Mais Ahmad Alamro1 , Khaled A. Ahmed1, Khaled M Khleifat1,2
, Khaled A. Ahmed1, Khaled M Khleifat1,2 , Belal almajali1
, Belal almajali1 , Usamah Sayed1
, Usamah Sayed1 , Abdullah Saleh Al-wajeeh3
, Abdullah Saleh Al-wajeeh3 and Hamid Ali Nagi Al-Jamal4*
and Hamid Ali Nagi Al-Jamal4*
1Department of Medical Laboratory Sciences, Faculty of Allied Medical Sciences, Al-Ahliyya Amman University, Amman, Jordan.
2Department of Medical Laboratory Sciences, Faculty of Science, Mutah University, Al-Karak, Jordan.
3Anti-Doping Lab Qatar, Doha, Qatar.
4School of Biomedicine, Faculty of Health Sciences, Universiti Sultan Zainal Abidin (UniSZA), Kuala Nerus, Terengganu, Malaysia.
Corresponding Author E-mail: aljamalhamid@unisza.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2932
Abstract
Hashimoto’s thyroiditis (HT) is an autoimmune disorder characterized by elevated thyroid-stimulating hormone (TSH) levels. This research investigates the complex interaction between HT and cardiovascular risk in adult Jordanian non-pregnant women aged 20-50. Through a study involving 50 HT subjects and 40 healthy subjects, the levels of lipoprotein-associated phospholipase A2 (PLA2), high sensitivity C-reactive protein (hs-CRP), and anti-thyroid peroxidase (anti-TPO) antibodies were compared using ELISA methods and enzymatic colorimetric assays for lipid profiles. The results revealed significantly higher serum levels of hs-CRP, PLA2, and Anti-TPO in Hashimoto's patients, coupled with elevated cholesterol, triglyceride, and low-density lipoprotein (LDL) levels. Conversely, reduced levels of high-density lipoprotein (HDL) were observed in Hashimoto’s patients compared to healthy subjects. The study establishes a noteworthy correlation between thyroid autoimmunity, thyroid disease, PLA2, hs-CRP, and lipid profile, underscoring an increased cardiovascular risk in individuals with Hashimoto’s thyroiditis. The findings emphasize the prevalence of Anti-TPO antibodies in adult Jordanian non-pregnant women with Hashimoto’s thyroiditis.
Keywords
Anti-thyroid peroxidase; Cholesterol; C-reactive protein; Hashimoto’s thyroiditis; Thyroid-stimulating hormone
Download this article as:| Copy the following to cite this article: Alamro M. A, Ahmed K. A, Khleifat K. M, Almajali B, Sayed U, Al-wajeeh A. S, Al-Jamal H. A. N, Lipoprotein-Associated Phospholipase A2 and hs-CRP are Correlated with Anti-TPO Antibodies in Jordanian Non-Pregnant Women with Hashimoto’s Thyroiditis. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Alamro M. A, Ahmed K. A, Khleifat K. M, Almajali B, Sayed U, Al-wajeeh A. S, Al-Jamal H. A. N, Lipoprotein-Associated Phospholipase A2 and hs-CRP are Correlated with Anti-TPO Antibodies in Jordanian Non-Pregnant Women with Hashimoto’s Thyroiditis. Biomed Pharmacol J 2024;17(2). |
Introduction
The thyroid gland, a vital endocrine organ, plays a central role in regulating metabolic, growth, and developmental processes in humans 1. Serving as a major target of autoimmune disorders, the thyroid continuously releases hormones, including thyroxine, triiodothyronine, and calcitonin, crucial for physiological homeostasis 2. Notably, thyroid hormones exert a profound influence on the cardiovascular system, and imbalances can lead to a spectrum of disorders from goiter to life-threatening conditions 3,4. Thyroid dysfunction, encompassing hyper- and hypothyroidism, can precipitate heart failure, fibrillation, and hypertension by impacting cardiac output, contractility, vascular resistance, and rhythm 4. Among women of reproductive age, thyroid diseases are particularly prevalent, with hypothyroidism, hyperthyroidism, and thyroid nodules/cancer being common manifestations 1,5,6. Hypothyroidism, characterized by reduced thyroid gland activity, has far-reaching effects on various physiological systems, including neuromuscular, gastrointestinal, and cardiovascular functions, as well as lipid metabolism associated with heart disease 7,8. Autoimmune thyroid disease (AITD), the most frequent thyroid dysfunction, encompasses disorders such as Hashimoto’s thyroiditis and Graves’ disease, manifesting through the production of thyroid autoantibodies like Anti-TPO and thyroglobulin antibodies (Tg) 9,10. In AITD, cardiovascular risks associated with thyroid hormone imbalance may result in hemodynamic, hormonal, and metabolic changes, influencing factors such as hs-CRP and PLA2 11. C-reactive protein, an acute-phase protein linked to inflammation, and lipoprotein-associated phospholipase A2 (Lp-PLA2), associated with subclinical cardiovascular disease, emerge as significant markers in understanding the cardiovascular implications of thyroid disorders 12,13. This comprehensive overview underscores the intricate relationship between thyroid function, autoimmune thyroid diseases, and cardiovascular health.
Methodology
Ethical approval
The institutional review board (IRB) of Faculty of Allied Medical Sciences at Al-Ahliyya Amman University approved this study.(IRB: AAU/3/9/2021-2022). Data and samples were collected after written consent was obtained from each participant before the start of data collection.
Sample Collection and Handling
Three to 4 ml of human blood samples were meticulously collected by standard venipuncture procedure from Talyah Medical Labs. To ensure standardization, samples were gathered after 8-12 hours of overnight fasting, with fasting times duly verified before specimen collection. The blood specimens underwent careful management in the dedicated blood withdrawal room before being promptly transported to the clinical laboratories at Talyah Medical Labs. The inclusion criteria encompass individuals aged between 20 and 50 years old, non-pregnant females, non-alcoholics, and individuals diagnosed with hypothyroidism. Conversely, the exclusion criteria involve diabetic patients, individuals with kidney or liver-related issues, as well as lactating women and those in menopause. Additionally, individuals with polycystic ovary syndrome are also excluded from the study.
Processing and Storage
Upon collection in plain tubes, whole blood was allowed to clot at room temperature for 5 minutes. The subsequent removal of the clot was achieved through centrifugation at 3,000-3500 rpm for 5 minutes. All serum samples were judiciously stored at −20°C to maintain sample integrity, ensuring optimal conditions for subsequent analyses.
Biochemical analysis
Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were measured using enzymatic colorimetric assays. In contrast, low-density lipoprotein cholesterol (LDL-C) levels were calculated using Friedewald’s formula, ensuring a comprehensive lipid profile assessment.
Diagnostic Biomarkers
Serum thyroid-stimulating hormone
Thyroid-stimulating hormone ELISA Kit from Italy company (Diametra-Catalogue number: DKO013) with a reference rang from (0.27-4.2 mIU/L) was used. This kit is used to measure TSH levels in different types of samples including: human serum and plasma. This kit is based on Direct ELISA which measures antibody concentrations using antigen-coated bounded in the microELISA (Alhajj & farhana, 2021). The micro-ELISA plate provided in this kit has been pre-coated with conjugated antibody specific to TSH. Standards or samples were added to the appropriate Micro-ELISA plate wells were combined with specific antibody. Then, a Horseradish Peroxidase (HRP)-conjugated antibody was added to each micro-ELISA plate well and incubated for 90 minutes at room temperature. After incubation, the free components were washed away.
Tetramethylbenzidine (TMB) substrate solution was added to each well followed by incubation for 20 minutes at room temperature. Only those wells that contain TSH and HRP conjugated-TSH antibody appeared as blue color and then turned into yellow after the addition of the stop solution. The optical density (OD) was measured spectrophotometrically using ELISA reader at a wavelength of 450 nm. The OD value is proportional to the concentration of TSH. Concentration of TSH in the samples was calculated by comparing the OD of the samples to the standard curve. The sensitivity of the test is 0.01 mIU/L.
Serum thyroid hormone
Free thyroxin hormone ELISA Kit was obtained from Italy company (Diametra- catalogue number: DKO038) with a reference rang from (12-22 pmol/l) and used for analysis. This kit is used to measure FT4 levels in different types of samples including: human serum, plasma. This kit is based on Direct enzyme-linked immunosorbent assay ELISA type. The micro-ELISA plate provided in this kit has been pre-coated with an antibody specific to FT4. Standards or samples were added to the appropriate Micro-ELISA plate wells were combined. A HRP-conjugated antibody specific for FT4 was added to each micro-ELISA plate well and incubated for 1 Hour at room temperature. After incubation, the free components were washed away.
TMB substrate solution was added to each well and incubate for 15 minutes at room temperature. Only those wells that contain FT4 and HRP conjugated-FT4 antibody appeared as blue color and then turned into yellow after the addition of the stop solution. The OD was measured spectrophotometrically using ELISA reader at a wavelength of 450 nm. The OD value is proportional to the concentration of FT4. Concentration of FT4 in the samples was calculated by comparing the OD of the samples to the standard curve. The sensitivity of the test is 0.11 pmol/L
Serum Phospholipase A2, Lipoprotein Associated
Serum PLA2, from USA company (MyBioSource-catalogue number: MBS035611) was used. This kit is based on Sandwich ELISA type which measures antigen concentrations using antibody-coated bounded in the microELISA (Alhajj & farhana, 2021). MicroELISA plate was pre-coated with an antibody specific to PLA2. Standards or samples are added to the appropriate microELISA plate wells and combined to the specific antibody. Horseradish Peroxidase-conjugated antibody specific for PLA2 was added to microELISA plate well and incubated for 60 minutes at 37°C and then the free components were washed away. Chromogen solution A&B was added to each well, then, was incubated for 15 minutes at 37°C. Only those wells that contain PLA2 and HRP conjugated PLA2 antibody appeared as blue color and then turned into yellow after the addition of the stop solution. The OD, was measured spectrophotometrically at a wavelength of 450 nm, was proportional to the PLA2 levels in the serum samples. The levels of PLA2 ranged from 6.25- 200 ng/ml for serum. The sensitivity of the test was 1.0 ng/ml.
Serum anti-thyroid peroxidase
Serum anti-TPO levels were measured using the human anti-TPO ELISA Kit for Germany company (Aesku- catalogue number: 3401) with a reference rang from (40 IU/ml) using Sandwich enzyme immunoassay-ELISA method. The Microelisa strip plate provided in this kit has been pre-coated with an antibody specific to anti-TPO. Standards or samples are added to the appropriate Microelisa strip plate wells and combined to the specific antibody. Then, a Horseradish Peroxidase-conjugated antibody specific for anti-TPO was added to Microelisa strip plate well and incubated for 30 minutes at room temperature and then the free components were washed away. The Conjugate was added to each well and incubated for 30 minutes at room temperature then the free components were washed away.
The TMB substrate solution was added to each well incubate for 30 minutes at room temperature. Only those wells that contain anti-TPO and HRP conjugated anti-TPO antibody appeared blue in color and then turned yellow after the addition of the stop solution Incubate 5 minutes minimum. The OD, was measured spectrophotometrically at a wavelength of 450 nm, was proportional to the anti-TPO levels in the serum samples. The levels of anti-TPO ranged from 0-3000 IU/ml for serum. The sensitivity of the test was 10 IU/ml.
Serum high sensitivity C-Reactive Protein
Serum High Sensitivity C-Reactive protein ELISA Kit for USA company (Monobind – catalogue number:3125-300) with a reference rang from (< 3 μg/ml) was used. This kit is used to measure hs-CRP levels in different types of samples including: human serum, and plasma. This kit is based on Sandwich assay ELISA type. MicroELISA plate provided in this kit has been pre-coated with an antibody specific to hs-CRP. Standards or samples were added to the appropriate wells of microELISA plate and combined with the specific antibody. A Horseradish Peroxidase (HRP)-conjugated antibody specific for hs-CRP was added to each microELISA plate well and incubated for 15 minutes at room temperature. After incubation, the free components were washed away.
TMB and hydrogen peroxide (H2O2) solution substrate was added to each well and incubated for 15 minutes at room temperature. Only those wells that contain hs-CRP and HRP conjugated-hs-CRP antibodies were appeared as blue in color and then turned into yellow after the addition of the stop solution. The OD was measured spectrophotometrically using ELISA reader at a wavelength of 450 nm. The OD value is proportional to the concentration of hs-CRP. The concentration of hs-CRP in the samples was calculated by comparing the OD of the samples to the standard curve. The sensitivity of the test is 0.014 μg/ml.
Statistical analysis
SPSS V.22 was used for data analysis, including Chi-square tests for demographic and clinical parameters, and independent t-tests for quantitative data with significance at p < 0.05. The study also investigated correlations between thyroid markers and cardiovascular risk factors using Pearson’s correlations in univariate linear regression analysis.
Results
Frequency of abnormal values of clinical parameters in patients and healthy subjects.
The study included 90 female participants aged 20 to 50, divided into a patient group (n = 50) and a healthy subjects group (n = 40) matched for age and inclusion criteria (table 1). Hypothyroidism patients exhibited a significantly higher frequency of decreased FT4 levels (***p=0.0001) and increased anti-TPO levels (***p=0.0001) compared to healthy individuals (figure 1). Additionally, hypothyroidism patients showed a higher prevalence of abnormal LDL cholesterol (***p=0.001), HDL levels (**p=0.040), and elevated hs-CRP (**p=0.005), indicating an association with cardiovascular and hypothyroidism risk factors (figure 2).
Table 1: Frequency of high-risk levels of thyroid gland abnormalities in the two groups of study hypothyroidism patients and healthy individuals.
|
Parameter |
Healthy subjects |
Hypothyroidism patients |
P.value |
Chi-Square |
|
|
FT4 (pmol/L) |
Normal |
39 (97.5%) |
17 (34.0%) |
0.0001*** |
38.120 |
|
High risk, < 12 |
1 (2.5%) |
33 (66.0%) |
|||
|
Anti-TPO (IU/ml) |
Normal |
33 (82.5%) |
14 (28.0%) |
0.0001*** |
26.454 |
|
High risk, > 40 |
7 (17.5%) |
36 (72.0%) |
|||
|
hs-CRP (μg/ml) |
Low risk, ≤ 3 |
38 (95.0%) |
36 (72.0%) |
0.005** |
8.042 |
|
High risk, > 3 |
2 (5.0%) |
14 (28.0%) |
|||
|
LDL (mg/dl) |
Normal |
25 (62.5%) |
14 (28.0%) |
0.001*** |
10.771 |
|
High risk,>100 |
15 (37.5%) |
36 (72.0%) |
|||
|
HDL (mg/dl) |
Normal |
30 (75.0%) |
27 (54.0%) |
0.040** |
4.220 |
|
High risk, < 45 |
10 (25.0%) |
23 (46.0%) |
|||
*For Significant P-values
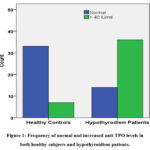 |
Figure 1: Frequency of normal and increased anti-TPO levels in both healthy subjects and hypothyroidism patients. |
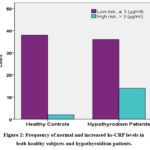 |
Figure 2: Frequency of normal and increased hs-CRP levels in both healthy subjects and hypothyroidism patients. |
Clinical and laboratory characteristics
Table 2 summarizes the clinical and laboratory characteristics of both hypothyroidism patients and healthy individuals. The mean age showed no significant difference between the two groups. However, hypothyroidism patients exhibited significantly higher levels of hs-CRP (1.27 ± 0.94 vs. 0.04 ± 0.02, p<0.0001), phospholipase-A2 (57.28 ± 21.96 vs. 47.15 ± 12.92, p=0.012), and anti-TPO (103.51 ± 91.80 vs. 30.32 ± 15.14, p<0.001) compared to the healthy subjects.
Furthermore, hypothyroidism patients displayed significantly elevated TSH levels (7.09 ± 2.81 vs. 2.06 ± 0.81, p<0.001) and lower FT4 levels (9.41 ± 4.68 vs. 16.19 ± 2.52, p<0.001) compared to the healthy group.
Regarding lipid profiles, hypothyroidism patients had higher mean levels of serum total cholesterol (207.84 ± 49.78 vs. 168.11 ± 47.27, p=0.0001) and LDL (137.51 ± 49.52 vs. 94.05 ± 41.62, p=0.0001) compared to the healthy group. However, there were no statistical differences in serum HDL and triglyceride concentrations between the two groups (p=0.26 and p=0.183, respectively).
Table 2: Comparison of laboratory data between Hypothyroidism patients and healthy subjects.
|
parameter |
Healthy subjects (No=36) |
Hypothyroidism patients (No=44) |
P-value |
|
Anti-TPO (IU/ml) |
30.32 ± 15.14 |
103.51 ± 91.80 |
<0.001٭** |
|
TSH (mIU/L) |
2.06 ± 0.81 |
7.09 ± 2.81 |
<0.001٭** |
|
FT4 (pmol/L) |
16.19 ± 2.52 |
9.41 ± 4.68 |
<0.001٭** |
|
PLA2 (ng/ml) |
47.15 ±12.92 |
57.28 ± 21.96 |
0.012٭ |
|
Hs-CRP (μg/ml) |
0.04 ± 0.02 |
1.27 ± 0.94 |
<0.001٭** |
|
LDL (mg/dl) |
94.05 ± 41.62 |
137.51 ± 49.52 |
<0.001**٭ |
|
HDL (mg/dl) |
52.77 ± 12.16 |
49.15 ± 16.67 |
0.26 |
|
TG (mg/dl) |
98.36 ± 41.70 |
113.90 ± 61.25 |
0.18 |
|
TC (mg/dl) |
168.11± 47.27 |
207.84 ± 49.78 |
<0.001٭ |
|
LDL-HDL |
1.90 ± 0.97 |
3.13 ± 1.62 |
< 0.001٭ |
TC; total cholesterol, TG; triglycerides, PLA2; phospholipase A2, TSH; thyroid stimulating hormone, HDL; high-density lipoprotein cholesterol, LDL; low-density lipoprotein cholesterol, hs-CRP; high sensitivity C-reactive protein, anti-TPO; anti-thyroid peroxidase, T4; thyroxine, *; significantly p-value.
Correlations of age with different parameters in hypothyroidism patients and healthy subjects.
Table 3 illustrates the correlation between age and various health parameters in both healthy subjects and hypothyroidism patients. Among healthy subjects, age showed no significant correlation with thyroid parameters (TSH, anti-TPO) and cardiovascular markers (PLA2, LDL, HDL). However, a significant positive correlation was observed between age and hs-CRP (r = -0.307, P = 0.030), while a significant negative correlation was found with FT4 (r = -0.335, P = 0.017).
In contrast, among hypothyroidism patients, there was no statistical correlation between age and TSH, PLA2, LDL, HDL, and anti-TPO.
Table 3: Correlations of age with different parameters in non-pregnant women with hypothyroidism and healthy subjects.
|
Parameters |
Healthy subjects |
Hypothyroidism patients |
||
|
Correlation Coefficient (r) |
P value |
Correlation Coefficient (r) |
P value |
|
|
Age vs FT4 |
0.109 |
0.504 |
-0.335 |
0.017 |
|
Age vs TSH |
0.184 |
0.256 |
0.145 |
0.314 |
|
Age vs Anti-TPO |
0.100 |
0.541 |
-0.017 |
0.906 |
|
Age vs Phospholipase A2 |
0.175 |
0.280 |
0.009 |
0.950 |
|
Age vs Hs-CRP |
-0.146 |
0.370 |
0.307 |
0.030 |
|
Age vs LDL |
0.017 |
0.919 |
-0.044 |
0.762 |
|
Age vs HDL |
-0.271 |
0.091 |
-0.060 |
0.678 |
Correlation between thyroid function biomarkers and cardiovascular risk factors in Hashimoto’s patients
Table 4 presents the correlation analysis results for various parameters. PLA2 demonstrates a moderate positive and significant correlation with TSH (r = 0.461, p=0.001), but no significant correlation is observed with FT4 and anti-TPO. Both LDL and HDL do not show significant correlations with thyroid gland function tests (FT4, TSH, anti-TPO).
Furthermore, hs-CRP exhibits a positive and significant correlation with anti-TPO (r= 0.352, p=0.012), while hs-CRP shows a negative and significant correlation with FT4. However, no significant correlation is observed between hs-CRP and TSH.
Table 4: Correlation analysis between thyroid function biomarkers and cardiovascular risk factors.
|
Cardiovascular parameter |
Thyroid markers |
|||||
|
FT4 |
TSH |
Anti-TPO |
||||
|
Correlation Coefficient (r) |
P-value |
Correlation Coefficient (r) |
P-value |
Correlation Coefficient (r) |
P-value |
|
|
PLA2 |
-0.032 |
0.823 |
0.461 |
0.001 |
0.147 |
0.308 |
|
hs-CRP |
-0.304 |
0.032 |
0.242 |
0.090 |
0.352 |
0.012 |
|
LDL |
-0.168 |
0.244 |
0.190 |
0.186 |
0.176 |
0.223 |
|
HDL |
0.084 |
0.564 |
0.214 |
0.136 |
-0.047 |
0.745 |
Discussion
Hashimoto’s thyroiditis, an autoimmune thyroid disease characterized by thyroid volume increase, parenchymal lymphocyte infiltration, and antibodies against thyroid antigens, has become the most prevalent disorder in thyroid gland dysfunction. Patients with Hashimoto’s thyroiditis (HT) are more prone to cardiovascular disease and malignant neoplasms. Our unique study, focusing specifically on non-pregnant women with hypothyroidism (mean age 32.1 years), aligns with demographic data from the HT community, emphasizing the distinctiveness of our study group. Notably, the prevalence of Hashimoto’s thyroiditis is higher in women than in males 14, and females are more likely to experience autoimmune thyroid disease (AITD) and hypothyroidism 15,16.
Diagnosis of Hashimoto’s thyroiditis involves clinical symptoms and laboratory results, including high TSH levels with normal to low thyroxine levels. Despite limited evidence that anti-thyroid peroxidase antibodies (anti-TPO) contribute to autoimmune thyroid disease onset, anti-TPO antibody treatments aid in complement function recovery. Our study underscores the importance of using anti-TPO as a first-tier test in conjunction with TSH and FT4 to prevent overlooking individuals with normal TSH but increased autoantibodies. Additionally, we explore the association between Hashimoto’s and cardiac markers such as PLA2, hs-CRP, and lipid profile, aiming to identify potential cost-effective strategies for long-term conditions like thyroid cancer and cardiovascular disease prevalent in women of childbearing age. Our findings reveal increased TSH levels and low thyroxine levels in Hashimoto’s disease, consistent with prior research 17, emphasizing the endothelium’s sensitivity to thyroid hormone action 18.
TSH levels serve as useful indicators of cardiovascular disease (CVD) in hypothyroid patients 19. Our study aligns with others, showing an increased cardiovascular risk in hypothyroidism with higher TSH levels 20. Notably, our research supports the significant increase in anti-TPO levels in Hashimoto’s patients, consistent with prior studies 9.
Demographic variations include higher TSH levels and antithyroid antibodies in females, influenced by age and higher in whites and Mexican Americans compared to blacks 21. Increased circulating thyroid autoantibodies are linked to an atherogenic lipid profile, with the potential link between autoimmune thyroiditis and dyslipidemia leading to heart disease 22,23.
Lipoprotein-associated phospholipase A2 (PLA2) is implicated in atherosclerosis progression by promoting cholesterol migration into arterial plaques. Clinical studies confirm increased PLA2 activity as a risk factor for cardiovascular disease 24. Our study identifies PLA2 as a potential predictor of cardiovascular disease risk. Elevated PLA2 levels, produced by inflammatory cells and linked to cardiovascular disease risk 25, theoretically induce atherogenesis by impairing endothelial function through inflammation, lipid abnormalities, oxidative stress, and blood pressure 26.
Increased hs-CRP levels, consistent with previous reports 27, highlight the importance of assessing inflammatory status in cardiovascular risk stratification. However, our study found no significant difference in hs-CRP levels between subclinical hypothyroidism patients and the healthy subjects group.
The study supports the link between hypothyroidism and dyslipidemia, with patients exhibiting hyperlipidemia and high cholesterol levels 28. However, conflicting evidence exists regarding the association between hypothyroidism and atherosclerosis risk 29. HDL reduction in Hashimoto’s disease, consistent with earlier findings, and its negative correlation with anti-TPO levels underscore the link between autoimmunity and lipid profile 12.
Positive correlations between TSH and cardiovascular risk markers, including PLA2-IIA and TG, support the concept that hypothyroidism increases the risk of atherosclerosis 30. The study confirms the link between hypothyroidism and inflammation, with significantly higher levels of PLA2-IIA and hs-CRP in the hypothyroidism group compared to the healthy subjects group. Previous research supports the correlation between Lp-PLA2, hs-CRP levels, and cardiovascular risk 24.
Strong evidence indicates that hypothyroidism raises cardiovascular disease and atherosclerosis risk by increasing LDL levels 31. Premenopausal women with hypothyroidism are more susceptible to cardiovascular disease than those with normal thyroid function 32. The study emphasizes the impact of autoimmune disease on the lipid profile, supporting the positive association between PLA2 and TSH, higher levels of LDL, cholesterol, and hs-CRP in Hashimoto’s disease patients, suggesting an increased risk of heart disease 33.
In conclusion, our findings in Hashimoto’s patients reveal significant correlations between thyroid disease, thyroid autoimmunity, PLA2, lipid profile, and hs-CRP, suggesting an elevated cardiovascular risk in adult non-pregnant women with Hashimoto’s thyroiditis. Anti-thyroid peroxidase antibody positivity is more common in this population compared to healthy subjects. The study establishes an association between Hashimoto’s disease and increased cardiovascular risk markers, specifically PLA2 and hs-CRP levels. This research contributes to the understanding of biochemical parameters in Hashimoto’s disease, especially in the context of Jordan, emphasizing the importance of comprehensive assessments for cardiovascular risk in this patient population.
Acknowledgment
The authors would like to thank all staff at the lab staff of Talyah Medical Laboratory, for their cooperation.
Funding source
This research was supported by Al-Ahliyya Amman University, Jordan with grant No.2023/17-5.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beynon ME, Pinneri K. An overview of the thyroid gland and thyroid-related deaths for the forensic pathologist. Acad Forensic Pathol. 2016;6(2):217-236. doi:10.23907/2016.036
CrossRef - Barbieri A, Prasad ML, Gilani SM. Thyroid tissue outside the thyroid gland: Differential diagnosis and associated diagnostic challenges. Ann Diagn Pathol. 2020;48:151584.
CrossRef - Yamakawa H, Kato TS, Noh JY, Yuasa S, Kawamura A, Fukuda K, Aizawa Y. Thyroid Hormone Plays an Important Role in Cardiac Function: From Bench to Bedside. Front Physiol. 2021;12:606931.
CrossRef - Awad SAS, Ashraf EM, Khaled AS, Salih BS, Yousef S, Abeer et al. The epidemiology of thyroid diseases in the Arab world: A systematic review. J Public Health Epidemiol. 2016;8(2):17-26. doi:10.5897/JPHE2015.0770
CrossRef - Brown EDL, Obeng-Gyasi B, Hall JE, Shekhar S. The Thyroid Hormone Axis and Female Reproduction. Int J Mol Sci. 2023;24(12):9815.
CrossRef - Burnett M. Injury Prevention Strategies for Pre-Professional and Professional Ballet Dancers [doctoral dissertation]. Am Univ. 2021.
CrossRef - Sharma A, Arya R, Mehta R, Sharma R, K Sharma A. Hypothyroidism and cardiovascular disease: factors, mechanism and future perspectives. Curr Med Chem. 2013;20(35):4411-4421. doi:10.2174/09298673113209990190
- Udovocic M, Pena RH, Patham B, Tabatabai L, Kansara A. Hypothyroidism and the Heart. Methodist DeBakey Cardiovasc J. 2017;13(2):55. doi:10.14797/mdcj-13-2-55
CrossRef - Siriwardhane T, Krishna K, Ranganathan V, Jayaraman V, Wang T, Bei K, et al. Significance of anti-TPO as an early predictive marker in thyroid disease. Autoimmune Dis. 2019;2019:1684074.
CrossRef - Liontiris MI, Mazokopakis EE. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients. Hell J Nucl Med. 2017;20(1):51-56.
CrossRef - Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-1562.
CrossRef - Nik MHS, Darabi M, Ziaee A, Hajmanoochehri F. Serum phospholipase A2-IIA, hs-CRP, and lipids in women with subclinical hypothyroidism. Int J Endocrinol Metab. 2014;12(3):e17652. doi:10.5812/ijem.17652.
CrossRef - Younus A, Humayun C, Ahmad R, Ogunmoroti O, Kandimalla Y, Aziz M, et al. Lipoprotein-associated phospholipase A2 and its relationship with markers of subclinical cardiovascular disease: A systematic review. J Clin Lipidol. 2017;11(2):328-337. doi:10.1016/j.jacl.2016.12.016
CrossRef - Gabrielson AT, Sartor RA, Hellstrom WJ. The impact of thyroid disease on sexual dysfunction in men and women. Sex Med Rev. 2019;7(1):57-70. doi:10.1016/j.sxmr.2018.06.003
CrossRef - Mammen JSR, Cappola AR. Autoimmune Thyroid Disease in Women. JAMA. 2021;325(23):2392-2393.
CrossRef - Hussein TA, Othman RA, Oudah MK. The prevalence of thyroid stimulating blocking antibodies TSBAbs in newly diagnosed patients with AITD. Prevalence. 2022;140(01).
- Mincer DL, Jialal I. Hashimoto Thyroiditis. 2023 Jul 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls.
- Jankauskas SS, Morelli MB, Gambardella J, Lombardi A, Santulli G. Thyroid hormones regulate both cardiovascular and renal mechanisms underlying hypertension. J Clin Hypertens (Greenwich). 2021;23(2):373-381.
CrossRef - Delitala AP, Fanciulli G, Maioli M, Delitala G. Subclinical hypothyroidism, lipid metabolism and cardiovascular disease. 2017;28040402.
CrossRef - Rodondi N, Den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh J et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365-1374. doi:10.1001/jama.2010.1361
CrossRef - Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
CrossRef - Diab N, Daya NR, Juraschek SP, Martin SS, McEvoy JW, Schultheiß UT, Köttgen A, Selvin E. Prevalence and Risk Factors of Thyroid Dysfunction in Older Adults in the Community. Sci Rep. 2019;9(1):13156.
CrossRef - Tipu SAA, Fantazy K. Exploring the relationships of strategic entrepreneurship and social capital to sustainable supply chain management and organizational performance. Int J Product Perform Manage. 2018.
CrossRef - Cengiz H, Demirci T, Varim C, Tamer A. The effect of Thyroid Autoimmunity on Dyslipidemia in patients with Euthyroid Hashimoto Thyroiditis. Pak J Med Sci. 2021;37(5):1365. doi:10.12669/pjms.37.5.3447591
CrossRef - Zhang L, Li Z, Li N. Serum IMA and LP-PLA2 Levels in Patients with Coronary Heart Disease and Their Correlation with the Degree of Myocardial Ischaemia and Their Diagnostic Value. Emerg Med Int. 2022;2022:35726302.
CrossRef - Clark K, Sharp S, Womack CJ, Kurti SP, Hargens TA. Increased sedentary time and decreased physical activity increases lipoprotein associated phospholipase A2 in obese individuals. Nutr Metab Cardiovasc Dis. 2022;1703-1710. doi:10.1016/j.numecd.2022.02.002
CrossRef - Kvetny J, Heldgaard PE, Bladbjerg EM, Gram J. Subclinical hypothyroidism is associated with a low‐grade inflammation, increased triglyceride levels and predicts cardiovascular disease in males below 50 years. Clin Endocrinol (Oxf). 2004;61(2):232-238. doi:10.1111/j.1365-2265.2004.02062.x
CrossRef - Dey A, Kanneganti V, Das D. A study of the cardiac risk factors emerging out of subclinical hypothyroidism. J Family Med Prim Care. 2019;8(7):2439. doi:10.4103/jfmpc.jfmpc_482_19
CrossRef - Liu H, Peng D. Update on dyslipidemia in hypothyroidism: the mechanism of dyslipidemia in hypothyroidism. Endocr Connect. 2022;11(2):e210002.
CrossRef - Chiche F, Jublanc C, Coudert M, Carreau V, Kahn JF, Bruckert E. Hypothyroidism is not associated with increased carotid atherosclerosis when cardiovascular risk factors are accounted for in hyperlipidemic patients. Atherosclerosis. 2009;203(1):269-276. doi:10.1016/j.atherosclerosis.2008.07.023
CrossRef - Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132(4):270-278. doi:10.7326/0003-4819-132-4-200002150-00004
CrossRef - Stamatouli A, Bedoya P, Yavuz S. Hypothyroidism: Cardiovascular endpoints of thyroid hormone replacement. Front Endocrinol (Lausanne). 2020;10:888. doi:10.3389/fendo.2019.00888
CrossRef - Joshi V. The Dyslipidemia and Inflammatory markers as the risk predictors for cardiovascular disease in newly diagnosed premenopausal hypothyroid women. J Med Biochem. 2022. doi:10.5937
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132(4):270-278. doi:10.7326/0003-4819-132-4-200002150-00004.
CrossRef








