Manuscript accepted on :12-08-2024
Published online on: 05-09-2024
Plagiarism Check: Yes
Reviewed by: Dr. Nadhim M. H and Dr. Avinash kumar Singh
Second Review by: Dr. Pravinkumar Darji
Final Approval by: Dr. Mariia Shanaida
Venkatesan Kotteeswaran* , Mrinalini Saravanakumar
, Mrinalini Saravanakumar , Roshelle Mary Alexander
, Roshelle Mary Alexander , Radhika S Nair
, Radhika S Nair![]() and Kavin M Ramnath
and Kavin M Ramnath![]()
Department of Biotechnology, SRM Institute of Science and Technology, Kattankulathur, India.
Corresponding Author E-Mail: venkatek@srmist.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2953
Abstract
The field of wound healing is currently experiencing a ground-breaking transformation with the introduction of nanofiber technology. This groundbreaking discovery has the potential to revolutionize regenerative medicine and tissue engineering worldwide, effectively meeting the growing need for innovative and affordable healthcare solutions. This comprehensive review explores the evolution and utilization of nanofibers to enhance wound healing. Nanofibers, known for their ability to mimic the extracellular matrix of human tissue, play a crucial role in facilitating cell growth and wound repair. The review explores sophisticated techniques like electrospinning and the integration of bioactive agents such as antibiotics and growth factors with nanofibers. It emphasizes on the precision with which these nanofibers are customized to address specific therapeutic requirements. Additionally, the review sheds light on the use of both natural and synthetic polymers in crafting biocompatible nanofibers, which significantly reduces healing time while ensuring optimal aesthetic recovery, meeting the expectations of patients with minimal scarring. It emphasizes the synergy of expertise from materials science, biology,and clinical practices in propelling nanofiber-based therapies from the laboratory to the forefront of clinical care. Through this succinct overview, we aim to underscore the immense potential of nanofibers in transforming wound care. We are offering a glimpse into a future where effective and efficient healing is within reach, marking a remarkable leap forward in the global quest for advanced healthcare solutions.
Keywords
Anti-Microbial; Nanofibers; Skin repair; Tissue engineering; Wound dressing; Wound healing
Download this article as:| Copy the following to cite this article: Kotteeswaran V, Saravanakumar M, Alexander R. M, Nair R. S, Ramnath K. M. Advancement of Nanofibers in Wound Healing: A Review. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Kotteeswaran V, Saravanakumar M, Alexander R. M, Nair R. S, Ramnath K. M. Advancement of Nanofibers in Wound Healing: A Review. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/47cT9KI |
Introduction
Wound healing is the natural process of restoring the integrity and functionality of tissue after an injury. The process is a biological response coordinated through a sequence of events, namely the four phases: hemostasis, inflammation, proliferation, and remodelling. The innate healing mechanism however might prove to be insufficient in complete restoration of tissue integrity and function, especially in cases of extensive injury or chronic wounds. Biomaterials provide a solution in such instances in facilitating and enhancing the wound healing.1,2
Wound Healing
The biological process is categorised into four continuous and overlapping phases hemostasis, inflammation, proliferation, and remodelling, which results in tissue restoration. Skin is the most commonly and widely affected organ in case of injury or wounds. Depending on the extent of the injury, skin wounds are the result of degradation of the epidermal, dermal and hypodermal layers of the skin. Acute wounds follow a structured wound healing process, usually regaining skin integrity in 4–12 weeks.3 In contrast, chronic wounds take longer to heal—more than 12 weeks—which increases the risk of infection. The optimal healing process involves rapid hemostasis; inflammation; mesenchymal cell differentiation, proliferation , and migrationand angiogenesis ; re-epithelialization; and synthesis, cross-linking, and alignment of collagen to provide structural and mechanical strength to the healing tissue. 4,5
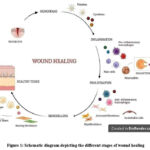 |
Figure 1: Schematic diagram depicting the different stages of wound healing |
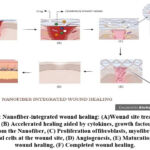 |
Figure 2: Nanofiber-integrated wound healing: (A)Wound site treated with Nanofiber, (B) Accelerated healing aided by cytokines, growth factors and drug released from the Nanofiber, |
Excessive bleeding is controlled by vascular constriction and fibrin clot formation, initiating hemostasis. This process releases cytokines and growth factors, which attract cells for tissue repair. In the inflammatory phase, neutrophils, macrophages, and lymphocytes migrate to the wound to clear debris and microbes. Macrophages then promote apoptosis and stimulate the activity of keratinocytes, fibroblasts, and angiogenesis. The proliferative phase involves epithelial cells proliferating and migrating over the wound site.
Fibroblasts are key cells in dermal repair, contributing to the extracellular matrix (ECM), capillary growth, collagen formation, and granulation tissue. In the final phase of remodeling, the ECM and vascular density return to their normal states, while contractile fibroblasts aid in the contraction of the wound. Multiple cell types participate in different stages of the healing process.2
Major Concerns Hindering Wound Healing
Bacterial infections are a major concern as they delay wound healing, leading to tissue deformation and increasing the risk of other diseases and infections. Stress, age, ischemia, diabetes, obesity etc are some other factors that affect wound healing.The diabetic population, for example, is prone to developing chronic diabetic foot ulcers (DFUs). The healing of DFUs is majorly impaired by the hypoxia that accompanies the disease. Hyperglycemia, a characteristic of diabetes also adds to the oxidative stress.
Diabetic wounds are also at risk of bacterial infections, dysfunction of fibroblasts and epidermal cells, neuropathy, impaired angiogenesis and neovascularization.6 In another instance, inadequate decontamination results in prolonged inflammation which subsequently induces the wound to enter the chronic state. Prolonged inflammation could in turn increase the levels of matric metalloproteases that could potentially degrade the ECM. Biofilms due to bacterial infection also accounts for antibiotics’ ineffectiveness in treatment of wounds. 7-9
Therapeutic Approaches
Therapeutic approaches to wound healing are often limited by the intricate and unique nature of each wound’s healing process. Acute wounds generally heal more quickly, while chronic wounds require more prolonged treatment. Surgical treatment of wounds is one of the most effective treatment methods. However, it involves a risk of general anaesthesia and potential damage to surrounding tissue. Skin grafts is a treatment method utilised in cases of extensive tissue loss. There are various shortcomings with thismethod such as, it cannot be utilised in patientswith less than 30 per cent wound coverage, side-effects of pain, increased contraction and scarring during healing, restricted supply of donor skin, potential immune rejection risk, riskof disease transmission in case of allografts andxenografts, etc. 10-13
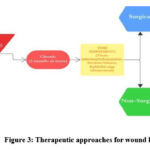 |
Figure 3: Therapeutic approaches for wound healing |
Non-surgical wound care therapies utilize synthetic materials, such as specialized dressings, to manage debridement, maintain moisture balance, support re-epithelialization, promote wound contraction, and prevent infection. Topical formulations are employed to effectively deliver drugs and antibiotics during the inflammatory phase. Formulations with collagenase and dexpanthenol promote faster healing by enhancing re-epithelialization and ECM remodeling. Recently, growth factors have been incorporated using nanoparticle encapsulation to improve stability and targeted delivery to thewound site. Recent studies showed improved collagen synthesis and angiogenesis upon topical application of growth factors (FFGF- 10)onto the wound site.14,15
Wound dressings are pivotal in providing protection against external risks and expediting the recovery process. An ideal wound dressing should possess key characteristics, including the ability to absorb excess exudate, prevent microbial infection, maintain a moist environment, facilitate gas exchange, be non- toxic, biocompatible, degradable, and easy to replace and remove. Different forms of dressings, including hydrogel, film, foam, sponge, andnanofiber membranes, are available which can be further elevated by incorporation of antimicrobial agents, growth factors and drugs. The healing process can also be accelerated by natural biomaterials which replicate the ECM environment of the skin. Composite dressings, made from various natural, synthetic, organic, and inorganic biomolecules, are also used in wound therapies. By integrating nanotechnology, these dressings are enhanced with nanoparticles, growth factors, antibiotics, and bioactive substances to increase their bioactivity. Stimuli-responsive dressings that are sensitive to pH, temperature, and oxygen, as well as 3D-printed or bio-printed substitutes, have recently acquired importance. 16-19
Nanofibers
The distinct structure of the nanofiber membrane with its adjustable physical and mechanical properties renders it an extremely viable and efficient treatment method. Traditionally, methods like drawing, self- assembly, and phase separation have been used to produce nanofibers. However, these techniques are often costly, time-consuming, and less efficient compared to electrospinning technology..20,21,22
Electrospun nanofibers, which can be produced with diameters in the nanometre to micrometre range, are notable for their high surface-to- volume ratio and microporosity. These characteristics make nanofibers highly versatile, enabling their use in scaffold preparation, drug delivery, and wound healing. By closely replicating the structure and function of the extracellular matrix (ECM), nanofibers provide a supportive framework that promotes tissue regeneration. Additionally, they combine the mechanical strength ofsynthetic polymers with the biocompatibility ofnatural polymers. The porous structure of the nanofiber membrane allows for the loading andcontrolled release of biologically active compounds, making them highly versatile and effective in medical applications.23,24 Nanofibers with targeted delivery capabilities promote tissue regeneration and prevent infection due to their biodegradability, which supports natural healing without additional intervention. They can be tailored to enhance both physical structure and biological functionsnecessary for tissue engineering, drug delivery,and infection control, thereby aiding wound healing effectively.25-28
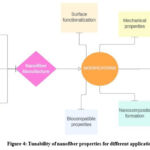 |
Figure 4: Tunability of nanofiber properties for different applications |
Synthesis of Nanofibers
There are several different methods available for nanofiber fabrication. The method is usually chosen based on the type of material used, the size required, the application, the amount of nanofibers to be produced, etc. Described below are some of the commonly used techniques for the synthesis of nanofibers.
Electrospinning
Electrospinning is a widely used technique for nanofiber synthesis.29 Electrospinning proves to be an efficient technique for producing nanofibers, typically with average diameters spanning from tens to hundreds of nanometers.30 Electrospinning is carried out using polymeric materials to draw continuous fibers under a high-voltage electric field. A traditional electrospinning setup consists of four main components: a high-voltage source, a needle or spinneret, a syringe pump, and a collector. The polymeric solution or polymer melt is transferred to the syringe and subsequently linked to the syringe pump. When a high voltage is applied to the pendant droplet formed when the liquid is extruded from the spinneret, the droplet will start to elongate into a conical shape known as a Taylor cone. This leads to the formation of a liquid jet that is directed towards the collector. As the liquid jet makes its way to the collector, the solvent existing in the polymer solution will evaporate, leading to the creation of solid fibers. On the other hand, the solid fibers will take shape as the polymer cools down.31 This is the basic principle behind electrospinning. One of the major advantages of electrospinning is the ability to use many different types of materials such as organic materials, inorganic materials, and even organic and inorganic hybrids 32. However conventional electrospinning has certain limitations such as low throughput. Certain materials with low solubility in various solvents or high electrical resistivity are not suitable for efficient use. Therefore, considerable amounts of research were done to develop more advanced spinning system. Needleless spinning systems have been developed to facilitate higher throughput. In such systems, the electric field is directly applied to the liquid surface with no needle involved. Even though throughput can be increased by using multiple nozzles, other problems arise such as unwanted interaction among formed jets due to interference of the electric fields. Also, the tips often get clogged which can affect the continuity of the spinning process. They also require more space for setting up the equipment which can lead to increased costs [32]. Due to all these limitations, needleless electrospinning is far better. A study was conducted where 3D-printed PCL scaffolds were fabricated. These scaffolds were coated with a layer of PCL/Gelatine nanofibers containing ε-PL. The nanofibers were synthesized by electrospinning. The cytocompatibility was assessed using human fibroblasts and HaCat cells through live/dead cell staining. The 3D-printed scaffold showed bright blue fluorescence indicating presence of live cells after 24h of coincubation. These results were far better when compared to the positive control and indicate the scaffold’s potential in wound healing applications.33
Centrifugal Spinning
Centrifugal spinning is an innovative technique that utilizes centrifugal force to draw out nanofibers. The spinneret involved will rotate with the help of a motor and a driveshaft. The liquid substance will be forced out of the nozzle of the spinneret due to the circular motion. The fibers are ejected out radially onto the collector [34]. The liquid form of the polymer is loaded into the rotating device to draw out the nanofibers. This technique does not involve the application of an electric field on the liquid substance, therefore materials with high electrical resistivity can be used. Centrifugal melt spinning utilizes melted polymers to produce nanofibers. With this technique, no solvents are required, and processing time is reduced 34. In an experiment, centrifugal spinning was used to produce gelatin nanofibrous mats incorporated with silver nitrate to provide antimicrobial properties to the nanofibrous mat. The nanofibrous biomat incorporated with silver showed significant antibacterial activity, indicating its potential for use in wound dressings35.
Self-assembly
Self-assembly represents a bottom-up approach to manufacturing, in which individual components are drawn together by intermolecular forces like hydrogen bonding, electrostatic interactions, and hydrophobic interactions. Such units will organize themselves to form nanofibers. Parameters such as pH, and van der Waals forces also play a role in the self-assembly process. This method can be used to make 3D aerogels, tissue engineering scaffolds, filters, etc. [36]. The main drawbacks of this method are the high costs involved, low productivity, and complex processing 37. Certain peptides can self-assemble into nanofibers, which can be used for tissue engineering, wound healing, and even promote angiogenesis. An experiment was conducted where a series of proangiogenic peptides that were designed to have self-assembling properties were self-assembled into nanofiber hydrogels. The angiogenic effects were studied using an in vitro (endothelial cell model (HUVEC)) and an in vivo model (subendothelial implantation experiment in mice). Both models showed proangiogenic results. The in vitro model showed many HUVEC-connected tubes on the nanofiber hydrogel. The in vivo analyses showed new blood vessel growth into the nanofiber hydrogel within 2 weeks of implantation 38.
Pressurized Gyration
Pressurized gyration is a nanofiber fabrication technique that combines centrifugal force and gas at high pressure to form nanofibers 39. Thinner fibers and higher fiber yields can be achieved with pressurized gyration systems when compared to centrifugal spinning and electrospinning systems. Pressurized gyration consists of three components: the spinning vessel, collector, and high-pressure gas system. The spinning vessel consists of several orifices through which the liquid polymer will be forced out to form nanofibers. When the spinning process begins, the liquid will spread in the vessel due to centrifugal forces. Once the gas flow is applied, the liquid polymer will reach the orifices of the spinning vessel. Due to forces such as centrifugal force and pressure differences, the polymer droplets will expand outwards and form a finger-like shape, in a process known as jet initiation. The polymer jets will continue to leave the orifice and move outward and stretch due to pressure differences and inertia. During the movement of the jet, the solvent will evaporate gradually. These dried fibers will get deposited on the collector 40. In a study, pressurized gyration was used to synthesize polyethylene oxide (PEO) nanofibers incorporated with antimicrobial peptides (AMPs). Two different AMPs were used at different concentrations. The study aimed to assess the effectiveness of the nanofibers in inhibiting the growth of Staphylococcus epidermidis. The nanofibers showed significant efficacy against the bacteria. A significant reduction in bacterial population was seen with increased AMP concentration. This shows that the nanofibers have great potential for use in wound dressing applications41.
Phase Separation
This technique utilizes the physical incompatibility between the solvent and the solute which leads to a separation of the components into two phases. The solvent phase will be removed and the polymer phase will remain [42]. It involves dissolving the polymer in a solvent to obtain a homogenous polymer solution. Then the solution will undergo gelation at a certain gelation temperature. After gelation, the solvent will be extracted with water and the gel will be freeze-dried to form the dried nanofiber product. The major drawback of this technique is that only certain polymers such as polylactide can be used since this process requires a gelation step and not all polymers have gelation capability [43]. An experiment was conducted where chitosan acetate nanofibers were synthesized with varying chitosan concentrations by solid-liquid phase separation. Since chitosan has commendable biocompatibility, these fibers have great potential in regenerative applications.44
Drawing
Drawing is a method used to synthesize nanofibers using a micropipette or a sharp tip to draw fibers from a polymer solution droplet. The tip is first inserted into the edge of the droplet and then withdrawn from the droplet at a constant rate to get a nanofiber. The solvent evaporates from the fibers and solid nanofibers will be obtained. To get a network of fibers, this process will be repeated several times. Parameters such as viscosity, drawing speed, polymer properties, etc. will determine the type of fiber quality and dimensions. This technique is cost-effective and simple, however, it has very low productivity and only viscoelastic materials can be used 45.
Gravity fiber drawing is another method that involves drawing of nanofibers from a polymer solution with the help of a sharp needle under the influence of gravity. In this technique, a polymer solution is pumped through a syringe needle which allows free-fall of the formed droplets. The solvent evaporates as the droplet falls, leading to formation of nanofibers, [46]. In a research endeavor, RAW 264.7 macrophages were cultured on a gravity fiber-drawn scaffold for 36 hours. Then the cells were observedusing a confocal microscope. This confirmed proper cell anchoring and proliferation, which indicates that this scaffold has great potential for tissue engineering applications.
Template synthesis
Template synthesis is a method that involves the usage of a template or a mould to get the desired nanofibers with the preferred shape and structure. The template used can be metal oxide membranes with nano-scale diameter pores. Polymers are extruded through the orifice of the design framework using hydropressure to manufacture nanofibers. The extruded polymer forms the desired nanofibers in contact with a solidifying solution [47]. Silica-based or aluminum oxide-based templates are commonly used. A major disadvantage associated with this technique is that only a few micrometers of fibers can be obtained 43. The advantage of template synthesis is that the diameter can be controlled with the selection of the template membrane, and therefore obtain more regular nanofibers. The template’s solubility is critical to ensure easy removal after synthesis. This can be seen as a disadvantage as it can reduce the practicality of the technique since additional steps like dissolution have to be carried out to remove the template 48. In a study, Poly(ε-caprolactone) (PCL) nanowires and nanofibers were synthesized by template synthesis. An aluminium oxide membrane was used to carry out template synthesis. PCL is also a biodegradable and biocompatible polymer, making it a suitable candidate for wound healing applications. The template synthesis method can also be utilized to load the nanofibers with drug molecules 49.
Table 1: The methods of synthesis of nanofibers and their parameters for fabrication.
|
Methods |
Material Used |
Diameter of Nanofibers Obtained |
Application |
Reference |
|
Electrospinning |
Poly(ε-caprolactone) (PCL)/Gelatine |
– |
Opportunities for utilization in wound healing and tissue engineering applications |
[33] |
|
|
PCL/Polylactic acid (PLA)/Nigella sativa herbal extract |
250nm – 694nm (Based on the concentration of the polymer components) |
Antibacterial activity |
[51] |
|
|
Polyvinylpyrrolidone (PVP)/Polyvinyl alcohol (PVA)/Gelatin/Ajwain essential oil |
564nm – 746nm (Based on the concentration of the polymer components) |
Accelerated wound healing for infected wounds |
[56] |
|
|
Cellulose acetate/PCL/Propolis |
50nm-400nm |
Nanofiber mat for wound healing with antimicrobial and antioxidant activity |
[57] |
|
Centrifugal Spinning |
Gelatin/silver nitrate |
3.23 ± 0.9 μm (At 7000 rpm rotation, 20wt% concentration) |
Antibacterial wound dressing |
[35] |
|
|
PLA/Gelatin/Ciprofloxacin |
513nm – 622nm |
Antibacterial wound dressing |
[93] |
|
Self-Assembly |
Peptides |
45nm – 60 nm |
Efficient wound healing with proangiogenic peptides |
[38] |
|
|
Peptides |
– |
Promotion of burn wound regeneration with heparin mimetic peptides |
[94] |
|
Pressurized Gyration |
Polyethylene oxide/Antimicrobial peptides |
191nm – 506nm |
Antimicrobial wound dressing |
[41] |
|
|
PCL/Ag |
14nm – 38nm |
Antimicrobial non- woven nanofiber scaffold for tissue engineering/wound healing |
[95] |
|
Phase Separation |
Chitosan |
50nm – 500nm |
Opportunities for utilization in wound healing and tissue engineering applications |
[44] |
|
|
Poly(l-lactide)/Chitosan |
50nm – 500nm |
Biomimetic nanofibrous scaffold for tissue engineering and wound healing |
[96] |
|
Drawing |
PCL |
1 μm – 100 μm |
Tissue engineering and wound healing |
[46] |
|
|
Polyvinyl acetate (PVAc), polyurethane (PU), PCL and polystyrene (PS) |
PVAc: 4 μm – 12 μm PU: 4 μm – 9 μm PCL: 9 μm – 40 μm PS: 0.9 μm – 4.16 μm |
Opportunities for utilization in wound healing and tissue engineering applications |
[97] |
|
Template Synthesis |
MoS2 |
50nm |
Opportunities for utilization in wound healing and tissue engineering applications |
[98] |
|
|
PCL |
– |
Tissue engineering and wound healing applications |
[49] |
Table 2: The advantages and disadvantages of nanofiber synthesis methods
|
Synthesis Methods |
Major Parameters |
Advantages |
Disadvantages |
|
Electrospinning |
Polymer concentration, voltage, the viscosity of the solution, the distance between tip and collector, type of solvent, flow rate |
Different types of materials can be used; a wide range of fiber diameters; simple process |
Materials with high electrical resistivity cannot be used; low throughput; complex setup; high costs |
|
Centrifugal Spinning |
Polymer concentration, surface tension, speed of spinneret, viscosity of solution |
High throughput; high voltage not required; no toxic chemicals involved; |
Uneven distribution of fibers; high costs; potential for bead formation |
|
Self-Assembly |
Hydrophobic interactions, Van der Waals forces, pH, hydrogen bonding |
Simple method; low cost |
Low productivity; complex process; highly sensitive to environmental factors |
|
Pressurized Gyration |
Spinning vessel speed, pressure, orifice size, viscosity of solution |
High production rate; good scalability; good uniformity of nanofiber diameter |
Complex set-up; limited control over fiber alignment |
|
Phase Separation |
Gelation temperature, polymer concentration, type of solvent |
Simple process; easy set up; cost- effective; biocompatible |
Continuous long fibers cannot be produced; only certain polymers can be used |
|
Drawing |
Viscosity of solution, polymer properties, drawing speed |
Simple process; cost-effective; good scalability |
Limited to certain polymers; Highly sensitive to processing conditions |
|
Template Synthesis |
Template size and shape, polymer properties |
Controlled morphology of fibers; tailored properties |
Removal of the template can be difficult |
Applications of Nanofibers
Plant-derived/herbal seeded nanofibers Soy
Antibiotics-infused membranes have been developed using biodegradable soy protein for wound dressing purposes as it shows promise in accelerating wound closure and reducing scar formation. The lack of mechanical strength in soy protein isolate-based nanofibers hinders its biomedical application. To surpass this limitation, soy protein isolates are combined with other substances.
Recent evolutionary research showcased an innovative biocompatible use of soy protein isolate (SPI) Nanofibers for chronic wound healing. It was combined with polycaprolactone (PCL), N-(3-sulfopropyl) chitosan salt (SPCS) to enhance its electrospinnability, mechanical strength and surface morphology. Furthermore, Zeolite imidazolate framework-8 nanoparticles (ZIF-8 NPs) and Matricaria chamomillai essential oil (MCEO) were incorporated in-situ toimprove the anti-bacterial properties of the nanofiber. The produced nanofibers exhibited commendable blood clotting abilities (30% clot formation), an impressive haemolysis rate and cell viability with favourable cell adhesion properties. Its remarkable anti-bacterial activity against a broad spectrum of bacterial strains is noteworthy. There is significant potential to raise interest in this eco-friendly approach.50
Black seed (Nigella Sativa)
Many Asian, Middle Eastern, and Far Eastern countries have a long-standing tradition of using Nigella sativa L seeds (NS), commonly also referred to as black seeds, in their traditional medicine to treat a variety of health conditions such as (headaches, abdominal pain, diarrhoea, rheumatism, and other diseases). Research has indicated that these seeds’ aqueous and oil extracts showcasedsignificant antioxidant, anti-inflammatory, anticancer, analgesic, and antibacterial properties.
NS herbal extract incorporated PCL and Poly- lactic acid (PLA) hybrid nanofibrous mats were used to study their wound healing capacity. Different combinations of nanofibers, namely PLA/NS, PCL/NS, PLA-PLC/NS, and PCL’PLA/NS were fabricated with random orientations and morphologies and investigated for their hydrophilicity , mechanical, chemical and cytotoxicity properties.
It was concluded that the nanofibers exhibited anti-bacterial behaviour, as evidenced by the formation of inhibition zones around the samples against both gram-positive and gram- negative bacteria. The MTT assay confirmed that reduced cytotoxicity and increased cell viability were observed with an increase in the concentration of the NS extract in the nanofibers. It is also worth mentioning that the extract also improved cell proliferation.52
Satureja and Oliveria
Prior studies 52,53 have indicated that significant quantities of thymol and carvacrol are present in the essential oils (EOs) of Satureja mutica (S. mutica) and Oliveria decumbens (O. decumbens). Their primary constituents were found to be oxygenated monoterpenes, which retain exceptional antioxidant and antimicrobial characteristics.
Nanofibers supplemented with the mentioned EOs demonstrated enhanced antimicrobial and antioxidative attributes. Nanofiber with a core layer of chitosan and PVA and a shell layer with polyvinylpyrrolidone and Maltodextrin was designed to aid in further study of the EOs’ behaviour. Upon testing the samples, an amplified antimicrobial efficacy was seen against P. aeruginosa, E. coli, S. aureus, C. dubliniensis, and C. albicans and at 80mg/ml, alleviated antioxidant activity from 18% to 61% for nanofibers with S. mutica and 64% for nanofibers with O. decumbens was observed. This core-shell nanofibrous scaffold shows great potential in wound dressing for dry wounds but needs to be further researched to be applied on moist wounds.54
Alfalfa (Medicago sativa)
Alfalfa, (Medicago sativa), also known as “father of all foods,” is a traditional herb used for its therapeutical nature for centuries, to treat cutaneous wounds. Bioactive components present in alfalfa like phytoestrogens and chlorophylls are found to be responsible for its healing qualities.
PCL/alfalfa composite nanofibers can mimic dermal ECM accurately and boast a super- hydrophilic surface. This surface property showed the potential to enhance cellular growth and maintain a viable environment to promote wound healing. The inclusion of alfalfa in the nanofibers resulted in increased adhesion of HEKa and HNDF on PCL/alfalfa nanofibers when compared to PCL nanofibers in-vitro .
Furthermore, in an experimental model involving mouse excisional wounds, the efficacy of PCL/alfalfa scaffolds was evaluated by comparing their performance not only against internal controls but also against commercially available wound dressings. To assess the effectiveness of a novel bioscaffold, the skin tissue architecture quality (STAQ) index was employed to compare the healing results of PCL/alfalfa scaffolds, control samples, and commercially accessible wound dressings based on histological analysis. The results showed that PCL/alfalfa scaffolds had a healing score of 96.9%, while PCL scaffolds had a score of 70.6%. This suggested that the presence of bioactive components from alfalfa in the nanofiber scaffolds improved healing outcomes.54
Ajwain (Trachyspermum ammi)
To reduce the healing time of bacteria-infected wound sites, an interesting study put forth the invaluable therapeutic effects of Ajwain Essential oil (EO), namely its ability to promote haemostasis, reduce inflammation and promote re-epithelialization. A core-shell nanofiber (core layer – PVP and Shell layer – PVA and Gelatin) loaded with Ajwain (Trachyspermum ammi) EO, as an antimicrobial agent for wound dressing was fabricated. The purpose of this innovative dressing was to expedite the healing process of wounds infected with various strains of bacteria and fungi. The addition of Ajwain EO resulted in the development of potent antimicrobial characteristics in the nanofiber mats, effectively combating both gram-positive and gram-negative bacteria as well as fungi.
Moreover, the release of antimicrobial agents from the mat was observed in both in-vitro and ex-vivo experiments, indicating its long-lasting effectiveness for up to 48 and 72 hours, respectively. The in vivo investigations conducted on rats revealed the promising capabilities of the S2/EO nanofiber mat (the sample with the smallest mean diameter with EO) in promoting wound healing and eradicating bacterial infections at the wound sites. Additionally, the histopathological analysis revealed the absence of any signs of inflammation, while observing an augmentation in the accumulation of collagen in the wound dressed by S2/EO mats on day 14.56
Propolis
Propolis is a natural compound known for its antioxidant, anti-cancer, wound-healing (anti- bacterial, anti-inflammatory, antifungal, anti- viral) capabilities. Its chemical composition depends on its source and is composed of over 300 different compounds. Hence, researchers have made efforts to incorporate propolis into wound dressings to enhance wound healing and treat infected wounds.
Propolis was incorporated into Cellulose acetate (CA) and PCL nanofibers and its physiochemical properties were characterized. It demonstrated effective antibacterial properties against both Gram-positive and Gram-negative bacteria. Furthermore, this mat displayed a comparable level of antioxidant activity to the mat that did not contain propolis. This particular mat can function as a surface that is both biodegradable and biocompatible, which makes it an ideal candidate for use in wound healing applications.57
Clove (Syzgium aromaticum)
Cloves also known as Syzgium aromaticum from the Myrtaceae family are widely recognized as an influential natural herb with antimicrobial properties. Essential oils made from cloves demonstrated extensive antibacterial, antifungal, antioxidant, analgesic, anaesthetic, and insecticidal activities.
Major components such as eugenol (acetate) and β-caryophyllene aid in enhancing the therapeutic effects of clove. Additionally, when formulated as nanofibers with different polymers, they demonstrate antibacterial properties, particularly effective against Staphylococcus aureus, Escherichia coli, Pseudomonas fluorescens, and Bacillus subtilis.
To investigate their characteristics further, a Chitosan (CS) and Polyethene oxide (PEO) formulated nanofiber loaded with clove oil to treat tropical wounds. The results indicated that the developed NFs exhibited a high degree of clove oil encapsulation and loading, with a favourable release profile at pH 5.5 and 7.4. This release pattern involved an initial burst release. It is then followed by sustained release. Additionally, the NFs demonstrated no cytotoxic effects on fibroblast cell lines, while also exhibiting notable antibacterial properties and promoting wound healing.58
Zataria multiflora
Zataria multiflora (ZM) is a plant that belongs to the Lamiaceae family and resembles thyme. According to various sources, the plant is well known for its medicinal and aromatic qualities. Recent research indicates that ZM also exhibits several other characteristics such as antibacterial, antifungal, anti-inflammatory, antinociceptive and spasmolytic effects. Studies have shown that the presence of carvacrols and thymol in ZM essential oil gives it the ability to combat microbes. These monoterpenes disrupt the cytoplasmic membrane, thereby causing increased cell membrane permeability and release of ions and ATP.
To explore the physiochemical, antibacterial, and cytotoxic characteristics of ZM essential oil it was integrated into the CS/PVA/Gel nanofiber mats with the electrospinning technique. The nanofiber mat’s nontoxicity was confirmed against a tested L929 mouse fibroblast cell line, and they demonstrated appropriate swelling and mechanical properties. The antimicrobial effect of the ZM essential oil is attributed to thymol, as shown in the GC-MS analysis. Their nanofiber mat with 10% ZM-oil demonstrated the complete inhibition of S.aureus, P.aeruginosa, and C. albicans (after 24 hrs incubation). All these qualities prove to be a promising alternative to traditional wound dressing. 59
Henna (Lawsonia inermis)/Lawsone
Henna, scientifically known as Lawsonia inermis, belongs to the Lythraceae family. It is a well-known medicinal herb that has been utilized for centuries. It is beneficial for the treatment of burns, skin infections, wounds, and ulcers due to its antimicrobial and anti- inflammatory characteristics.
A wound dressing using gelatin and oxidized starch impregnated with Lawsonia inermis (henna) was fabricated. It was seen that the incorporation of henna into the nanocomposite significantly improved fibroblast proliferation and attachment, along with collagen secretion. The histological analysis demonstrated that using henna in the treatment of burn wounds had a positive impact by reducing inflammation and promoting the proliferation of fibroblasts. When compared to other studies, the formation of well-organized collagen was significantly better in this one. Faster wound closure was observed in the mice wound models that used a nano-matrix having a higher concentration of henna. It further revealed clear re- epithelialization , angiogenesis, the presence of hair follicles and sebaceous glands, and well- organized collagen in the burn wounds treated with G/OST (H30) after four days.
Compared to the other treated samples, the burn wound sites treated with henna-loaded mats exhibited a milder inflammatory response, with fewer macrophages present at the burn site as indicated by CD68 immunohistochemical staining. These results suggested that the development of a henna-loaded G/OST nano- fibrous mat could hold promise as an effective wound dressing for the treatment of burn wounds.60
Another study in 2019, incorporated different concentrations of Lawsone into electrospun mats made of shell-core polymers of PCL/ Gelatin. The fabricated mats were then evaluated for their morphology, biodegradability, release profile of lawsone, and mechanical properties of the nanofibers. The impact of the mats on the expression of healing-related genes, antibacterial activity, compatibility with HGF cells and wound healing efficacy on a rat model excision was examined. The research demonstrated that the addition of a 1% concentration of lawsone to PCL/gel composite resulted in increased growth and proliferation of fibroblast cells. Two genes associated with healing processes, TGF-B1 and COL1 showed a substantial increase in expression upon exposure to nanofibers made of PCL/Gel/Law at concentrations of 0.5% and 1%.61
Neem (Azadirachta indica)
Neem, scientifically known as Azadirachta indica, is a well-known plant with medicinal properties that are used in the Indian subcontinent widely. A wide range ofproperties such as anti-ulcer, antibacterial, anti- inflammatory, anti-malarial, anti-fungal, anti- viral, antioxidant, anti-mutagenic, and anti- carcinogenic nature was seen due to its main active ingredient, Azadirachtin. However, this plant, inherently, is not too efficient in healing wounds. This challenge is addressed by implementing techniques such as electrospinning, electrospraying, solution blow spinning and film casting. By using these methods, we can utilize plant extracts for the development of products to use in wound dressing applications.
PVA and Neem extract were electrospun to produce novel nanofiber mats. The goal was to assess the properties and composition of the resulting mat. The moisture management and thermal properties of the PVA-neem nanofibrous mats, which were created using a solution ratio of 80:20 (Neem: PVA), were significantly improved. This improvement can be attributed to the antibacterial components present in the neem extract. When compared to the PVA nanofiber alone, the developed samples exhibited a 20 mm inhibition zone against the same bacteria, indicating the effectiveness of the neem extract in preventing bacterial growth.62
The exceptional therapeutic benefits of A. indica and the advancements inelectrospinning technology were used to produce biopolymer- based nanofibers that are capable of carrying neem extract in a controlled and eco-friendly manner. The neem leaf extractwas incorporated into biopolymer-based nanofibers, resulting in nanofibers that possessed both antioxidant and antifungal properties. These nanofibers exhibited a high swelling rate and a sustained release of phytoconstituents. A novel method called single-fluid electrospinning was used to fabricate these medicated nanofibers, with a sophisticated double-phase release profile. These prepared nanofibers can be potentially utilized as wound dressings, soft tissue scaffolds , and transdermal carriers.63
Curcumin (Curcuma longma)
Curcumin a chemical compound naturallyfound in Curcuma longma has been utilized forwound healing for centuries. It possessesantimicrobial, anti-inflammatory, and antioxidant properties, making it an effective treatment option. Nevertheless, the limited solubility of curcumin limits its potential in biomedical applications.
A curcumin-loaded thiocarbohydrazide gelatin (CTCHGel) nanofiber was fabricated to examine the potential antibacterial effects of curcumin. This study aimed to determine the suitability of CTCHGel for use in wound healing applications. An amorphous nanosolid dispersion of curcumin was made in 50% acetic acid with the help of TCHGel fibers, this approach was effectively safer and more environmentally friendly as it eliminated the need for hazardous fluorinated solvents. The result of the antibacterial assay showcased that the spun nanofiber exhibited antibacterial properties against E. coli. Moreover, the CTCHGel fiber and the crosslinked 1–3% CCTCHGel fiber mat demonstrated excellent fibroblast migration and cell migration in monocytic cells (THP-1 cells). Considering these positive outcomes, the nanofibrous mats blended with curcumin/ gelatin (1% CCTCHGel) can serve as a potent remedy for promoting the healing of acute wounds.64
Aloe vera And Hypericum perforatum
Aloe vera extract has long been recognized as a supplementary herbal treatment for a range of ailments. Its anti-inflammatory, antibacterial, and anti-diabetic properties are well- documented. Moreover, Aloe vera offers significant advantages, including its ability to inhibit wound formation, enhance immunomodulatory activity, and contribute to myogenic activities.
Hypericum perforatum is another extensively utilized plant for its medicinal properties, has been commonly applied topically to expedite the recovery process of burns and various types of wounds in individuals. It has been suggested that the plant’s antibacterial activity contributes to its therapeutic effects. It has been documented that Hypericum perforatum oil exhibits anti-inflammatory properties by enhancing the body’s ability to fight infections, promoting the migration of fibroblasts, and facilitating the accumulation of collagen.
In 2021, a research endeavour involved the comparison of the oxidative and antioxidative impact of nanofiber dressings loaded with Aloe vera and Hypericum perforatum. on diabetic wounds during the healing process. Additionally, assessed the potential of Fourier transform infrared spectroscopy by utilizing FTIR signatures to evaluate the progress of wound healing in diabetic patients. The study demonstrated that the oils of Hypericum perforatum and Aloe vera had a significant impact on the total oxidative stress level in streptozotocin rats.
Interestingly, when comparing the oxidative stress index, it was observed that the Hypericum perforatum oil group exhibited a greater decrease in this parameter compared to the Aloe vera group, in comparison with the diabetic model on Wistar rats. The FTIR spectra analysis revealed that the chemical structure of blood and serum collected from streptozotocin rats resembled that of the control group more closely when treated with Hypericum perforatum oil, as opposed to Aloe vera oil. These differences were particularly evident in the PO2- groups from phospholipids and in the vibrations of amide- I, II, and III. Hypericum perforatum oil appeared to yield better results for bio-nano applications.65
Table 3: Medicinal Plants and their biological activities to promote wound healing
|
S.No |
Medicinal Plants |
Source |
Polymers used with |
Biological Activities |
Reference |
|
1. |
Soy Protein Isolate |
Seed |
N-3-sulfopropyl chitosan salt Poly-Lactic acid (PLA) Zeolite imidazolate framework-8 Nanoparticles Matricaria chamomilla essential oil |
Accelerates wound closure Reduces Scar formation |
[50] |
|
2. |
Black Seed (Nigella sativa) |
Seed |
Poly-Lactic acid (PLA) Poly-caprolactone (PCL) |
Antioxidant Anti-inflammatory Anticancer Analgesic Antibacterial |
[51] |
|
3. |
Satureja mutica |
Flowers and Leaves |
PVA and Chitosan (Core layer) PVP and Maltodextrin (Shell layer) |
Antioxidant Antimicrobial |
[54] |
|
4. |
Oliveria decumbens |
Flowers |
PVA and Chitosan (Core layer) PVP and Maltodextrin (Shell layer) |
Antioxidant Antimicrobial |
[54] |
|
5. |
Alfalfa (Medicago sativa) |
Leaves |
PCL |
Antibacterial Tissue remodeling |
[55] |
|
6. |
Ajwain (Trachyspermum ammi) |
Seed |
PVP (Core layer) PVA and Gelatin (Shell layer) |
Antibacterial Antioxidant Accelerates Cell Proliferation and growth |
[56] |
|
7. |
Propolis |
Secretion from Hive |
PCL Cellulose Acetate |
Antibacterial Anti-inflammatory Antifungal Antiviral Anticancer |
[57] |
|
9. |
Clove (Syzgium aromaticum) |
Flowers |
Chitosan and Polyethylene oxide |
Analgesic Anti-inflammatory Antibacterial |
[58] |
|
10 |
Zataria multiflora |
Flowers |
Chitosan PVA Gelatin |
Antibacterial, Antifungal, anti-inflammatory, Antinociceptive and spasmolytic effects. |
[59] |
|
11. |
Henna (Lawsonia inermis) |
Leaves and roots |
Gelatin Oxidized Starch Gelatin PCL |
Antibacterial Anti-inflammatory |
[60] [61] |
|
12. |
Neem (Azadirachta indica) |
Leaves |
PVA Sodium Alginate Chitosan PEO Acetic acid |
Antibacterial Antifungal Antioxidant |
[62] [63] |
|
12. |
Turmeric (Curcumin longa) |
Rhizomes |
Thioocarbohydrazide Gelatin |
Antimicrobial Anti-inflammatory Antioxidant |
[64] |
|
13. |
Aloe (Aloe vera) |
Leaves |
PCL Gelatin |
Anti-inflammatory Antibacterial Inhibit wound formation Enhance immunomodulatory activity |
[65] |
|
14. |
Hypericum perforatum |
Flowers, Leaves and Stem heads |
PCL Gelatin |
Anti-inflammatory Migration of Fibroblasts Synthesis of Collagen |
[65] |
Stem Cell- Seeded Nanofibers Adipose Stem Cells
The potential of Adipose stem cells (ADSCs) to differentiate into multiple cell lineages, including adipocytes, osteoblasts, nerve cells, and glial cells, is well-established. They secrete various pro-angiogenic and anti-apoptotic factors that can provide anti-inflammatory, anti-oxidative, and resistance to oxygen-free radical damage. These properties make ADSCs a potential solution for repairing damaged tissues and organs. In recent times, several research studies have suggested that ADSCs possess the ability to aid in the healing of wounds by preventing the formation of scars, these qualities have garnered significant attention in both basic research and clinical applications.
Recent research explored the effect of nanofiber-based meshes on the paracrine behaviour of ADSCs. They devised a set of these meshes and collected conditional mediums from different cultures. Fibroblasts and endothelial cells were grown in-vitro by using this medium, thus allowing the researchers to evaluate the impact on cell movement and angiogenesis. The optimal scaffold material of ADSCs carriers wasutilized for skin wound repair in rats in this study.67
In this study, the rate of wound healing, number of inflammatory cells, and collagen density were some of the tissue repair indicators that were observed and recorded. It was seen that the nanofiber-based meshes affected the paracrine behaviour of the ADSCs distinctly. They showed better fibroblast and endothelial cell migration, and improved angiogenesis, proving that they are an invaluable tool foraffecting cell behaviour in wound healing and tissue engineering applications. 66
Bone Marrow Stem Cells
Mesenchymal stromal cells derived from bone marrow (BMSCs) possess the ability to differentiate into a variety of cell types such as fibroblasts, endothelial cells, cartilage, bone, muscle, and neuronal cells. They are known to secrete a significant amount of growth factors and cytokines that play a crucial role in the healing process of damaged tissues.67,68
A study 69, aimed to create a both radically and vertically aligned 3D nanofiber scaffold, that were used in the transplantation of bone marrow mesenchymal stem cells (BMSCs). It revealed that such scaffolds are highly effective in promoting the formation of granulation tissue, angiogenesis, and collagen deposition.
They also observed that the immune responses were shifted towards a pro-regenerative direction. The establishment of the anti- contraction wound healing model in type 2 diabetic mice enabled the prevention of wound closure resulting from skin contraction. This model facilitated the healing of wounds through the processes of granulation and re- epithelialization, closely resembling the natural healing process observed in human skin wounds.
In another experiment 70, they electrospun the cytomembrane from Lipopolysaccharide (LPS)/IFN-γ activated macrophages with PLGA nanofibers to create a novel material, which was then analyzed for their biophysical properties such as diameter, hydrophilicity,and degradation rate. The study revealed that the fibers promoted the growth and proliferation of BMMSCs and migration of keratinocytes when subjected to in vitro oxidative stress. LPS/IFN- γ activated human THP-1 cells derived cytomembrane was used to modify the nanofibers (referred to as TCM-fibers) and it was seen that they have almost identical impact in stimulating human BMMSCs (hBMMSCs) growth and keratinocyte movement under oxidative stress.
It was further seen that TCM-fiber-hBMMSCs, which are cell membrane-coated electrospun nanofibers, offer significant advantages in healing diabetic wounds thanks to their living nature. These nanofibers not only boost the growth of bone marrow-derived mesenchymal stem cells (BMMSCs) under oxidative stress but also speed up the healing process in diabetic mice. They do this by improving immune response, aiding the movement of skin cells, remodeling skin structure, decreasing stress at the cellular level, and fostering the formation of new blood vessels in the affected area. This discovery could lead to better treatment strategies for chronic wounds in diabetes patients.
Epidermal Stem Cells
Epidermal stem cells (ESCs) have collected significant interest over the years, and have recently raised curiosity over its use in wound healing. It maintains and protects the patient’s skin from further damage and reduces pain.
Recently, they differentiated stem cells from human-exploited deciduous (SHED) into ESCs and proceeded to seed them onto PVA/Silk fibroin (SF) nanofibers and studied their use in in-vivo wound healing. The primary objectives of the study were to assess the epithelial differentiation of SHED and the impact of the resulting cells on wound healing.
When SHED cells were grown with theaddition of certain specific growth factors, theyexhibited successful differentiation into ESCs and expressed their distinctive molecular markers, namely p63, K14, and α6 integrin. ESCs are located in layers found deep in the skin and travel to the site of injury to facilitate the repair of skin. Therefore, it was thought thatpromoting the differentiation of SHED cells into ESCs beforehand could potentially enhance the healing process of wounds. The statement is consistent with the results of the research, showing that the groups who underwent ESCs and SHED cell transplants experienced faster wound healing in comparison to the control group. Specifically, the PFSM/ESCs group exhibited a 1.86-fold faster wound closure rate, while the PFSM/SHED group experienced a 1.57-fold faster closure rate, both compared to the blankcontrol group. Furthermore, the rate of wound closure using solely PFSM material exhibited a1.35-fold increase in speed by day 5. 71
Endometrial Stem Cells
There has been significant focus on adult mesenchymal stem cells (MSCs) as the primary candidate for skin regeneration research. However, there is currently no documented evidence of utilizing human endometrial stem cells (hEnSCs) seeded on PCL or PCL/collagen scaffolds for skin regeneration.
The endometrium, a highly regenerative tissue, contains stromal progenitor cells of mesenchymal origin. These cells have shown the ability to repair the uterine tissue through their robust angiogenic capabilities. As a result, they have the potential to be a promising option for enhancing angiogenesis in wound repair.
In a novel experiment aimed at advancing skin tissue engineering, researchers proposed using human endometrial stem cells (hEnSCs) integrated into a collagen-enhanced PCL (polycaprolactone) scaffold. This innovative approach was hypothesized to offer a superior method for effective wound healing. The rationale behind this hypothesis is rooted in the belief that hEnSCs, when combined with a collagen-coated PCL structure, could outperform traditional mesenchymal stem cells (MSCs) by better promoting angiogenesis— key to healing skin ailments that suffer from poor blood vessel growth. The findings of the study confirmed that the collagen-coated PCL scaffold not only provides an optimal support and nano-environment for the hEnSCs in vitro but also leads to promising skin repair outcomes, highlighting its potential as a valuable therapeutic strategy in the field of regenerative medicine.72
Nanoparticle Seeded Nanofibers Chitosan Nanoparticles
Chitosan (CS), has been looked into for its therapeutic properties for a while now, however, its extensive medicinal nature is still being probed upon.
In an experiment, chitosan nanoparticles were loaded into polycaprolactone (PCL), and utilized as protein carriers by making it into a scaffold by electrospinning and its effect on drug release on proliferation and migration of fibroblast cells at the wound site was analyzed. Proliferation and migration of the cells were observed using agarose migration assay, performed in regards to PDGF-BB, PDGF-BB- incorporated scaffolds and Fetal Bovine Serum. By measuring the distance that the cells travelled from the central well border on days 3 and 6 of the experiment, it was seen that the loaded scaffold showed a significantly enhanced chemotactic effect in terms of cell mass growth and migration. When PDGF-BB was incorporated into the scaffold, these cell populations grew and migrated in a specific direction rapidly. 73
In another experiment, chitosan tripolyphosphate nanoparticles were loaded with curcumin (CURCSNPs) to extend and enhance the sustained release of quercetin in the wound site. The CURCSNPs were electrosprayed into the PCL/CS/CUR electrospun membranes, which increased its bioavailability, tissue retention and transdermal delivery. It was also probed that there was no impact on the average diameter of the CURCSNPs. They noted favourable characteristics such as high-water vapour transmission rate and appropriate swelling behaviour. Moreover, CURCSNPs hydrophilic nature contributed to an accelerated degradation rate of PCL/CS/CUR scaffold and its functionalization such as its remarkable antibacterial efficacy against MRSA (99.3% reduction) and an increase in antioxidant function upto 89%. It also exhibited a significant improvement on the proliferation rate of human dermal fibroblasts (HDF) cells.
Additionally, a 15-day wound closure assaywas conducted to assess the wound healing percentage which showcased results of 98.5% and 96.4% for non-infected and MRSA- infected wounds, respectively. It was further confirmed with histological analysis that the wounds reacted with this specific scaffold demonstrated a complete and organized healing process. These characteristic results highlighted the potential application of this novel wound dressing as an effective solution with significant antibacterial activity.74
Heparin Nanoparticles
Heparin is a well-known bioactive substance that aims to promote skin regeneration by interacting with various growth factors, cytokines, and chemokines involved in the wound healing processes. Being a highly sulphated glycosaminoglycan plays a crucial role in signalling for promoting differentiation, migration, proliferation, and maintenance of cells. Heparin not only alleviates pain, but also enhances blood circulation, speeds up the healing process, and prevents clotting and is also anti-inflammatory, but it also influences the proliferation of various cell types, contributing to wound healing.
Researchers developed 3D wound dressings combining sericin and gelatin polymers for skin tissue engineering. They used a wet electrospinning technique to fabricate these fibres which are designed to carry heparin- loaded nanoparticles. Heparin nanoparticles are encapsulated in PLGA (Poly Lactic-co- Glycolic Acid) and then loaded into the Ser/gel scaffold, to investigate the cytotoxicity and cell viability of the scaffold. The heparin-loaded scaffold showed no cytotoxic effects on L929 cells upon exposure and greater cell viability than unloaded scaffolds, suggesting that the fabricated wound dressings can be used for skin and tissue engineering purposes. However, further research is required to assess the potential of it in wound healing and skin regeneration.75
Bioactive Glass Nanoparticles
A recent study, investigated the bioavailability, antibacterial efficacy, regenerative potential and biomedical application of glass nanoparticles incorporated in cellulose acetate nanofiber composite. Both in-vitro and in-vivo studies were conducted using diabetic rat models and it is shown that there is a significant decrease in size of induced wounds, a progressive healing process within a short period, and enhanced antibacterial activity against a broad spectrum of microorganisms. (including both gram-negative and gram- positive bacteria).76
Silver Nanoparticles
In light of previously mentioned information, a trial presented research of a novel variety of wound dressing of PVP-CIP (polyvinylpyrrolidone-ciprofloxacin)/EC- AgNPs (ethyl cellulose- silver nanoparticles) Janus nanofibers. In-vitro, dissolution tests demonstrated that Janus fibers containing PVP- CIP were able to release over 90% of their CIP within 30 minutes and they also demonstrated superior antibacterial activity against both S. aureus and E. coli. The proposed mechanism producing this synergistic effect is yet to be elucidated. The Janus fibers’ PVP-CIP exhibits a strong initial antibacterial effect due to the rapid release of CIP. In contrast, the EC-AgNPs indicate a more prolonged and sustained period of antibacterial activity. This implies that the two distinct aspects of the Janus fibers’ sides work together to provide a comprehensive antimicrobial solution that is both immediate and long-lasting.
Shielding wounds against infection is considered a crucial attribute of an optimal wound dressing. Recent studies have particularly focused of the use of silver nanoparticles (AgNPs) for their enhanced antibacterial properties compared to antibiotics.77
In another research they believed that the combination of PVA nanofibers and PCL/Gum Arabic (GA) nanofibers loaded with AgNPs could effectively inhibit various microbial strains, making it a suitable option for wound dressings. The PCL-coated GA-PVA-AgNPs mat displayed a growth inhibition zone against both Gram-negative and Gram-positive bacteria, as well as a fungal strain, indicating its antimicrobial activity. Furthermore, the mat showed excellent biocompatibility and promoted fibroblast proliferation. These results suggest that the recommended mats are promising for further development of wound dressings for treating infectious wounds.78
In 2022, group of researchers, successfully created a novel electrospun chitosan nanofiber composite. This material contained Cur@β- CD/AgNPs nanoparticles composed of a combination of silver and curcumin. The researchers found that this composite material exhibited remarkable synergistic effects in terms of antibacterial activity and wound healing.
The functionalized silver nanoparticles demonstrated significant activity against both Gram-negative and Gram-positive bacteria. In- vivo experiments revealed that the chitosan dressing with Cur@β-CD/AgNPs had superior wound closure rates than the commercially available AquacelAg. Additionally, this dressing contributed to a more uniform distribution of collagen, as demonstrated by Masson’s trichrome staining. These findings highlight the potential of the Cur@β- CD/AgNPs chitosan dressings as a promising therapeutic option for wound healing applications.79
Similarly, a different group focused on improving cell adhesion and proliferation, by using a simple and environmentally friendly approach. They employed chitosan hydrogel as both a reducing and stabilizing agent, to reduce harmful chemicals from being formed during the synthesis of Ag nanoparticles.
The researchers specifically recognized the following unique characteristic of bacteria- infected wounds: its acidic pH. Cellular abnormalities in these sites serve as triggers for drug release. The incorporation of Ag nanoparticles into the nanofibers increased surface area and thereby promoted stronger interaction with the drug. Consequently, this remarkably improved the loading and entrapment of curcumin into the prepared nanofibers. The antibacterial activity of both gram-positive and gram-negative bacteria, observed through MIC analysis on E. coli and S. aureus, was significantly influenced by the concentration of chitosan. The cytocompatibility and efficacy of the developed nanofibers on adhesion, growth and proliferation of NIH 3T3 fibroblasts were demonstrated by MTT assay.80
Zinc Oxide Nanoparticles
Zinc oxide (ZnO) is an extremely valuable metallic nanoparticle with great potential in the field of medicine. It is very useful for its wide range of therapeutic properties, including antibacterial and anti-inflammatory effects. Zinc serves as an important cofactor for approximately 10% of the body’s proteins involved in various cellular functions and regulatory processes. These proteins contribute to important metabolic activities such as transcriptional regulation, DNA repair, and ECM regulation. However, the potential for skin regeneration in this particular context has yet to be fully explored.
A study reported that the combination of two specific polymers, PLGA and silk fibroin (SF), encapsulating Zinc Oxide (ZnO) NPs, could serve as a versatile scaffold for wound healing. To test this hypothesis, they successfully prepared nanofibers composed of PLGA/SF, as well as PLGA/SF with varying concentrations of ZnO (1%, 2%, and 3% v/v), referred to as PS, PSZ1, PSZ2, and PSZ3 respectively. These nanofibers underwent extensive analysis to evaluate their physicochemical properties, in pseudo-biological settings such as in vitro cell culture, antiseptic behaviour, antioxidant activity, and in-vivo wound healing.
The physicochemical analysis demonstrated that NF membranes possessed favourable morphological properties and significant mechanical and thermal stability, making it suitable for use a as wound dressing. The results of the cytotoxicity studies indicatedthat the use of ZnO NPs at up to 3% concentration is safe for biomedical applications. Furthermore, the presence of mild antioxidant activity was primarily attributed to silk fibroin. As a result of antibacterial testing,it was revealed that the NF with a concentration of 3% ZnO concentration exhibited an inhibitory effect on both Gram-positive and negative bacteria. Moreover, the in vivo wound healing tests provided further evidence that the PSZ3-NF membranes effectively promoted wound contraction, re- epithelialization, cell migration, collagen deposition, and the formation of capillary networks.81
Another group of researchers created a Polymethyl methacrylate/ polyethylene glycol (PMMA/PEG) nanofiber containing ZnO and glucosamine (GA) that could potentially enhance the healing process of injured skin. The addition of ZnO nanoparticles and GA to the electrospun nanofibers was considered beneficial to assess wound healing efficiency by strengthening the injured tissue. In this study, the composite nanofiber grafts were found to exhibit broad-spectrum anti-bacterial activity in both Gram-positive and Gram- negative bacterial strains. Moreover, the nanofiber grafts demonstrated promising antioxidant properties, suitable for wounddressings.
The results of the in-vitro cytotoxicity assay displayed that the ZnO- and GA-grafted PMMA/PEG nanofibers themselves did not hinder the rise in L929 fibroblasts. The results from in vivo wound healing and cytopathological examinations also indicated that the composite nanofibrous graft exhibited improved wound healing, leading to the complete re-epithelialization of new tissues, and facilitating rapid wound closure.82
Gelatin Nanoparticles
A study in 2021, fabricated a nanofiber incorporated with peppermint extract for its antibacterial and anti-inflammatory properties and optimized its release by crosslinking it with gelatin nanoparticles (CGN). Furthermore, F127 pluronic was added to the polymer matrix to enhance the fibre’s absorption ability and controlled release of the extract.
The findings showcased the continuous release of the extract from the nanofiber over a period of 144 hours. The wound dressing displayed a maximum absorption capacity of 410.65% and demonstrated a strong antibacterial activity of 99.9% against S. aureus and E. coli bacteria.
The cytopathological studies indicated that the wound treated with the extract-containing nanofibers exhibited a significant reduction in inflammation. This group displayed characteristics more similar to normal skin, including the presence of a thin epidermis, normal rete ridges, and rejuvenation of skin appendages. The wounds treated with the extract-containing nanofibrous wound dressing showed higher levels of neovascularization and collagen deposition compared to the other groups.83
Copper /Copper Oxide Nanoparticles
Copper (Cu) is one of the most widely used metals due to its high electrical conductivity and antibiotic properties. The tendency of Cu Nanoparticles (NPs) to aggregate when mixed with a polymer matrix and its eco-destructive behaviour threaten its significance in biomedical and environmental applications. However, its antibacterial properties were too strong to ignore for usage in skin tissue engineering.
An experiment was conducted where antimicrobial PVA/Cu nanofiber membranes were successfully synthesized by incorporating the Cu nanoparticles onto PVA nanofibers. The PVA/Cu nanofiber membranes displayed inhibition zones of 17 and 15 against Gram- negative Escherichia coli and Gram- positive Staphylococcus aureus bacteria, respectively, which are comparable to the inhibition zones of Rifampicin. These results indicate that the nanofiber is a good candidate for wound dressing.85
Previous studies have provided evidence indicating that copper oxide nanoparticles can stimulate the proliferation of dermal fibroblasts. These nanoparticles are shown to demonstrate the upregulation of collagen and elastin fibre components, promote angiogenesis, increase the activity of copper-dependent enzymes, and enhance the expression of integrins.
A study was conducted where nanofibers were created by infusing biosynthesized Copper Oxide NPs into a blend of Polycaprolactone (PCL) and Gelatin (Gel). The resulting nanofibers (NFs) were characterized to assess their antibacterial properties and biocompatibility. Furthermore, an MTT assay was performed to analyze the proliferation of fibroblast cells at various time intervals, specifically 1, 3, and 5 days. The results revealed that as the incubation period extended, there was a noticeable increase in the augmentation of fibroblast cells on the coverslips coated with PCL/Gel and CuO Nanofiber. The FESEM image captured on day 5 displayed that the incorporation of CuO NPs into the NF did not initiate any cytotoxic effects. However, a slight increase in toxicity was noted when 1% (w/w) CuO nanoparticles were incorporated. 86
To address this issue, another study was conducted by a team where Copper Oxide nanoparticles were carried by a natural extract called Momordica charantia (MC). This extract possesses weak antibacterial properties. Hence, the introduction of CuO nanoparticles to MC extract is expected to amplify its compatibility and improve its mechanical strength. PVA/MC nanofibres that were synthesized with a 0.6% (w/w) concentration of CuO nanoparticles outmatched the other samples regarding wound dressing applications. It is worth noting, that these nanofibers exhibited excellent antiradical and antiseptic behaviour. Furthermore, this study showcased cytocompatibility, with cell growth exceeding 100% in comparison to the control group. The fabricated scaffolds showed all the essential therapeutic features to serve as an effective wound dressing. 87
Conclusion
The process of wound healing is complex and can be influenced by various factors, especially in chronic cases. Advanced therapeutic approaches such as biomaterials and nanofiber technology offer promising solutions to enhance tissue regeneration and improve healing outcomes. Nanofibers possess mechanical and structural properties that make them effective in wound healing applications, enabling integration into the biological system and effective regulation of the skin’s response.[87] The synthesis of nanofibers involves various techniques, including electrospinning, centrifugal spinning, self- assembly, and template synthesis, each offering distinct advantages and limitations depending on the material properties and intended applications. However, to prove effective, several fine-tuning measures are needed. Prospects include 3D printing of nanofiber scaffolds, bioactive nanofibers, self-healing nanofiber, nanofiber sensors and biodegradable nanofibers 88.
Prospects for nanofibers include 3D printing of nanofiber scaffolds, bioactive nanofibers, self- healing nanofibers, nanofiber sensors, and biodegradable nanofibers.[88] 3D printing allows customization of nanofibers to meet the specific needs of each patient. 3D printing is an innovative technology that creates three-dimensional objects by adding material layer by layer. Known for its precision and accuracy, it is an excellent method for making nanofibrous scaffolds. This technology can revolutionize personalized wound dressings by mimicking the complex structures of natural tissues. By using biomaterials, cells, and bioactive molecules, 3D printing can replicate the intricate details of our body’s tissues, making it a powerful tool for advanced medical treatments.89
Bioactive scaffolds are loaded with living cells or bioactive molecules that respond to environmental cues and take necessary measures. 90 Nanofiber sensors can monitor parameters such as pH levels, glucose levels, temperature, and infection status of a patient, enabling real- time communication with healthcare professionals 91.
Electrospun nanofibers are used in wound dressings, aiding in the healing process due to their net-like makeup, which promotes healing processes like cell growth, absorption, and proliferation.[92] This paper highlights the potential of plant-derived nanofiber matrices/scaffolds, particularly those infused with bioactive compounds, in enhancing wound healing through improved antibacterial properties, mechanical strength, and biocompatibility.
Furthermore, it underscores the significant potential of various stem cell types, including adipose, bone marrow, epidermal, and endometrial stem cells, in enhancing wound healing and tissue regeneration through innovative scaffold designs and paracrine mechanisms. Nanoparticle-based wound dressings, particularly those incorporating chitosan, heparin, silver, and zinc oxide, demonstrate enhanced antibacterial properties, improved healing rates, and favourable biocompatibility, suggesting their viability for effective wound management and tissue engineering applications.
Acknowledgement
We acknowledge the Department of Biotechnology, and the management of SRMIST.
Conflict of Interest
The author(s) declares no conflict of interest
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
References
- Herman TF, Bordoni B. Wound Classification. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2023. PMID: 32119343
- Guo S, DiPietro LA. Factors affecting wound healing. Journal of Dental Research. 2010;89(3):219-229. doi:10.1177/0022034509359125
CrossRef - Kuhlmann M, Wigger-Alberti W, Mackensen YV, et al. Wound healing characteristics of a novel wound healing ointment in an abrasive wound model: A randomised, intra-individual clinical investigation. Wound Medicine. 2019;24(1):24-32. doi:10.1016/j.wndm.2019.02.002
CrossRef - Gosain A, DiPietro LA. Aging and Wound Healing. World Journal of Surgery., 2004; 28:321–6. doi: 10.1007/s00268-003-7397-6
CrossRef - Mathieu, D. (Ed.). Handbook on hyperbaric medicine. New York:: Springer., 2006; 27.
- Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocrine Reviews. 2004;25(4):612-628. doi:10.1210/er.2003-0019
CrossRef - Edwards R, Harding KG. Bacteria and wound healing. Current Opinion in Infectious Diseases., 2004;17:91– 6.
CrossRef - Davis SC, Ricotti C, Cazzaniga A, Welsh E, Eaglstein WH, Mertz PM. Microscopic and physiologic evidence for biofilm‐associated wound colonization in vivo. Wound Repair and Regeneration. 2007;16(1):23-29. doi:10.1111/j.1524- 475x.2007.00303.x
- Bjarnsholt T, Kirketerp‐Møller K, Jensen PØ, et al. Why chronic wounds will not heal: A novel hypothesis. Wound Repair and Regeneration. 2007;16(1):2-10. doi:10.1111/j.1524- 475x.2007.00283.x
CrossRef - Kolimi P, Narala S, Nyavanandi D, Youssef AA, Dudhipala N. Innovative treatment strategies to accelerate wound healing: Trajectory and recent advancements. Cells. 2022;11(15):2439. doi:10.3390/cells11152439
CrossRef - Ligresti C, Bo F. Wound bed preparation of difficult wounds: an evolution of the principles of TIME. International Wound Journal. 2007;4(1):21-29. doi:10.1111/j.1742-481x.2006.00280.x
CrossRef - Gushiken LF, Beserra FP, Bastos JK, Jackson CJ, Pellizzon CH. Cutaneous wound healing: An update from physiopathology to current therapies. Life. 2021;11(7):665. doi:10.3390/life11070665
CrossRef - Varkey M, Ding J, Tredget E. Advances in skin substitutes—potential of tissue engineered skin for facilitating anti-fibrotic healing. Journal of Functional Biomaterials. 2015;6(3):547-563. doi:10.3390/jfb6030547
CrossRef - Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds. Journal of the American Academy of Dermatology., 2016;74:607–25. doi:10.1016/j.jaad.2015.08.070
CrossRef - Xu K, Chai B, Zhang K, et al. Topical application of fibroblast growth factor 10- PLGA microsphere accelerates wound healing via inhibition of ER stress. Oxidative Medicine and Cellular Longevity. 2020;2020:1-13. doi:10.1155/2020/8586314
CrossRef - Zeng R, Lin C, Lin Z, et al. Approaches to cutaneous wound healing: Basics and future directions. Cell and Tissue Research. 2018;374(2):217-232. doi:10.1007/s00441- 018-2830-1
CrossRef - Still J, Glat P, Silverstein P, Griswold J, Mozingo D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns. 2003;29(8):837-841.
CrossRef - He J, Shi M, Liang Y, Guo B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chemical Engineering Journal. 2020;394:124888.
CrossRef - Liu X, Xu H, Zhang M, Yu D-G. Electrospun medicated nanofibers for wound healing: Review. Membranes. 2021;11(10):770. doi:10.3390/membranes11100770
CrossRef - Jao D, Beachley VZ. Continuous Dual- Track fabrication of polymer Micro-/Nanofibers based on direct drawing. ACS Macro Letters. 2019;8(5):588-595. doi:10.1021/acsmacrolett.9b00167
CrossRef - Shin S, Menk F, Kim Y, et al. Living Light- Induced Crystallization-Driven Self- Assembly for rapid preparation of semiconducting nanofibers. Journal of the American Chemical Society. 2018;140(19):6088-6094. doi:10.1021/jacs.8b01954
CrossRef - Qin W, Li J, Tu J, Yang H, Chen Q, Liu H. Fabrication of porous chitosan membranes composed of nanofibers by low temperature thermally induced phase separation, and their adsorption behavior for Cu2+. Carbohydrate Polymers. 2017;178:338-346. doi:10.1016/j.carbpol.2017.09.051
CrossRef - Keshvardoostchokami M, Majidi SS, Huo P, Ramachandran R, Chen M, Liu B. Electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue engineering. Nanomaterials. 2020;11(1):21. doi:10.3390/nano11010021
CrossRef - Augustine R, Rehman SR, Ahmed R, et al. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. International Journal of Biological Macromolecules. 2020;156:153-170. doi:10.1016/j.ijbiomac.2020.03.207
CrossRef - Sabra S, Ragab DM, Agwa MM, Rohani S. Recent advances in electrospun nanofibers for some biomedical applications. European Journal of Pharmaceutical Sciences. 2020;144:105224. doi:10.1016/j.ejps.2020.105224
CrossRef - Mozaffari A, Gashti MP, Mirjalili M, Parsania M. Argon and Argon–Oxygen plasma surface modification of gelatin nanofibers for tissue engineering applications. Membranes. 2021;11(1):31. doi:10.3390/membranes11010031
CrossRef - Luraghi A, Peri F, Moroni L. Electrospinning for Drug Delivery Applications: A Review. Journal of Controlled Release. 2021;334:463-484. doi:10.1016/j.jconrel.2021.03.033
CrossRef - Bootdee K, Nithitanakul M. Poly(d,l-lactide-co- glycolide) nanospheres within composite poly(vinyl alcohol)/aloe vera electrospun nanofiber as a novel wound dressing for controlled release of drug. International Journal of Polymeric Materials. 2019;70(4):223-230. doi:10.1080/00914037.2019.1706512
CrossRef - Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. International Journal of Nanomedicine., 2006; 1:15– 30.doi: 10.2147/nano.2006.1.1.15
CrossRef - Cui C, Sun S, Wu S, Chen S, Ma J, Zhou F. Electrospun chitosan nanofibers for wound healing application. Engineered Regeneration. 2021;2:82-90. doi:10.1016/j.engreg.2021.08.001
CrossRef - Khan M, Jerold S, Chelladurai S, et al. Recent developments in nanofibers research.; 2023. doi:10.5772/intechopen.100676
CrossRef - Song J, Kim M, Lee H. Recent advances on Nanofiber Fabrications: Unconventional state-of-the-art spinning techniques. Polymers. 2020;12(6):1386. doi:10.3390/polym12061386
CrossRef - Ghomi ER, Chellappan V, Neisiany RE, et al. An innovative tunable bimodal porous PCL/gelatin dressing fabricated by electrospinning and 3D printing for efficient wound healing and scalable production. Composites Science and Technology. 2024;247:110402. doi:10.1016/j.compscitech.2023.110402
CrossRef - Marjuban SM, Rahman M, Duza SS, et al. Recent advances in centrifugal spinning and their applications in tissue engineering. Polymers. 2023;15(5):1253. doi:10.3390/polym15051253
CrossRef - Gungor M, Sagirli MN, Calisir MD, Selcuk S, Kilic A. Developing centrifugal spun thermally cross‐linked gelatin based fibrous biomats for antibacterial wound dressing applications. Polymer Engineering and Science. 2021;61(9):2311-2322. doi:10.1002/pen.25759
CrossRef - Anusiya G, Jaiganesh R. A review on fabrication methods of nanofibers and a special focus on application of cellulose nanofibers. Carbohydrate Polymer Technologies and Applications. 2022;4:100262. doi:10.1016/j.carpta.2022.100262
CrossRef - Nemati S, Kim S, Shin YM, Shin H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Convergence. 2019;6(1). doi:10.1186/s40580-019-0209-y
CrossRef - Chu B, He JM, Liu LL, et al. Proangiogenic peptide nanofiber hydrogels for wound healing. ACS Biomaterials Science & Engineering. 2021;7(3):1100-1110. doi:10.1021/acsbiomaterials.0c01264
CrossRef - Huesmann D. Pressurized Gyration for the Mass Production of Polymeric Fibers. Advanced Science News., 2018.https://www. advancedsciencenews.co m/pressurized-gyration-for-the-mass- production-of-polymeric-fibers/
- Dai Y, Ahmed J, Edirisinghe M. Pressurized gyration: fundamentals, advancements, and future. Macromolecular Materials and Engineering. 2023;308(7). doi:10.1002/mame.202300033
CrossRef - Afshar A, Yuca E, Wisdom C, et al. Next‐ generation Antimicrobial Peptides (AMPs) incorporated nanofibre wound dressings. Medical Devices & Sensors. 2020;4(1). doi:10.1002/mds3.10144
CrossRef - Alghoraibi I, Alomari S. Different methods for nanofiber design and fabrication. In: Springer eBooks. ; 2018:1-46. doi:10.1007/978-3-319-42789-8_11-2
CrossRef - Bayrak E. Nanofibers: Production, characterization, and Tissue Engineering Applications. 21st Century Nanostructured Materials – Physics, Chemistry, Classification, and Emerging Applications in Industry, Biomedicine, and Agriculture. Published online April 20, 2022. doi:10.5772/intechopen.102787
CrossRef - Zhao J, Han W, Chen H, et al. Preparation, structure and crystallinity of chitosan nano-fibers by a solid–liquid phase separation technique. Carbohydrate Polymers. 2011;83(4):1541-1546. doi:10.1016/j.carbpol.2010.10.009
CrossRef - Sabzehmeidani MM, Ghaedi M. Adsorbents based on nanofibers. In: Interface Science and Technology. ; 2021:389-443. doi:10.1016/b978-0-12-818805- 7.00005-9
CrossRef - Yadavalli NS, Asheghali D, Tokarev A, Zhang W, Xie J, Minko S. Gravity drawing of micro‐ and nanofibers for additive manufacturing of Well‐ Organized 3D‐Nanostructured scaffolds. Small. 2020;16(11). doi:10.1002/smll.201907422
CrossRef - El-Sayed ESA, El-Sakhawy M, El-Sakhawy MAM. Non-wood fibers as raw material for pulp and paper industry. Nordic Pulp & Paper Research Journal. 2020;35(2):215-230. doi:10.1515/npprj-2019-0064
CrossRef - Aleshin AN. Polymer nanofibers and nanotubes: charge transport and device applications. Advanced Materials. 2005;18(1):17-27. doi:10.1002/adma.200500928
CrossRef - Tao SL, Desai TA. Aligned Arrays of Biodegradable Poly(ε-caprolactone) Nanowires and Nanofibers by Template Synthesis. Nano Letters. 2007;7(6):1463- 1468. doi:10.1021/nl0700346
CrossRef - Masrour S, Motavalizadehkakhky A, Hosseiny M, Mehrzad J, Zhiani R, Kazeminava F. Soy Protein Isolate-Based Hybrid Electrospun Nanofibers: An Enhanced Antimicrobial Bio-platform for Potential Wound Healing. Journal of Polymers and the Environment. 2023;31(8):3433-3444. doi:10.1007/s10924-023-02812-2
CrossRef - Sharifi M, Bahrami SH, Nejad NH, Milan PB. Electrospun PCL and PLA hybrid nanofibrous scaffolds containing Nigella sativa herbal extract for effective wound healing. Journal of Applied Polymer Science. 2020;137(46). doi:10.1002/app.49528
CrossRef - Esmaeili H, Karami A, Maggi F. Essential oil composition, total phenolic and flavonoids contents, and antioxidant activity of Oliveria decumbens Vent. (Apiaceae) at different phenological stages. Journal of Cleaner Production. 2018;198:91-95. doi:10.1016/j.jclepro.2018.07.029
CrossRef - Ghorbanpour M, Hadian J, Hatami M, Salehi- Arjomand H, Aliahmadi A. Comparison of Chemical Compounds and Antioxidant and Antibacterial Properties of Various Satureja Species Growing Wild in Iran. DOAJ (DOAJ: Directory of Open Access Journals). Published online August 1, 2016. https://doaj.org/article/ce549dffa9174b9a9b8c1aee25d de6c1
- Barzegar S, Zare MR, Shojaei F, et al. Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. International Journal of Pharmaceutics. 2021;597:120288. doi:10.1016/j.ijpharm.2021.120288
CrossRef - Ahn S, Ardoña HAM, Campbell PH, Gonzalez GM, Parker KK. Alfalfa Nanofibers for Dermal Wound Healing. ACS Applied Materials & Interfaces. 2019;11(37):33535-33547. doi:10.1021/acsami.9b07626
CrossRef - Zare MR, Khorram M, Barzegar S, et al. Antimicrobial core–shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. International Journal of Pharmaceutics. 2021;603:120698. doi: 10.1016/j.ijpharm.2021.120698
CrossRef - Khoshnevisan K, Maleki H, Samadian H, Doostan M, Khorramizadeh MR. Antibacterial and antioxidant assessment of cellulose acetate/polycaprolactone nanofibrous mats impregnated with propolis. International Journal of Biological Macromolecules. 2019;140:1260-1268. doi:10.1016/j.ijbiomac.2019.08.207
CrossRef - Hameed M, Rasul A, Waqas M, et al. Formulation and Evaluation of a Clove Oil- Encapsulated Nanofiber Formulation for Effective Wound-Healing. Molecules. 2021;26(9):2491. doi:10.3390/molecules26092491
CrossRef - Ardekani NT, Khorram M, Zomorodian K, Yazdanpanah S, Veisi H, Veisi H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. International Journal of Biological Macromolecules. 2019;125:743-750. doi:10.1016/j.ijbiomac.2018.12.085
CrossRef - Hadisi Z, Nourmohammadi J, Nassiri SM. The antibacterial and anti-inflammatory investigation of Lawsonia Inermis-gelatin- starch nano-fibrous dressing in burn wound. International Journal of Biological Macromolecules. 2018;107:2008-2019. doi:10.1016/j.ijbiomac.2017.10.061
CrossRef - Adeli-Sardou M, Yaghoobi MM, Torkzadeh-Mahani M, Dodel M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. International Journal of Biological Macromolecules. 2019;124:478-491. doi:10.1016/j.ijbiomac.2018.11.237
CrossRef - Ali A, Shahid MdA. Polyvinyl Alcohol (PVA)–Azadirachta indica (Neem) Nanofibrous Mat for Biomedical Application: Formation and Characterization. Journal of Polymers and the Environment. 2019;27(12):2933-2942. doi:10.1007/s10924-019-01587-9
CrossRef - Hameed A, Rehman TU, Rehan ZA, et al. Development of polymeric nanofibers blended with extract of neem (Azadirachta indica), for potential biomedical applications. Frontiers in Materials. 2022;9. doi:10.3389/fmats.2022.1042304
CrossRef - Kulkarni AS, Gurav DD, Khan AA, Shinde VS. Curcumin loaded nanofibrous mats for wound healing application. Colloids and Surfaces B Biointerfaces. 2020;189:110885. doi:10.1016/j.colsurfb.2020.110885
CrossRef - Guleken Z, Depciuch J, Ege H, et al. Spectrochemical and biochemical assay comparison study of the healing effect of the Aloe vera and Hypericum perforatum loaded nanofiber dressings on diabetic wound. Spectrochimica Acta Part a Molecular and Biomolecular Spectroscopy. 2021;254:119639. doi:10.1016/j.saa.2021.119639
CrossRef - Liu N, Zhou Z, Ning X, et al. Enhancing the paracrine effects of adipose stem cells using nanofiber-based meshes prepared by light-welding for accelerating wound healing. Materials & Design. 2023;225:111582. doi:10.1016/j.matdes.2022.111582
CrossRef - Tavakoli S, Kisiel MA, Biedermann T, Klar AS. Immunomodulation of Skin Repair: Cell-Based Therapeutic Strategies for Skin Replacement (A Comprehensive Review). Biomedicines. 2022;10(1):118. doi:10.3390/biomedicines10010118
CrossRef - Sethuram L, Thomas J. Therapeutic applications of electrospun nanofibers impregnated with various biological macromolecules for effective wound healing strategy – A review. Biomedicine & Pharmacotherapy.2023;157:113996. doi:10.1016/j.biopha.2022.113996
CrossRef - Chen S, Wang H, Su Y, et al. Mesenchymal stem cell- laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomaterialia. 2020;108:153-167. doi:10.1016/j.actbio.2020.03.035
CrossRef - Gao S, Chen T, Wang Z, et al. Immuno-activated mesenchymal stem cell living electrospun nanofibers for promoting diabetic wound repair. Journal of Nanobiotechnology. 2022;20(1). doi:10.1186/s12951- 022-01503-9
CrossRef - Huang TY, Wang GS, Tseng CC, Su WT. Epidermal cells differentiated from stem cells from human exfoliated deciduous teeth and seeded onto polyvinyl alcohol/silk fibroin nanofiber dressings accelerate wound repair. Materials Science and Engineering C. 2019;104:109986. doi:10.1016/j.msec.2019.109986
CrossRef - Sharif S, Ai J, Azami M, et al. Collagen‐coated nano‐ electrospun PCL seeded with human endometrial stem cells for skin tissue engineering applications. Journal of Biomedical Materials Research Part B Applied Biomaterials. 2017;106(4):1578-1586. doi:10.1002/jbm.b.33966
CrossRef - Piran M, Vakilian S, Piran M, Mohammadi- Sangcheshmeh A, Hosseinzadeh S, Ardeshirylajimi A. In vitrofibroblast migration by sustained release of PDGF-BB loaded in chitosan nanoparticles incorporated in electrospun nanofibers for wound dressing applications. Artificial Cells Nanomedicine and Biotechnology. 2018;46(sup1):511-520. doi:10.1080/21691401.2018.1430698
CrossRef - Fahimirad S, Abtahi H, Satei P, Ghaznavi-Rad E, Moslehi M, Ganji A. Wound healing performance of PCL/chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydrate Polymers. 2021;259:117640. doi:10.1016/j.carbpol.2021.117640
CrossRef - Başaran DDA, Gündüz U, Tezcaner A, Keskin D. Topical delivery of heparin from PLGA nanoparticles entrapped in nanofibers of sericin/gelatin scaffolds for wound healing. International Journal of Pharmaceutics. 2021;597:120207. doi:10.1016/j.ijpharm.2021.120207
CrossRef - Sharaf SS, El-Shafei AM, Refaie R, Gibriel AA, Abdel-Sattar R. Antibacterial and wound healing properties of cellulose acetate electrospun nanofibers loaded with bioactive glass nanoparticles; in-vivo study. Cellulose. 2022;29(8):4565-4577. doi:10.1007/s10570-022-04570-1
CrossRef - Yang J, Wang K, Yu DG, Yang Y, Bligh SWA, Williams GR. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Materials Science and Engineering C. 2020;111:110805. doi:10.1016/j.msec.2020.110805
CrossRef - Eghbalifam N, Shojaosadati SA, Hashemi- Najafabadi S, Khorasani AC. Synthesis and characterization of antimicrobial wound dressing material based on silver nanoparticles loaded gum Arabic nanofibers. International Journal of Biological Macromolecules. 2020;155:119-130. doi:10.1016/j.ijbiomac.2020.03.194
CrossRef - Liu C, Zhu Y, Lun X, Sheng H, Yan A. Effects of wound dressing based on the combination of silver@curcumin nanoparticles and electrospun chitosan nanofibers on wound healing. Bioengineered. 2022;13(2):4328-4339. doi:10.1080/21655979.2022.2031415
CrossRef - Foroushani PH, Rahmani E, Alemzadeh I, et al. Curcumin Sustained Release with a Hybrid Chitosan-Silk Fibroin Nanofiber Containing Silver Nanoparticles as a Novel Highly Efficient Antibacterial Wound Dressing. Nanomaterials. 2022;12(19):3426. doi:10.3390/nano12193426
CrossRef - Khan AUR, Huang K, Jinzhong Z, et al. Exploration of the antibacterial and wound healing potential of a PLGA/silk fibroin based electrospun membrane loaded with zinc oxide nanoparticles. Journal of Materials Chemistry B. 2021;9(5):1452- 1465. doi:10.1039/d0tb02822c
CrossRef - Balakrishnan SB, Alam M, Ahmad N, Govindasamy M, Kuppu S, Thambusamy S. Electrospinning nanofibrous graft preparation and wound healing studies using ZnO nanoparticles and glucosamine loaded with poly(methyl methacrylate)/polyethylene glycol. New Journal of Chemistry. 2021;45(18):7987-7998. doi:10.1039/d0nj05409g
CrossRef - Almasian A, Najafi F, Eftekhari M, Ardekani MRS, Sharifzadeh M, Khanavi M. Preparation of Polyurethane/Pluronic F127 Nanofibers Containing Peppermint Extract Loaded Gelatin Nanoparticles for Diabetic Wounds Healing: Characterization, In Vitro, and In Vivo Studies. Evidence-based Complementary and Alternative Medicine. 2021;2021:1-16. doi:10.1155/2021/6646702
CrossRef - Khan MQ, Kharaghani D, Nishat N, et al. The fabrications and characterizations of antibacterial PVA/Cu nanofibers composite membranes by synthesis of Cu nanoparticles from solution reduction, nanofibers reduction and immersion methods. Materials Research Express.2019;6(7):075051. doi:10.1088/2053-1591/ab1688
CrossRef - Karuppannan SK, Ramalingam R, Khalith SBM, et al. Copper oxide nanoparticles infused electrospun polycaprolactone/gelatin scaffold as an antibacterial wound dressing. Materials Letters. 2021;294:129787. doi:10.1016/j.matlet.2021.129787
CrossRef - Sarwar MN, Ali HG, Ullah S, et al. Electrospun PVA/CuONPs/Bitter Gourd Nanofibers with Improved Cytocompatibility and Antibacterial Properties: Application as Antibacterial Wound Dressing. Polymers. 2022;14(7):1361. doi:10.3390/polym14071361
CrossRef - Chen S, Liu B, Carlson MA, Gombart AF, Reilly DA, Xie J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine. 2017;12(11):1335- 1352. doi:10.2217/nnm-2017-0017
CrossRef - Samadian H, Zamiri S, Ehterami A, et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: in vitro and in vivo studies. Scientific Reports. 2020;10(1). doi:10.1038/s41598-020-65268-7
CrossRef - Yayehrad AT, Siraj EA, Matsabisa M, Birhanu G. 3D printed drug loaded nanomaterials for wound healing applications. Regenerative Therapy. 2023;24:361-376. doi:10.1016/j.reth.2023.08.007
CrossRef - Pereira RF, Barrias CC, Granja PL, Bartolo PJ. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine. 2013;8(4):603-621. doi:10.2217/nnm.13.50
CrossRef - Halicka K, Cabaj J. Electrospun Nanofibers for Sensing and Biosensing Applications—A Review. International Journal of Molecular Sciences. 2021;22(12):6357. doi:10.3390/ijms22126357
CrossRef - Liu M, Duan XP, Li YM, Yang DP, Long YZ. Electrospun nanofibers for wound healing. Materials Science and Engineering C. 2017;76:1413-1423. doi:10.1016/j.msec.2017.03.034
CrossRef - Xia L, Lu L, Liang Y, Cheng B. Fabrication of centrifugally spun prepared poly(lactic acid)/gelatin/ciprofloxacin nanofibers for antimicrobial wound dressing. RSC Advances. 2019;9(61):35328-35335. doi:10.1039/c9ra07826f
CrossRef - Yergoz F, Hastar N, Cimenci CE, et al. Heparin mimetic peptide nanofiber gel promotes regeneration of full thickness burn injury. Biomaterials. 2017;134:117-127. doi:10.1016/j.biomaterials.2017.04.040
CrossRef - Xu Z, Mahalingam S, Basnett P, et al. Making Nonwoven Fibrous Poly(ε‐ caprolactone) Constructs for Antimicrobial and Tissue Engineering Applications by Pressurized Melt Gyration. Macromolecular Materials and Engineering. 2016;301(8):922-934. doi:10.1002/mame.201600116
CrossRef - Zhao J, Han W, Tu M, et al. Preparation and properties of biomimetic porous nanofibrous poly(l-lactide) scaffold with chitosan nanofiber network by a dual thermally induced phase separation technique. Materials Science and Engineering C. 2012;32(6):1496-1502. doi:10.1016/j.msec.2012.04.031
CrossRef - Jao D, Beachley VZ. Continuous Dual- Track Fabrication of Polymer Micro-/Nanofibers Based on Direct Drawing. ACS Macro Letters.2019;8(5):588-595. doi:10.1021/acsmacrolett.9b00167
CrossRef - Zelenski CM, Dorhout PK. Template Synthesis of Near- Monodisperse1Microscale Nanofibers and Nanotubules of MoS2. Journal of the American Chemical Society. 1998;120(4):734-742. doi:10.1021/ja972170q
CrossRef







