectively) vs. NTG-C (Figure 1B).
Effects on Thermal hyperalgesia
A one-way ANOVA of the thermal allodynia data in terms of the AUC of PWL revealed a significant treatment effect [F(5, 138) = 181.8, P < 0.001]. A 71% decrease (P < 0.001) was observed in the NTG-C group compared to the VC group. A statistically significant (P < 0.001) increase was observed in the PWL of the SUMA and INDCA-NS (10, 30, or 100) groups (115%, 121%, 255%, and 254% increase, respectively) compared with the NTG-C group ((Figure 1C).
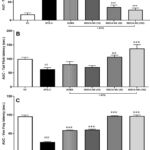 |
Figure 1: Effects of treatments on pain-related responses (A) Total Grimace score, (B) Tail Flick latency and (C) von Fray latency. |
Effects on Photophobia – AUC of Time Spent in Light Chamber
A one-way ANOVA of the time spent in the light chamber in terms of AUC during the light-dark test revealed a significant treatment effect [F(5, 66) = 12.80, P < 0.001]. The 44% decrease (P < 0.05) in NTG-C group than VC. The SUMA and INDCA-NS (10) groups did not show statistically significant effects, whereas INDCA-NS (30) and INDCA-NS (100) showed 119% (P < 0.001) and 186% increases (P < 0.001), respectively, compared to the NTG-C group (Figure 2A).
Effects on Photophobia – Ratio of Time Spent in the Light: Dark Chamber
A one-way ANOVA of the ratio of time spent in the light:dark chamber as a measure of photophobia revealed a significant treatment effect [F(5, 66) = 3.002, P < 0.05]. Dunnett’s multiple comparisons test showed significant increase (P < 0.05) in NTG-C group than VC. Significant decreases of 83% (P < 0.05) and 91%, 89%, and 95% (P < 0.001) were observed for the SUMA and INDCA-NS (10,30 or 100) treatments, respectively (v/s. NTG-C) (Figure 2B).
Effects on Photophobia – Number of Transitions during the Light-dark Test
One-way ANOVA of the number of transitions during the light-dark test revealed a significant treatment effect [F(5, 66) = 5.937, P < 0.001]. Dunnett’s multiple comparisons test showed 34% decrease (P < 0.05) in NTG-C group than VC. The SUMA and INDCA-NS (10) groups did not show statistically significant effects, whereas INDCA-NS (30) and INDCA-NS (100) showed 68% (P < 0.01) and 59% increases (P < 0.05), respectively, compared to the NTG-C group (Figure 2C).
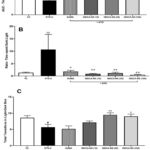 |
Figure 2: Effects of treatments on NTG-induced photophobia in Light-dark paradigm (A) Time spent in light chamber (B) Ratio of time spent in dark v/s light chamber and (C) Total transitions between chambers. |
Effects on Biochemical Markers
Brain PACAP
One-way ANOVA of brain PACAP levels showed a significant treatment effect [F(5, 18) = 311.89, P < 0.001]. There was a significant increase (158%, P < 0.01) in the NTG-C group compared to the VC group. A significant decrease of 100% (P < 0.001) and 99%, 94%, and 99% (P < 0.001) was observed in the SUMA and INDCA-NS (10,30 or 100) treatments, respectively, compared to the NTG-C group (Figure 3A).
Plasma PACAP
One-way ANOVA of plasma PACAP levels revealed significant treatment-related effects [F(5, 50) = 2.148, P < 0.05]. A significant increase (169%, P < 0.05) in the Plasma PACAP levels was observed in the NTG-C group (vs. VC). The SUMA and INDCA-NS (10, 30, or 100) groups showed a decline (not significant) in plasma PACAP levels compared with the NTG-C group (Figure 3B).
Nitric oxide in the brain
One-way ANOVA of brain Nitric oxide levels showed no significant treatment-related effects [F(5, 35) = 1.159, not significant]. Furthermore, Dunnett’s multiple comparison tests did not reveal significant changes in plasma NO levels in the NTG-C group (vs. VC), SUMA 980), or INDCA-NS-treated groups (vs. NTG-C group) (Figure 3C).
Nitric Oxide in Plasma
One-way ANOVA of plasma NO levels showed a significant treatment effect [F(5, 20) = 6.446, P < 0.001]. There was a significant increase (8%, P < 0.05) in the NTG-C group compared with that in the VC group. The SUMA group showed a 5.4% (not significant) decrease compared with the VC group. INDCA-NS (10,30 or 100) treatments are shown. The plasma NO levels decreased by 11% (P < 0.01), 12% (P < 0.001), and 10% (P < 0.01), respectively( Figure 3D).
 |
Figure 3: Effects of treatment on NTG-induced pain-related biomarkers: (A) Brain PACAP, (B) Plasma PACAP, (C) Brain NO (D) serum NO. |
Brain Cortisol
One-way ANOVA of brain cortisol levels showed a significant treatment effect [F(5, 18) = 11.00, P < 0.001]. Dunnett’s multiple comparisons test showed a huge and significant increase (773%, P < 0.01) in NTG-C group than VC. A significant decrease of 98% (P < 0.001), 96% ( P < 0,001), 65% ( P < 0.01), and 64% (P < 0.01) was observed in the SUMA and INDCA-NS (10,30 or 100) treatments, respectively, compared to the NTG-C group (Figure 4A).
Serum Cortisol
One-way ANOVA of serum cortisol levels showed significant treatment effects [F(5, 35) = 5.130, P < 0.01]. Dunnett’s multiple comparisons test showed significant increase (127%, P < 0.01) in NTG-C group than VC. A significant decrease of 47% (P < 0.01) and 48% (P < 0.01) was observed in the SUMA and INDCA-NS (100) groups, respectively, compared to the NTG-C group. However, the 20% and 19% decrease in serum cortisol levels observed in the INDCA-NS (10) and INDCA-NS (30) groups, respectively, compared to the NTG-C group, were not statistically significant (Figure 4B).
 |
Figure 4: Effects of treatments on NTG-induced stress biomarker (A) Brain cortisol (B) Serum cortisol VC- Vehicle Control. |
Discussion
Many medications are used to manage migraine attacks and to reduce the intensity and duration of pain. However, migraine prophylaxis is challenging due to the limited availability of effective and safe options. Current prophylactic treatments often involve repurposed antihypertensives or antiepileptic medications, each with its own set of side effects and usage limitations 38.
The present study evaluated the efficacy of INDCA-NS against NTG-induced migraine-like pain in rats mimicking chronic recurrent migraine. The intense pulsating pain of migraine is progressive and is often accompanied by various stress-related symptoms 39, 40. In the present study, INDCA-NS prevented TG-induced symptoms of pain (facial expression, mechanical, and thermal allodynia) and stress- (photophobia) related behavior and biochemical markers (PACAP, NO, and cortisol) in rats.
Intraperitoneal administration of NTG mimics some key features of migraine, including trigeminal neuron activation and nociceptive behavior, owing to its vasodilatory effects 39. NTG-induced migraine-like pain in animals is accompanied by allodynia 41, involves brainstem regions such as human migraine attacks 42, and is blocked by known prophylactic agents 43. Therefore, NTG has been extensively used to induce migraine-like symptoms in experimental animals 44.
However, studies on NTG-induced migraine with a single injection do not fully represent clinical migraine in terms of induction frequency (episodes) or behavioral endpoints 45 and have poor clinical relevance 26. Recent reports have confirmed the need for recurrent NTG episodes, such as light sensitivity and photophobia, to produce the clinically relevant symptoms of chronic migraine such as light sensitivity, photophobia 31. Repeated NTG injections in rodents have been reported to produce clinically relevant features, such as frequency and clinical symptoms of recurrent migraine26, and are suitable for evaluating agents against chronic migraine conditions 26, 31.
Intranasal administration of a single SUMA was effective against acute migraine attacks in two clinical studies 46. Recently, SUMA nasal spray has been approved for the treatment of acute migraine with or without aura 47. Therefore, intranasal SUMA was used as a positive control in the present study.
Migraineurs experience hypersensitivity to various sensory modalities including light, sound, and touch. The present study employed the RGS as an indicator of facial pain in a rodent model of migraine 25. The RGS is a valuable tool for assessing nociceptive behavior in rodents, exhibiting good construct validity and inter-rater reliability 34. NTG induction showed enhanced RGS scores, which correlated well with clinical symptoms such as orbital tightening, ne/cheek flattering, ear changes, and whisker changes related to hypersensitivity and altered nociceptive processing in migraine 25. Treatment with INDCA-NS resulted in a marked reduction in RGS scores, indicating a reduction in nociceptive processing.
Episodic migraine is associated with allodynia during or after a migraine attack 48. These include mechanical allodynia, in which nociceptive signaling of the trigeminal nerve is involved 49. Mechanical allodynia refers to the perception of pain in response to normally non-painful stimuli, and the von Frey hair test allows for the quantification of this hypersensitivity 25. The roles of signaling molecules, neurotransmitters, neuroinflammation, neuronal excitability, and sex differences make mechanical allodynia a multifaceted phenomenon 50-52.
The efficacy of INDCA-NS in reducing mechanical allodynia and improving pain latency in this study indicates migraine prophylaxis efficacy through neuronal excitability and sensitization, perhaps similar to topiramate 53.
Thermal hyperalgesia is a significant feature of the clinical pathophysiology of migraines, reflecting altered pain-processing mechanisms 54, 55. The latency of the pain response, tail flick, by rodents after touching the tail with a heat source, is a reliable and validated procedure for the quantitative measurement of thermal hyperalgesia 25. INDCA-NS prevented thermal hyperalgesia in a dose-dependent manner, with higher tail-flick latencies in rats.
The induction of thermal hyperalgesia by intraperitoneal treatment with NTG in rodents 56 and its prevention by SUMA, a serotonin antagonist, has been reported 57. Similar thermal hyperthermia induction by serotonin and prevention of serotonin-induced thermal hyperalgesia by SUMA suggest a role for serotonin receptors in thermal hyperalgesia 58. Therefore, serotonin may have contributed to the alleviation of INDCA-NS-induced NTG-induced thermal hyperalgesia.
Photophobia, an extreme aversion to light, is a commonly reported clinical feature of migraine, especially during the aura and headache phases. Enhanced sensitivity to light in photophobia significantly reduces the quality of life. NTG injections have been reported to produce photophobia 59. In this study, subacute treatment with INDCA-NS prevented NTG-induced photophobia in a dose-dependent manner, as indicated by light-dak box measurements. All photophobia parameters (light-dark box paradigm) in the NTG group were reversed in the INDCA-NS group but not in the SUMA group.
Recently, “calcitonin gene-related peptide” (CGRP) injections were reported to stimulate posterior thalamic nuclei and induce photophobic behavior in mice 60. In contrast, CGRP antagonists are highly effective for migraine treatment 61. Therefore, the role of CGRP in the anti-photophobic action of INDCA-NS cannot be ruled out.
PACAP has been identified as a promising target in migraine management 62. The variation in PACAP levels in migraine patients during attacks compared to controls suggests its potential as a biomarker 63. PACAP has been reported to induce migraine-like symptoms in humans and rodents, including light aversion and tactile allodynia, possibly via vasodilatory mechanisms 64.
Existing evidence indicates a direct 65 or indirect 66 influence of SUMA on circulating PACAP levels. In this study, NTG administration in rats increased the PACAP levels in the brain and plasma. SUMA inhibits neuronal activity through 5-HT1B/D receptors and causes vasoconstriction of cranial blood vessels to reduce migraine-like pain 67. In contrast, PACAP acts as a vasodilator and potentially contributes to migraine by influencing trigeminal nerve sensitization and inflammatory processes 64. A recent clinical study showed that SUMA prevented PACAP-induced migraine-like pain and strongly suggested a vasodilator or neuromodulator role for PACAP in the efficacy of SUMA65. SUMA and INDCA-NS significantly prevented NTG-induced elevation of PACAP in the brain, but not in the plasma, suggesting the prevention of cranial vasodilation without affecting peripheral blood vessels to prevent NTG-induced migraine-like pain. NO is a potent vasodilator that triggers migraine headache attacks when administered as a donor, such as nitroglycerin, in humans and rodents 68.
NTG-derived NO activates different brain regions and increases nitrite levels, thereby inducing vasodilation and nociceptive transmission 59. In the present study, NTG showed enhanced NO levels in the serum but not in the brain. In the present study, subcutaneous SUMA administration showed a trend (not statistically significant), indicating the prevention of NTG-induced elevated NO levels by intravenous SUMA, as previously reported 69. In addition, INDCA-NS prevented NTG-induced increases in serum NO levels. These results are in line with the NO-reducing or modulating properties of C. asiatica and its triterpenoid constituents 70-72, especially asiaticoside 73 and madecassoside 74, which are markers of INDCA-NS.
In addition, preventing PACAP increase in the brain causes internalization of serotonin receptors in cortical neurons and attenuates the antinociceptive action of serotonin agonists 75. Moreover, the upregulation of PACAP with chronic stress drives anxiety-like behaviors 76. By reducing PACAP levels in the brain, as shown in the present study, INDCA-NS may prevent PACAP’s functional dampening of antinociception and stress reduction.
Serotonin also plays a crucial role in smell perception, beyond mood regulation 77. The serotonergic system is known to regulate odor-sensing and olfactory processing 77, 78. In addition, disruption of serotonin signaling pathways within the olfactory bulb can lead to impaired odor detection 79. The serotonergic properties of INDCA-NS can protect rodents against anosmia (loss of smell) and potential side effects of the nasal route of administration. In fact, protection from anosmia offered by INDCA-NS (100 µg/rat/day) has been confirmed in a subacute repeated-dose toxicology study 80
Oral supplementation with INDCA has been reported to ameliorate NTG-induced migraine-like pain through its 5-HT1A/1 B agonist properties 19. Evidence suggests a dynamic interplay between 5-HT1A activity and migraine susceptibility. 81, 82. The role of hypersensitivity of central 5HT1A receptors in migraine and associated behavioral symptoms, including anxiety and depression, has been reported. 83-85. Therefore, the migraine prophylaxis efficacy of INDCA-NS shown in this study can be attributed, at least in part, to selective 5HT1A agonist properties.
Cortisol, a stress hormone, influences the onset and severity of migraines 49, 86. Stress releases cortisol, which, in turn, can modulate pain perception and trigger migraine episodes 87. In addition, stress reduction techniques can positively affect migraine frequency and severity by potentially modulating stress response and cortisol levels 88. Repeated NTG injections have been reported to produce chronic migraine and stress symptoms including depression and anxiety 89. Repeated NTG injections enhanced brain and plasma cortisol levels and induced photophobia, as observed in the present study. In contrast, subacute co-treatment of SUMA- or INDCA-NS with NTG prevented cortisol elevation in the brain, plasma, and photophobia (light-dark box paradigm), indicating the stress-reducing properties of SUMA and INDCA-NS. Clinical photophobia is associated with psychiatric disorders such as anxiety and depression, hypothalamic-pituitary-adrenal axis dysregulation, and elevated cortisol levels 90. Moreover, the present results strongly support the anti-stress properties of orally administered INDCA against stress-induced behavioral conditions such as depression and anxiety in animal studies 14, 18.
Elevated cortisol levels are associated with a considerable increase in serotonin uptake during chronic stress 91 and depression 92. In addition, the cortisol reduction properties of INDCA-NS might have increased serotonin availability through serotonin transporter downregulation 83-85 and prevented chronic stress-induced anxiety or photophobia, as shown in the present study.
The robust safety profile of the subacute administration of INDCA-NS 80, including the cardiovascular system 93, with the prophylactic efficacy shown in the present study, makes INDCA-NS a suitable option for migraine prophylaxis. However, a well-designed clinical study of patients with migraine is useful.
Conclusion
In conclusion, INDCA-NS showed prevention of NTG-induced migraine-like symptoms such as pain, photophobia, and stress, and can be developed as a promising option for migraine prophylaxis after clinical studies.
Acknowledgment
The authors would like to acknowledge Dr. K.R. Mahadik, Ex-Principal, Poona College of Pharmacy, Bharati Vidyapeeth deemed university, Pune, India
Conflict of Interest
The authors declare no conflicts of interest.
Funding Sources
The study was supported by Indus Biotech Limited, Pune, India (Grant Project No: IBS433)
Authors Contributions
Prasad Thakurdesai: Conceptualization, Methodology, Writing – review and editing, Visualization, Supervision, Funding acquisition. Pooja Bhalerao: Investigation, Formal analysis. Urmila Aswar: Methodology, Software, Validation, Formal analysis, Resources, Data curation, Writing – original draft, Writing – Review and editing, Supervision, Project administration
References
- Gupta J, Gaurkar SS. Migraine: An Underestimated Neurological Condition Affecting Billions. Cureus. 2022;14(8):e28347.
CrossRef - Weatherall MW. The diagnosis and treatment of chronic migraine. Ther Adv Chronic Dis. 2015;6(3):115-23.
CrossRef - Urits I, Gress K, Charipova K, Zamarripa AM, Patel PM, Lassiter G, et al. Pharmacological options for the treatment of chronic migraine pain. Best Pract Res Clin Anaesthesiol. 2020;34(3):383-407.
CrossRef - Di Lorenzo C, Coppola G. Obesity, diet and nutraceuticals. J Headache Pain. 2015;16(1):A28.
CrossRef - Lombard L, Farrar M, Ye W, Kim Y, Cotton S, Buchanan AS, et al. A global real-world assessment of the impact on health-related quality of life and work productivity of migraine in patients with insufficient versus good response to triptan medication. J Headache Pain. 2020;21(1):1-16.
CrossRef - IHS. Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211.
CrossRef - Krishnan A, Chowdhury D. Burden, disability and public health importance of headache disorders in india. Neurol India. 2021;69(7):4.
CrossRef - Šagud M, Klinar I. Psychiatric Comorbidity in Migraine. Medicus. 2021;30(1 Migrena):105-.
- Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. 2019;20(1):117.
CrossRef - D’Amico D, Tepper SJ, D’Amico D. Prophylaxis of migraine: general principles and patient acceptance. Neuropsychiatr Dis Treat. 2008;4(6):1155.
CrossRef - Assadpour S, Shiran MR, Asadi P, Akhtari J, Sahebkar A. Harnessing intranasal delivery systems of sumatriptan for the treatment of migraine. Biomed Res Int. 2022;2022:1-9.
CrossRef - Orhan IE. Centella asiatica (L.) Urban: from traditional medicine to modern medicine with neuroprotective potential. Evid Based Complement Alternat Med. 2012;2012:946259.
CrossRef - James JT, Dubery IA. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules. 2009;14(10):3922-41.
CrossRef - Thakurdesai P. Centella asiatica (Gotu kola) leaves: potential in neuropsychiatric conditions. In: Ghosh D, editor. Nutraceuticals in Brain Health and Beyond. 1st ed. London: Elsevier, Inc; 2021. p. 307-27.
CrossRef - Aswar M, Yanna V, Aswar U, Thakurdesai P, Mohan V, editors. Asiaticoside (INDCA) ameliorates cognitive impairment in chronic mild stress (CMS) model in wistar rats. 48th Annual Conference of Indian Pharmacological Society, IPSCON2015; 2015 Dec 28-20, 2005; Saurashtra University, Rajkot, India: Indian Pharmacological Society.
- Aswar U, Kalshetty P, Thakurdesai PA, Mohan V, editors. Evaluation of standardized extract of Centella asciatica leaves on suicidal behavior related traits in laboratory rats [PZD-3 received PC Dandiya Award]. 46th Annual Conference of Indian Pharmacological Society and International Conference on Translational Medicine; 2013 December 16-18, 2013; Bangalore, India: Indian Pharmacological Society.
- Mukhrjee A, Sinha A, Kandhare A, Bodhankar S, Mohan V, Thakurdesai P, editors. Evaluation of potential of standardized extract of Centella asiatica (L.) Urban leaves in epilepsy and associated post-ictal depression. 2nd International Congress of Society for Ethnopharmacology (SFEC – 2015); 2015 Februory 20 – 22,2O15; Nagpur: Society for Ethnopharmacology.
- Kalshetty P, Aswar U, Mohan V, Bodhankar SL, Arulmozhi S, Thakurdesai PA. Antidepressant effects of standardized extract of Centella asiatica L in olfactory bulbectomy model. Biomedicine & Aging Pathology. 2012;2(2):48-53.
CrossRef - Bobade V, Bodhankar SL, Aswar U, Vishwaraman M, Thakurdesai P. Prophylactic effects of asiaticoside-based standardized extract of Centella asiatica (L.) Urban leaves on experimental migraine: Involvement of 5HT1A/1B receptors. Chin J Nat Med. 2015;13(4):274-82.
CrossRef - Keller LA, Merkel O, Popp A. Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Deliv Transl Res. 2022;12(4):735-57.
CrossRef - Giunchedi P, Gavini E, Bonferoni MC. Nose-to-Brain Delivery. Pharmaceutics. 2020;12(2):138.
CrossRef - Katare P, Pawar Medhe T, Nadkarni A, Deshpande M, Tekade RK, Benival D, et al. Nasal drug delivery system and devices: An overview on health effects. J Che Health Saf. 2024:3c00069.
CrossRef - Johnson NJ, Hanson LR, Frey WH. Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol Pharm. 2010;7(3):884-93.
CrossRef - Derry CJ, Derry S, Moore RA. Sumatriptan (intranasal route of administration) for acute migraine attacks in adults. Cochrane Database Syst Rev. 2012;2019(5):CD009663.
CrossRef - Kim S-J, Yeo J-H, Yoon S-Y, Kwon S-G, Lee J-H, Beitz AJ, et al. Differential development of facial and hind paw allodynia in a nitroglycerin-induced mouse model of chronic migraine: role of capsaicin sensitive primary afferents. Biol Pharm Bull. 2018;41(2):172-81.
CrossRef - Harris HM, Carpenter JM, Black JR, Smitherman TA, Sufka KJ. The effects of repeated nitroglycerin administrations in rats; modeling migraine-related endpoints and chronification. J Neurosci Methods. 2017;284:63-70.
CrossRef - International Headache Society. Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808.
CrossRef - Goyal RK, Bhise SB, Srinivasan BP, Rao CM, Sen T, Koneri R. Curriculum for pharmacology in pharmacy institutions in India: opportunities and challenges. Indian J Pharmacol. 2014;46(3):241-5.
CrossRef - CPCSEA. CPCSEA guidelines for laboratory animal facility. Indian J Pharmacol. 2003:257-74.
- Davis ME. The effect of sumatriptan on clinically relevant behavioral endpoints in a recurrent nitroglycerin migraine model in rats [Honors Degree]. Oxford: University of Mississippi; 2014.
- Sufka KJ, Staszko SM, Johnson AP, Davis ME, Davis RE, Smitherman TA. Clinically relevant behavioral endpoints in a recurrent nitroglycerin migraine model in rats. J Headache Pain. 2016;17(1):40.
CrossRef - Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci. 2011;50(5):600-13.
- Harris HM, Carpenter JM, Black JR, Smitherman TA, Sufka KJ. The effects of repeated nitroglycerin administrations in rats; modeling migraine-related endpoints and chronification. Journal of Neuroscience Methods. 2017;284:63-70.
CrossRef - Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55.
CrossRef - Farkas S, Bölcskei K, Markovics A, Varga A, Kis-Varga Á, Kormos V, et al. Utility of different outcome measures for the nitroglycerin model of migraine in mice. J Pharmacol Toxicol Methods. 2016;77:33-44.
CrossRef - Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43(5):645-57.
CrossRef - Ozbey U, Erisir M, Seyran A, Benzer F. Changes in plasma nitric oxide levels during migraine initial and attack periods in migraine patients. Afr J Pharm Pharmacol. 2013;7:822-6.
- Sprenger T, Viana M, Tassorelli C. Current prophylactic medications for migraine and their potential mechanisms of action. Neurotherapeutics. 2018;15(2):313-23.
CrossRef - Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev. 2017;97(2):553-622.
CrossRef - Korkmaz S, Kazgan A, Korucu T, Gönen M, Yilmaz MZ, Atmaca M. Psychiatric symptoms in migraine patients and their attitudes towards psychological support on stigmatization. J Clin Neurosci. 2019;62:180-3.
CrossRef - de Tommaso M, Libro G, Guido M, Difruscolo O, Losito L, Sardaro M, et al. Nitroglycerin induces migraine headache and central sensitization phenomena in patients with migraine without aura: a study of laser evoked potentials. Neurosci Lett. 2004;363(3):272-5.
CrossRef - Bahra A, Matharu MS, Buchel C, Frackowiak RSJ, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet. 2001;357(9261):1016-7.
CrossRef - Tvedskov JF, Thomsen LL, Thomsen LL, Iversen HK, Williams P, Gibson A, et al. The effect of propranolol on glyceryltrinitrate-induced headache and arterial response. Cephalalgia. 2004;24(12):1076-87.
CrossRef - Demartini C, Greco R, Zanaboni AM, Sances G, De Icco R, Borsook D, et al. Nitroglycerin as a comparative experimental model of migraine pain: From animal to human and back. Prog Neurobiol. 2019;177:15-32.
CrossRef - Mathew NT, Kailasam J, Seifert T. Clinical recognition of allodynia in migraine. Neurology. 2004;63(5):848-52.
CrossRef - Ryan R, Elkind A, Baker CC, Mullican W, DeBussey S, Asgharnejad M. Sumatriptan nasal spray for the acute treatment of migraine. Neurology. 1997;49(5):1225-30.
CrossRef - Martin V, Hoekman J, Aurora SK, Shrewsbury SB. Nasal delivery of acute medications for migraine: The upper versus lower nasal space. J Clin Med. 2021;10(11):2468.
CrossRef - Costa A, Smeraldi A, Tassorelli C, Greco R, Nappi G. Effects of acute and chronic restraint stress on nitroglycerin-induced hyperalgesia in rats. Neurosci Lett. 2005;383(1-2):7-11.
CrossRef - Levy D, Strassman AM, Burstein R. A critical view on the role of migraine triggers in the genesis of migraine pain. Headache. 2009;49(6):953-7.
CrossRef - LoPinto C, Young W, Ashkenazi A. Comparison of dynamic (brush) and static (pressure) mechanical allodynia in migraine. Cephalalgia. 2006;26(7):852-6.
CrossRef - Chen Y, Zhou Y, Li X-C, Ma X, Mi W-L, Chu Y-X, et al. Neuronal GRK2 regulates microglial activation and contributes to ectroacupuncture analgesia on inflammatory pain in mice. Biol Res. 2022;55(1):5.
CrossRef - Della Pietra A, Gómez Dabó L, Mikulenka P, Espinoza-Vinces C, Vuralli D, Baytekin I, et al. Mechanosensitive receptors in migraine: a systematic review. J Headache Pain. 2024;25(1):6.
CrossRef - Krusz JC. Prophylaxis for chronic daily headache and chronic migraine with neuronal stabilizing agents. Curr Pain Headache Rep. 2002;6(6):480-5.
CrossRef - Sand T, Zhitniy N, Nilsen K, Helde G, Hagen K, Stovner L. Thermal pain thresholds are decreased in the migraine preattack phase. Eur J Neurol. 2008;15(11):1199-205.
CrossRef - Meents JE, Neeb L, Reuter U. TRPV1 in migraine pathophysiology. Trends Mol Med. 2010;16(4):153-9.
CrossRef - Tassorelli C, Greco R, Wang D, Sandrini M, Sandrini G, Nappi G. Nitroglycerin induces hyperalgesia in rats—a time-course study. Eur J Pharmacol. 2003;464(2-3):159-62.
CrossRef - Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, et al. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 2009;30(2):170-8.
CrossRef - Loyd DR, Chen PB, Hargreaves KM. Anti-hyperalgesic effects of anti-serotonergic compounds on serotonin- and capsaicin-evoked thermal hyperalgesia in the rat. Neuroscience. 2012;203:207-15.
CrossRef - Sureda-Gibert P, Romero-Reyes M, Akerman S. Nitroglycerin as a model of migraine: Clinical and preclinical review. Neurobiol Pain. 2022;12:100105.
CrossRef - Sowers LP, Wang M, Rea BJ, Taugher RJ, Kuburas A, Kim Y, et al. Stimulation of posterior thalamic nuclei induces photophobic behavior in mice. Headache. 2020;60(9):1961-81.
CrossRef - Mohanty D, Lippmann S. CGRP Inhibitors for Migraine. Innov Clin Neurosci. 2020;17(4-6):39-40.
- Waschek JA, Baca SM, Akerman S. PACAP and migraine headache: immunomodulation of neural circuits in autonomic ganglia and brain parenchyma. J Headache Pain. 2018;19(1):23.
CrossRef - Kuburas A, Russo AF. Shared and independent roles of CGRP and PACAP in migraine pathophysiology. J Headache Pain. 2023;24(1):34.
CrossRef - Tanaka M, Szabo A, Kortesi T, Szok D, Tajti J, Vecsei L. From CGRP to PACAP, VIP, and beyond: Unraveling the next chapters in migraine treatment. Cells. 2023;12(22):2649.
CrossRef - Wienholtz NKF, Christensen CE, Zhang DG, Coskun H, Ghanizada H, Al-Karagholi MA-M, et al. Early treatment with sumatriptan prevents PACAP38-induced migraine: A randomised clinical trial. Cephalalgia. 2021;41(6):731-48.
CrossRef - Hansen JM, Fahrenkrug J, Petersen J, Wienecke T, Olsen KS, Ashina M. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) in the circulation after sumatriptan. Scand J Pain. 2013;4(4):211-6.
CrossRef - Miner J, Moore J. Subcutaneous delivery of sumatriptan in the treatment of migraine and primary headache. Patient Prefer Adherence. 2012;6:27.
CrossRef - Neri M, Frustaci A, Milic M, Valdiglesias V, Fini M, Bonassi S, et al. A meta-analysis of biomarkers related to oxidative stress and nitric oxide pathway in migraine. Cephalalgia. 2015;35(10):931-7.
CrossRef - Suwattanasophon C, Phansuwan-Pujito P, Srikiatkhachorn A. 5-HT1B/1D serotonin receptor agonist attenuates nitroglycerin-evoked nitric oxide synthase expression in trigeminal pathway. Cephalalgia. 2003;23(8):825-32.
CrossRef - Chanana P, Kumar A. Possible involvement of nitric oxide modulatory mechanisms in the neuroprotective effect of centella asiatica against sleep deprivation induced anxiety like behaviour, oxidative damage and neuroinflammation. Phytother Res. 2016;30(4):671-80.
CrossRef - Sari DCR, Aswin S, Susilowati R, Ar-Rochmah M, Prakosa D, Romi M, et al. Ethanol extracts of Centella asiatica leaf improves memory performance in rats after chronic stress via reducing nitric oxide and increasing Brain-Derived Neurotrophic Factor (BDNF) Concentration. GSTF Journal of Psychology (JPsych). 2014;1(1):9.
CrossRef - Bunaim MK, Kamisah Y, Mohd Mustazil MN, Fadhlullah Zuhair JS, Juliana AH, Muhammad N. Centella asiatica (L.) Urb. prevents hypertension and protects the heart in chronic nitric oxide deficiency rat model. Front Pharmacol. 2021;12:742562.
CrossRef - Bhaumik SK, Paul J, Naskar K, Karmakar S, De T. Asiaticoside induces tumour-necrosis-factor-α-mediated nitric oxide production to cure experimental visceral leishmaniasis caused by antimony-susceptible and-resistant Leishmania donovani strains. J Antimicrob Chemother. 2012;67(4):910-20.
CrossRef - Liu S, Li G, Tang H, Pan R, Wang H, Jin F, et al. Madecassoside ameliorates lipopolysaccharide-induced neurotoxicity in rats by activating the Nrf2-HO-1 pathway. Neurosci Lett. 2019;709:134386.
CrossRef - Hayata-Takano A, Shintani Y, Moriguchi K, Encho N, Kitagawa K, Nakazawa T, et al. PACAP–PAC1 Signaling Regulates Serotonin 2A Receptor Internalization. Front Endocrinol (Lausanne). 2021;12:732456.
CrossRef - Boucher MN, May V, Braas KM, Hammack SE. PACAP orchestration of stress-related responses in neural circuits. Peptides. 2021;142:170554.
CrossRef - Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci. 2009;12(6):784-91.
CrossRef - Fomin-Thunemann N, Garaschuk O. Role of serotonin in modulating the development and function of adult-born neurons in the olfactory bulb. Neural Regen Res. 2022;17(6):1253-4.
CrossRef - Sen A. Does serotonin deficiency lead to anosmia, ageusia, dysfunctional chemesthesis and increased severity of illness in COVID-19? Med Hypotheses. 2021;153:110627.
CrossRef - Thakurdesai P, Nimse S, Deshpande P. Characterization and preclinical toxicity assessment of intranasal administration of standardized extract of Centella asiatica (l.) Urban leaves (INDCA-NS) in laboratory rats. Toxicol Int. 2023:391-407.
CrossRef - Tanaka M, Török N, Vécsei L. Are 5-HT1 receptor agonists effective anti-migraine drugs? Expert Opin Pharmacother. 2021;22(10):1221-5.
CrossRef - Katalinic D, Vcev A, Smolic M, Aleric I. Serotonin receptor agonists in the treatment of migraine: A meta-analysis considering possible connection with paresthesia. Ann Indian Acad Neurol. 2022;25(3):332.
CrossRef - Hamel E, Currents H. Serotonin and migraine: Biology and clinical implications. Cephalalgia. 2007;27(11):1293-300.
CrossRef - Aswar U, Shende H, Aswar M. Buspirone, a 5-HT1A agonist attenuates social isolation-induced behavior deficits in rats: a comparative study with fluoxetine. Behav Pharmacol. 2022;33(5):309-21.
CrossRef - Pancheri C, Maraone A, Roselli V, Altieri M, Di Piero V, Biondi M, et al. The role of stress and psychiatric comorbidities as targets of non-pharmacological therapeutic approaches for migraine. Riv Psichiatr. 2020;55(5):262-8.
- Lippi G, Mattiuzzi C. Cortisol and migraine: a systematic literature review. Agri. 2017;29(3):95-9.
CrossRef - Marin PA. Pharmacologic management of migraine. J Am Acad Nurse Pract. 1998;10(9):407-12.
CrossRef - Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: A race against the development of cutaneous allodynia. Ann Neurol. 2004;55(1):19-26.
CrossRef - Farajdokht F, Babri S, Karimi P, Mohaddes G. Ghrelin attenuates hyperalgesia and light aversion-induced by nitroglycerin in male rats. Neurosci Lett. 2016;630:30-7.
CrossRef - Beech EL, Riddell N, Murphy MJ, Crewther SG. Sex and stress hormone dysregulation as clinical manifestations of hypothalamic function in migraine disorder: A meta-analysis. Eur J Neurosci. 2023;58(4):3150-71.
CrossRef - Tafet GE, Idoyaga-Vargas VP, Abulafia DP, Calandria JM, Roffman SS, Chiovetta A, et al. Correlation between cortisol level and serotonin uptake in patients with chronic stress and depression. Cogn Affect Behav Neurosci. 2001;1(4):388-93.
CrossRef - Tafet GE, Toister-Achituv M, Shinitzky M. Enhancement of serotonin uptake by cortisol: A possible link between stress and depression. Cogn Affect Behav Neurosci. 2001;1(1):96-104.
CrossRef - Uchale P, Patil V, Arulmathi S, Mahadik KR, Thakurdesai PA, editors. Safety pharmacology of intranasal administration of standardized extract of Centella asiatica leaves (INDCA-NS) on central nervous system and cardiovascular system using ICH guidelines in Wistar rats. International Conference on Emerging Trends in Delivery of Phytoconstituents and Ethnopharmacology – Validation of Traditional Medicine -II; 2019 29-30 November, 2019; Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune: Society of Ethnopharmacology, Pune Chapter.
Abbreviation List
5-HT : Serotonin; ANOVA : Analysis of Variance; AUC : Area under the curve; CA : Centella asiatica (L.) Urban; CPCSEA: Guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals; EDTA : ethylenediaminetetraacetic acid; ELISA : Enzyme-Linked immunosorbent Assay; IAEC : Institutional Animal Ethics Committee; INDCA-NS: Triterpenoids based standardized extracts of CA leaves – Nasal solution; NEM: N-ethylmaleimide; NO: nitric oxide; NTG: Nitroglycerine; NTG-C: Nitroglycerin control; PACAP: Pituitary Adenylate Cyclase-Activating Polypeptide; PWL: Paw withdrawal latency; RGS: Rat Grimace Scale; SUMA: Sumatriptan Succinate; VC: Vehicle control







