Abdullah H. Maad1* , Abdullah H. AL-Gamli2
, Abdullah H. AL-Gamli2 , Khaled Sh. Shamarekh3
, Khaled Sh. Shamarekh3 , Moath Refat4
, Moath Refat4 and Mohammed E. Shayoub5
and Mohammed E. Shayoub5
1Department of Pharmaceutical, College of Pharmacy, University of Al-Ameed, Karbala, Iraq
2Department of Pharmaceutics, Faculty of Pharmacy, University of Science and Technology, Yemen
3Department of Pharmaceutics, Faculty of Medical Science, Al-Nasser University, Sana’a, Yemen
4Department of Biochemistry and Molecular Biology, The Key Laboratory of Environment and Genes Related to Disease of Ministry of Education, Health Science Center, Xi’an Jiaotong University, Xi’an, China.
5Department of Pharmaceutics, Faculty of Pharmacy, Khartoum University, Khartoum city, Sudan.
Corresponding Author E-mail:dr.ph.abdullah.maad@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3001
Abstract
Colorectal cancer ranks as the fourth most frequent cause of cancer-related fatalities on a global scale. The present study aims at assessing the anti-proliferative and apoptotic effects of Solenostemma argel extract on colorectal cancer cells (HCT-116). The antiproliferative activity was investigated using Sulfo-Rhodamine-B (SRB) assay and the apoptotic effects were demonstrated utilizing acridine orange/ ethidium bromide (AO/EB) staining method. The antiproliferative results demonstrated that the extract exhibited dose-dependent antiproliferative activity, with an IC50 value of 85.3 μg/ml. The apoptosis results clearly demonstrated the ability of the methanolic extract of Solenostemma argel in inducing apoptosis in HCT-116 cancer cells. In conclusion, the investigation highlights the considerable antiproliferative and apoptotic impacts of Solenostemma argel leaf extract on HCT-116 colorectal cancer cells. This underscores its potential as a promising chemopreventive agent specifically targeting HCT-116 colon cancer cells.
Keywords
Antiproliferation; Apoptosis; Colorectal cancer; HCT-116; Solenostemma argel
Download this article as:| Copy the following to cite this article: Maad A. H, AL-Gamli A. H, Shamarekh K. S, Refat M, Shayoub M. E. Antiproliferative and Apoptotic Effects of Solenostemma argel Leaf Extracts on Colon Cancer Cell Line HCT-116. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Maad A. H, AL-Gamli A. H, Shamarekh K. S, Refat M, Shayoub M. E. Antiproliferative and Apoptotic Effects of Solenostemma argel Leaf Extracts on Colon Cancer Cell Line HCT-116. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/4ftvsBy |
Introduction
Colorectal cancer (CRC) ranks as the third most prevalent cancer. In 2023, approximately 1.63 million new cases of colorectal cancer were diagnosed globally, resulting in 694,000 deaths. Morbidity and mortality rates from this disease continue to rise rapidly in less developed countries, while highly developed countries tend to be seen stable or decrease, albeit remaining among the highest globally. The global burden is anticipated to surge by 60%, reaching over 2.2 million new cases and 1.1 million deaths by 2030 1, 2. In Sudan, colon cancer is the fifth most common cancer with an incidence rate of 7.1 per 100,000 of 6548 registered cancer cases during the 2009-2010 periods. Its incidence rate is increased per year, according to data collected at the Radiation and Isotope Centre in Khartoum (RICK) from 2009-2013(3). Generally, the incidence of CRC is increased due to increasing age, family history, smoking, excessive alcohol consumption, obesity, and dietary factors 3.
Conventional therapies of CRC are surgery, chemotherapy, radiotherapy and targeted therapy. However, their dangerous adverse effects and the developing resistance by tumor cells as well as the increasing mortality rate associated with colorectal cancer highlight the need to search for more effective therapies4,5. Plant-based products have provided a valuable resource for finding and developing of unique anticancer drugs that act on several signaling pathways in tumor cells and have limited or no adverse effects. These include the alkaloids Vinca, Vinblastine and Vincristine 6,7.
In Sudan, the utilization of medicinal and aromatic plants and their derivatives is deeply ingrained in daily life, playing a vital role across various aspects of society and culture8. Solenostemma argel (locallyknown in Sudan as “Hargel”) belongs to the family Asclepiadaceae. It is widely distributed in Sudan which is its richest source and in Egypt9. It stands as a crucial Sudanese medicinal plant, widely employed in traditional medicine to address various aliments including diabetes, jaundice, measles, syphilis, gastrointestinal disorders, urinary tract infections, and some diseases of liver and kidney10,11.
Recently, there are a lot of evidences about a wide spectrum of pharmacological effects of S. argel and its active compounds with low toxicity include analgesic, anti-ulcerogenic, hypoglycemic, antioxidant and antispasmodic effects12,14. Additionally, several previous studies reported that S. argel extracts have anticancer activity against different types of cancer, e.g., Ehrlich ascites carcinoma (EACC), acute myeloid leukemia (AML), acute lymphocyte leukemia (ALL), human hepatocellular cancer (HepG2) and Kaposi’s sarcoma cell 15-18. Therefore, based on the previous studies, it can be expected that S. argel also has antitumor activity on other types of human cancer. Therefore, the objective of this study is to assess the in vitro anticancer potential of the methanolic extract from S. argel leaves against the colon cancer cell line (HCT-116).
Materials and Methods
Plant material
The dried Solenostemma argel leaves were procured and meticulously identified, and authenticated by the herbarium of the Medicinal and Aromatic Plants Research Institute (MAPRI) in Sudan.
Chemicals and Reagents
Folin–Ciocalteau reagents, gallic acid, quercetin, aluminum chloride hexahydrate and potassium acetate were obtained from Sigma Chemical Co., St. Louis, MO, USA. Human colorectal carcinoma cells (HCT-116) were acquired from ATCC, USA. The cell culture materials, including penicillin G sodium, Fetal bovine serum (FBS), streptomycin sulphate, L-glutamine, amphotericin B, trypsin/ EDTA and dimethyl sulphoxide (DMSO) were obtained from Cambrex BioScience (Copenhagen, Denmark). Acridine orange/ ethidium bromide (AO/EB) was acquired from Biovision (Mountain View, CA, USA).
Cell Culture
Human colon cancer cells (HCT-116) were used to assess the antiproliferative and apoptotic effect of the plant extract. The cells were cultured in McCoy’s 5a modified medium which was enhanced by L glutamine (2 mM), penicillin G sodium (100 units/ml), fetal bovine serum (10%), 250 ng/ml amphotericin B and streptomycin sulphate (100 units/ml). The cells were stored in an atmosphere of 5% CO2 and 37°C. For subculture, cell monolayers were harvested after trypsin/ EDTA treatment. Dimethyl sulphoxide (DMSO) was used to dissolve the extract and diluted to the intended concentration for the test. All experiments were repeated three times unless otherwise stated.
Extraction of Solenostemma argel (Hargel) leaves
The dried Hargel leaves were cleared from other plant pieces and milled manually. After that, 100 grams of these leaves were extracted using 80% methanol via a Soxhlet apparatus. The solvent was then removed through evaporation under pressure using a Rotary evaporator. The resulting extract was stored at a temperature of 4ᵒC and maintained in a dark place. The obtained extract showed a yield of 20%19.
Preliminary Phytochemical analysis
The preliminary phytochemical tests for the methanolic extract of S. argel were conducted following the procedures outlined by Trease and Evans20. Briefly, a preliminary screening was carried by the application of various testing methods of Draggendorff’s, Liebermann-Bur chard test, Foam formation test, Lead acetate test, Keller-Killani test, Borntrager’s test and Braymer’s test for determining the presence of alkaloids, terpenoid, saponins, flavonoids, cardiac glycoside, anthraquinone and tannins, respectively.
Determination of the total content of phenols and flavonoids
Folin Ciocalteu reagent was used for analysis of total phenolics content21. Briefly, 0.5 ml of the extract was mixed with 0.5 ml of Folin-Ciocalteu reagent. The solution was kept at 25oC for 5-8 min before adding 2 ml of sodium carbonate solution 7.5 % and adjusting the volume to 8 ml with water. After 2 h, the absorbance was measured at 725 nm. Gallic acid was used as standard for the calibration curve. Total phenolic content was expressed as mg gallic acid equivalents per gram of sample (mg/g). All samples were analyzed in triplicate.
The total flavonoid content was measured by a colorimetric assay 22. In brief, 50 μL of crude extract (1 mg/mL ethanol) were made up to 1 mL with methanol, mixed with 4 mL of distilled water. Then, 0.3 ml of 5% NaNO2 was added. After 5 min, 0.3 ml of 10% aluminium chloride was added, and the mixture was allowed to stand for 6 minute. Then, 2 ml of 1 M sodium hydroxide was added to the mixture, and the final volume of the mixture was brought to 10 mL with distilled water. The absorbance was determined at 420 nm versus a blank. Quercetin was used as standard for the calibration curve. The total flavonoid content of the extract was calculated from a calibration curve, and the result was expressed as mg quercetin equivalents per gram of sample (mg/g). All samples were analyzed in triplicate.
Antiproliferative activity assay by Sulfo-Rhodamine-B (SRB)
The SRB assay23 was performed to evaluate the antiproliferative efficacy of the leaf extract on HCT-116 cell lines. Cells were seeded in a 96-multiwell plate at a density of 104 cells per well, 24 hours prior to treatment, allowing for attachment to the plate. Various concentrations of the extract (0, 25, 50, 100, and 200 µg/ml) were added to the cells, with triplicate monolayer wells established for each concentration. Following treatment, the cells were cultured for 48 hours under standard conditions of 37°C and 5% CO2. Subsequently, the cells were fixed and stained using Sulfo Rhodamine B stain. Excess stain was removed with acetic acid, and the remaining stain was dissolved with Tris EDTA buffer. Absorbance measurements were taken via ELISA. Survival curves for each tumor cell line were generated by plotting the relationship between surviving fraction and drug concentration.
Detection of Apoptosis by Acridine Orange/ Ethidium Bromide (AO/EB) Staining
To investigate the onset of apoptosis in HCT-116 cells, a series of treatments were administered using Solenostemma argel leaf extract at varying concentrations: 85.3 µg/ml (IC50), as well as lower and higher concentrations of 50 and 200 µg/ml, respectively. Following a 48-hour incubation period, cells were meticulously washed with cold PBS and subsequently stained with a mixture containing 20 μg/ml ethidium bromide and 20 μg/ml acridine orange, immediately preceding microscopic examination. A small aliquot (10 μL) of the gently agitated cell suspension was then placed onto microscope slides, covered with glass slips, and observed under a fluorescence microscope. For each data point, 300 cells were counted in randomly selected fields and quantified in duplicate24.
Statistical Analysis
Statistical analysis was conducted on all experiments, which were performed in triplicate. The results were then averaged to obtain a representative value. The cell viability percentages were plotted using Microsoft Office Excel 2010 to create graphical representations of the data.
Results
Phytochemical Analysis
The phytochemical analysis of the methanolic extract of S. argel (see Table 1) revealed the presence of various groups of secondary metabolites, including saponins, flavonoids, alkaloids, and tannins, all of which possess medicinal significance.
Table 1: Phytochemical screening of the methanol extract of S. argel leaves
|
Class of compound |
Result |
|
Terpenoids |
+ |
|
Tannins |
+ |
|
Saponins |
+ |
|
Flavonoids |
+ |
|
Alkaloids |
+ |
|
Anthraquinone |
– |
|
Cardiac glycosides |
+ |
(+): present, (–): absent.
Total phenolic and flavonoid contents in the methanolic extract of S. argel
Data on total phenolic and flavonoid contents in the methanolic extract of S. argel are presented in Table 2. The results showed that the total phenolic was (24.53 ± 0.35 mg), while the total flavonoid was (8.73 ± 0.025 mg).
Table 2: Total phenolic and flavonoids contents of S. argel methanolic extract
|
Test |
Amount |
|
Total Phenolics |
24.53 ± 0.35 mg GAE /g of dry extract |
|
Total Flavonoids |
8.73 ± 0.025 mg QE /g of dry extract |
The data are presented as the mean ± standard deviation (SD) of three replicates; GAE: Gallic acid equivalent; QE: Quercetin equivalent.
Antiproliferative effect of Hargel extract
The antiproliferative activity of S. argel leaf extract was assessed through the SRB assay, and the half-maximal inhibitory concentration (IC50) values were determined from the dose-response curves (refer to Figure 1). HCT-116 cells were subjected to varying concentrations of S. argel methanolic extract (25, 50, 100, and 200 μg/ml). Analysis of the SRB assay results revealed a concentration-dependent reduction in the proliferation of HCT-116 cells upon treatment with the methanolic extract of S. argel, with an IC50 value of 85.3 ± 0.15 μg/ml. As depicted in Fig. 1, treatment with the leaf extract at a concentration of 100 μg/ml resulted in a 37% inhibition of cancer cell proliferation after 48 hours, while at double the concentration (200 μg/ml), the inhibitory effect diminished, although the leaf extract continued to induce cell growth arrest.
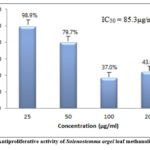 |
Figure 1: Antiproliferative activity of Solenostemma argel leaf methanolic extract |
Hargel extract induced Apoptosis
The Acridine orange/ ethidium bromide (AO/EB) assays were conducted to discern between live and dead cells, revealing that S. argel leaf extract induced cell death in a dose-dependent manner across the studied cell lines.
Human colon cells (HCT-116) were cultured with leaf extract of Solenostemma argel at concentrations of 50, 85.3, and 200 μg/ml. To detect apoptosis, cells were fluorescently stained with acridine orange/ethidium bromide (AO/EB) and observed under a fluorescence microscope. As depicted in Fig. 2, no significant apoptosis was observed in the control group (Fig. 2A). However, in the experimental group (Fig. 2B), early-stage apoptotic cells characterized by crescent-shaped or granular yellow-green AO nuclear staining were evident, with staining localized asymmetrically within the cells. Interestingly, increasing the concentration and duration of treatment resulted in a higher number of early-stage apoptotic cells. Furthermore, late-stage apoptotic cells were also detected, exhibiting concentrated and asymmetrically localized orange nuclear EB staining (Fig. 2C). Necrotic cells exhibited increased volume and displayed irregular orange-red fluorescence at their periphery, with cells appearing disintegrated (Fig. 2D).
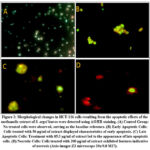 |
Figure 2: Morphological changes in HCT-116 cells resulting from the apoptotic effects of the methanolic extract of S. argel leaves were detected using AO/EB staining. |
Discussion
Chemical components of plants or crude extracts are known to be biologically active components. They are directly responsible for various activities such as antioxidant, antimicrobial and anticancer effects 25,26. Therefore, the phytochemical screening is essential to understand their role on treatment of colorectal cancer and subsequently may lead to drug discovery and development. In our study, Solenostemma argel methanolic extract was analyzed using the method described by Trease and Evans 20. The obtained results exhibited the existence of terpenoids, flavonoids, tannins, alkaloids, saponins, and cardiac glycosides compounds (Table 1), which is in agreement with previous studies 12,13. Indeed, flavonoids are recognized for their diverse biological activities, which include exhibiting anticancer properties 27-29 Numerous studies have reported on the anticancer activities of flavonoids against various types of cancers 30-32. Given that the methanolic extract of S. argel leaves contains these secondary metabolite compounds, it is plausible to infer that it possesses a wide range of pharmacological benefits, such as antioxidant, anti-inflammatory, and anticancer activities. This suggests the potential of S. argel extract as a valuable resource in the development of therapeutic interventions for cancer and other related conditions.
The phenolic and flavonoid compounds are renowned for their diverse biological activities, including antioxidant, anti-inflammatory, and anticancer properties33,34; hence it is important to determine the total phenolic and flavonoid contents of S. argel leaves extract. In our study, the total phenolic content was measured by the Folin-Ciocalteu reagent method and described as GAE (Gallic Acid Equivalent), whereas the total flavonoid content was estimated using the aluminum chloride colorimetric method and described as QE (Quercetin Equivalent). The total phenolic content in methanolic extract of S. argel leaves was found to be 24.53 ± 0.35 mg/g GAE, while the total content of flavonoid was 8.73 ± 0.025 mg/g QE (Table 2). This finding is almost near to results obtained by Muddathir et al. (2017) 35, who stated that the amount of total phenolic content measured in methanolic extract of S. argel (grown in Sudan) was 32.90 ± 3.42 mg/g GAE. Inconsistently, a previous study conducted in Egypt reported different quantities of total phenolic and total flavonoid in the methanolic extract of S. argel leaves, measuring at 6.32 mg/g GAE and 2.40 mg/g QE, respectively36. Discrepancies in these quantities may be attributed to variations in environmental factors such as temperature, UV radiation, day length, rainfall, altitude, and climate. These factors significantly influence plant development and subsequently affect the biosynthesis of secondary metabolites with biological activity 37. Therefore, differences in the environmental conditions between regions could contribute to variations in the composition of phenolic and flavonoid compounds in S. argel leaves. Furthermore, the total phenolic and flavonoid content can be significantly influenced by the extraction method and the choice of solvent 38,39. Indeed, several studies have indicated a positive correlation between the total content of phenols and flavonoids and the biological effects of the extracts 40-42. Therefore, it is reasonable to speculate that phenolic and flavonoid compounds play a crucial role in the observed anticancer activity of S. argel leaves in vitro.
The present study also evaluated the antiproliferative activity of the methanolic extract of Solenostemma argel leaves on colorectal cancer cells (HCT-116) using the Sulfo-Rhodamine B (SRB) assay. The obtained results showed that the extract exhibited antiproliferative effects dependent on doses with IC50 of 85.3 ± 0.15μg/ml. This finding is consistent with the results reported by Alaa Eldin (2008) 43, who observed a significant reduction in colorectal cancer cell proliferation with increasing doses of the methanolic extract of S. argel. Furthermore, Nassr-Allah et al. (2009) reported that both aqueous and ethanolic extracts of S. argel reduced tumor growth induced by Ehrlich ascites carcinoma cells and prolonged animal survival by 29 days (15). In addition, other plant extracts such as ginger leaf extract, Chinese herbal extract and turmeric root extract have exhibited anticancer properties on HCT-116 cells 44-46 in agreement with these findings.
Although crude extracts can have greater effects than a single ingredient, combinations of ingredients in plant extracts can be very important for the final biological activity; its different fractions contain compounds with different chemical compositions, and therefore, can exhibit different types of activities 47. The previous study conducted by Plaza et al. (2005) found that seven novel 15-keto pregnane glycosides, isolated from Solenostemma argel, exhibited the ability to decrease the proliferation of Kaposi’s sarcoma cells induced by vascular endothelial growth factor (VEGF) in a dose-dependent manner supporting our results 18. Previous phytochemical screening revealed that leaf extracts of Solenostemma argel contain various bioactive compounds like kaempferol and quercetin 48,49. Several previous studies have reported that Kaempferol has antiproliferation activities in various human cancer cell lines, including those derived from colon cancer 50-52. Furthermore, the combination of quercetin and kaempferol exhibited a greater cytotoxic efficacy on HCT-116 cells than did either quercetin or kaempferol alone53.
Apoptosis is a natural and intricate process of cellular self-destruction, occurring to eliminate damaged or atypical cells 24. However, Cancer cells have the ability to resist apoptosis, allowing them to continue rapidly growing without control. As a result, it would be ideal to have a compound that can effectively modulate apoptosis as a potential treatment for cancer 54. Many plant-based chemicals have been discovered to possess the capacity to trigger programmed cell death in a wide range of cancerous cells derived from humans 5-57. In the present study, the apoptotic effects of Solenostemma argel leaf methanolic extract on colorectal cancer cells (HCT-116) were assessed using the acridine orange/ethidium bromide (AO/EB) staining method. As shown in Fig. 2, treatment of HCT-116 cells with the Hargel extract at concentrations corresponding to the IC50 value (85.3µg/ml), as well as lower and higher concentrations (50 and 200 µg/ml), resulted in the staining of early and late apoptotic cells with condensed nuclei, appearing bright green in color at concentrations of extract ≤ 85.3 µg/ml (Figure 2b & c). Necrotic cells, on the other hand, appeared red in color at the higher concentration of extract (200 µg/ml) (Figure 2d). The results of the current study suggest that the methanolic extract of S. argel induces apoptosis in HCT-116 cells in a concentration-dependent manner. The apoptotic effect observed with the methanolic extract of S. argel leaves in our study is consistent with previous findings reported by Hanafi and Mansour (2010) (16), they demonstrated that the aqueous extract of S. argel leaves induced widespread zones of apoptotic cells in Ehrlich carcinoma tissue. Moreover, several previous studies investigating pure compounds isolated from S. argel have also been conducted to evaluate their apoptotic effects, with results confirming their ability to induce apoptosis in various cancer cells (58, 59), findings supported this result. Since apoptosis is considered a new target in cancer drug discovery, this result confirms the potential of S. argel as an agent with chemotherapeutic and cytostatic effects against colorectal cancer cells.
Conclusion
In conclusion, S. argel leaf methanolic extract exhibited antitumor effect on colorectal cancer cells (HCT-116) and this effect was shown to be dose dependent. The extract also induced significant apoptosis on HCT-116 cells. Therefore, the leaf extract of Solenostemma argel has a promising potential as preventive chemotherapy agent against colorectal cancer cells.
Acknowledgment
The authors would like to express their gratitude to all staff members of the Laboratory of Cancer Research at the National Cancer Institute from Egypt for providing the infrastructural facilities for conducting the experiments.
Conflict of Interests
The authors declare no conflict of interest.
Funding Source
There is no funding sources
Authors’ Contribution
AHM, AHA, and KS performed the experimental work, analyzed the findings, and wrote the manuscript. MS supervised the research. MR revised the manuscript and edited the final version.
Data availability
Data will be available upon request.
References
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA: a cancer journal for clinicians. 2023;73(3):233-54.
CrossRef - Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683-91.
CrossRef - Saeed ME, Cao J, Fadul B, Kadioglu O, Khalid HE, Yassin Z, et al. A five-year survey of cancer prevalence in Sudan. Anticancer research. 2016;36(1):279-86.
- PV Shekhar M. Drug resistance: challenges to effective therapy. Current cancer drug targets. 2011;11(5):613-23.
CrossRef - Zhou H, Liu Z, Wang Y, Wen X, Amador EH, Yuan L, et al. Colorectal liver metastasis: molecular mechanism and interventional therapy. Signal transduction and targeted therapy. 2022;7(1):70.
CrossRef - Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. Journal of ethnopharmacology. 2005;100(1-2):72-9.
CrossRef - Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer letters. 2008;269(2):352-62.
CrossRef - El-Ghazali GEB. “Promising Sudanese Medical Plants,” National Center for research, Khartoum, Sudan. 1997.
- Fayed MNE-HaA. “Material for Excoriation flora of Egypt,” Cairo University Herbarium, Taeckholmia,. 1995.
- El Kamali H, Khalid SA. The most common herbal remedies in Central Sudan. Fitoterapia. 1996;67:301-6.
- Boulos L. Medicinal Plants of North Africa. Medicinal plants of North Africa. 1983.
- Mudawi MM, Chidrawar VR, Yassin AY, Habeballa RS, E l-wahab MFA, Sulaiman MI. Analgestic Activity of Solenostemma argel by Modulating Pain Nociception Pathway in Mice. World Journal of Pharmaceutical Research. 2015;4(4):187-97.
- Mohammed Hamad A, Mohammed OY, Sania Shaddad AI, AM HI. Anti-ulcerogenic Activity of The Crude Methanolic Extract of Solenostemma argel Hyne. Journal of Pharmaceutical and Biomedical Sciences. 2014;04(12):1084-9.
- Al-Deen AT, Al-Naqeb G. Hypoglycemic effect and in vitro antioxidant activity of methanolic extract from Argel (Solenostemma Argel) plant. International Journal of Herbal Medicine. 2014;2(2 Part C):128-31.
- Nassr-Allah AA, Aboul-Enein AM, Aboul-Enein KM, Lightfoot DA, Cocchetto A, El-Shemy HA. Anti-cancer and anti-oxidant activity of some Egyptian medicinal plants. Journal of Medicinal Plants Research. 2009;3(10):799-808.
- Hanafi N, Mansour S. Antitumor Efficacy of salenostemma argel and/or y-irradiation against Ehrlich carcinoma. Journal of Biological Sciences. 2010;10(6):468-79.
CrossRef - Aboul-Enein AM, El-Ela FA, Shalaby EA, El-Shemy HA. Traditional medicinal plants research in Egypt: studies of antioxidant and anticancer activities. Journal of Medicinal Plants Research. 2012;6(5):689-703.
- Plaza A, Perrone A, Balestrieri C, Balestrieri ML, Bifulco G, Carbone V, et al. New antiproliferative 14, 15-secopregnane glycosides from Solenostemma argel. Tetrahedron. 2005;61(31):7470-80.
CrossRef - Horborne J. Phytochemical Methods, A Guide to Modern Techniques of Plant Analysis 3rd Eds. Chapman and Hall, London; 1998.
- Trease G, Evans W. Phytochemical screening. Textbook of pharmacognosy London: Tindal Limited. 1989:541.
- Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. Journal of agricultural and food chemistry. 2003;51(3):609-14.
CrossRef - Baba SA, Malik SA. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. Journal of Taibah university for science. 2015;9(4):449-54.
CrossRef - Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI: Journal of the National Cancer Institute. 1990;82(13):1107-12.
CrossRef - Aigner T. Apoptosis, necrosis, or whatever: how to find out what really happens? The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2002;198(1):1-4.
CrossRef - Ahmad Nejhad A, Alizadeh Behbahani B, Hojjati M, Vasiee A, Mehrnia MA. Identification of phytochemical, antioxidant, anticancer and antimicrobial potential of Calotropis procera leaf aqueous extract. Scientific Reports. 2023;13(1):14716.
CrossRef - Hossain MA, Nagooru MR. Biochemical Profiling and Total Flavonoids Contents of Leaves Crude Extract of Endemic Medicinal Plant Corydyline terminalis L. Kunth. Pharmacognosy Journal. 2011;3(24).
CrossRef - Chahar MK, Sharma N, Dobhal MP, Joshi YC. Flavonoids: A versatile source of anticancer drugs. Pharmacognosy reviews. 2011;5(9):1.
CrossRef - Thangavel P, Viswanath B, Kim S. Synthesis and characterization of kaempferol-based ruthenium (II) complex: A facile approach for superior anticancer application. Materials Science and Engineering: C. 2018;89:87-94.
CrossRef - Rajasekar M, Bhuvanesh P, Varada P, Selvam M. Review on Anticancer activity of flavonoid derivatives: recent developments and future perspectives. Results in Chemistry. 2023:101059.
CrossRef - Khan AU, Dagur HS, Khan M, Malik N, Alam M, Mushtaque M. Therapeutic role of flavonoids and flavones in cancer prevention: Current trends and future perspectives. European Journal of Medicinal Chemistry Reports. 2021;3:100010.
CrossRef - Cárdenas M, Marder M, Blank VC, Roguin LP. Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorganic & medicinal chemistry. 2006;14(9):2966-71.
CrossRef - Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12(2):457.
CrossRef - Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnology reports. 2019;24:e00370.
CrossRef - Kumar N, Gupta S, Chand Yadav T, Pruthi V, Kumar Varadwaj P, Goel N. Extrapolation of phenolic compounds as multi-target agents against cancer and inflammation. Journal of Biomolecular Structure and Dynamics. 2019;37(9):2355-69.
CrossRef - Muddathir A, Yamauchi K, Batubara I, Mohieldin E, Mitsunaga T. Anti-tyrosinase, total phenolic content and antioxidant activity of selected Sudanese medicinal plants. South African Journal of Botany. 2017;109:9-15.
CrossRef - El-Beltagi HS, Abdel-Mobdy YE, Abdel-Rahim E. Toxicological influences of cyfluthrin attenuated by Solenostemma argel extracts on carbohydrate metabolism of male albino rats. Fresen Environ Bull. 2017;26:1673-81.
- Khalid H, Abdalla WE, Abdelgadir H, Opatz T, Efferth T. Gems from traditional north-African medicine: medicinal and aromatic plants from Sudan. Natural products and bioprospecting. 2012;2(3):92-103.
CrossRef - Dirar A, Alsaadi D, Wada M, Mohamed M, Watanabe T, Devkota H. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. South African Journal of Botany. 2019;120:261-7.
CrossRef - Ibrahim EA, Gaafar AA, Salama ZA, El Baz FK. Anti-inflammatory and antioxidant activity of Solenostemma argel extract. International Journal of Research in Pharmacology and Phytochemistry. 2015;7:635-41.
- Fathi Hafshejani S, Lotfi S, Rezvannejad E, Mortazavi M, Riahi‐Madvar A. Correlation between total phenolic and flavonoid contents and biological activities of 12 ethanolic extracts of Iranian propolis. Food Science & Nutrition. 2023.
CrossRef - Sant’Ana LDO, Buarque Ferreira AB, Lorenzon MCA, Berbara RLL, Castro RN. Correlation of total phenolic and flavonoid contents of Brazilian honeys with colour and antioxidant capacity. International Journal of Food Properties. 2014;17(1):65-76.
CrossRef - Vamanu E, Nita S. Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. BioMed Research International. 2013;2013.
CrossRef - Alaa Eldin DMA. The effect of methanolic extract of Solenostemma argel [argel] on colorectal cancer in rats.: Master Thesis, Faculty of Pharmacy. University of Khartoum- Sudan.; 2008.
- Malmir S, Ebrahimi A, Mahjoubi F. Effect of ginger extracts on colorectal cancer HCT-116 cell line in the expression of MMP-2 and KRAS. Gene Reports. 2020;21:100824.
CrossRef - de Lima RMT, Dos Reis AC, de Menezes AAPM, Santos JVdO, Filho JWGdO, Ferreira JRdO, et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]‐gingerol in cancer: A comprehensive review. Phytotherapy research. 2018;32(10):1885-907.
CrossRef - Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chinese medicine. 2011;6(1):27.
CrossRef - Tingting Z, Xiuli Z, Kun W, Liping S, Yongliang Z. A review: extraction, phytochemicals, and biological activities of rambutan (Nephelium lappaceum L) peel extract. Heliyon. 2022.
CrossRef - El Kamali H. Botanical and Chemical Studies on Solenostemma argel (Del) Hayne Grown in Khartoum. M Sc, University of Khartoum, Sudan. 1991.
- Shafek R, Shafik N, Michael H. Glycosides Isolated from Solenostemma argel Stem Extract. Asian journal of plant sciences. 2012;11(3):143-7.
CrossRef - Almatroudi A, Allemailem KS, Alwanian WM, Alharbi BF, Alrumaihi F, Khan AA, et al. Effects and Mechanisms of Kaempferol in the Management of Cancers through Modulation of Inflammation and Signal Transduction Pathways. International Journal of Molecular Sciences. 2023;24(10):8630.
CrossRef - Riahi-Chebbi I, Souid S, Othman H, Haoues M, Karoui H, Morel A, et al. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Scientific Reports. 2019;9(1):195.
CrossRef - Yoshida T, Konishi M, Horinaka M, Yasuda T, Goda AE, Taniguchi H, et al. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochemical and Biophysical Research Communications. 2008;375(1):129-33.
CrossRef - Jaramillo-Carmona S, Lopez S, Abia R, Rodriguez-Arcos R, Jimenez A, Guillen R, et al. Combination of Quercetin and Kaempferol enhances in vitro Cytotoxicity on Human Colon Cancer (HCT-116) Cells. Records of Natural Products. 2014;8(3).
- Tor YS, Yazan LS, Foo JB, Armania N, Cheah YK, Abdullah R, et al. Induction of apoptosis through oxidative stress-related pathways in MCF-7, human breast cancer cells, by ethyl acetate extract of Dillenia suffruticosa. BMC complementary and alternative medicine. 2014;14(1):55.
CrossRef - Kiran A, Altaf A, Sarwar M, Malik A, Maqbool T, Ali Q. Phytochemical profiling and cytotoxic potential of Arnebia nobilis root extracts against hepatocellular carcinoma using in-vitro and in-silico approaches. Scientific Reports. 2023;13(1):11376.
CrossRef - Wang H, Xu Y, Sun J, Sui Z. The novel curcumin derivative 1g induces mitochondrial and ER-stress-dependent apoptosis in colon cancer cells by induction of ROS production. Frontiers in oncology. 2021;11:644197.
CrossRef - Shiezadeh F, Mousavi SH, Amiri MS, Iranshahi M, Tayarani-Najaran Z, Karimi G. Cytotoxic and apoptotic potential of Rheum turkestanicum Janisch root extract on human cancer and normal cells. Iranian journal of pharmaceutical research: 2013;12(4):811.
- Smiljkovic M, Stanisavljevic D, Stojkovic D, Petrovic I, Vicentic JM, Popovic J, et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. EXCLI journal. 2017;16:795.
- Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer chemotherapy and pharmacology. 2011;68(6):1449-57.
CrossRef







