Alaa Saadi Abbood* , Anwer Jaber Faisal
, Anwer Jaber Faisal and Mokhtar jawad Al-Imam
and Mokhtar jawad Al-Imam
Iraqi center for cancer and medical genetic research / Mustansiriyah university. Baghdad, Iraq.
Corresponding Author E-mail: Alaasabbood@uomustansiriyah.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/2948
Abstract
The herb Arte(also known as Artemisia V.) is widely used to treat a variety of illnesses, including gastrointestinal disorders, infections, and fungal infections. The antibacterial component that makes this ingredient so beneficial and essential for treating septicemia is the prebiotic. This study looked at how Artemisia vulgaris L. protected rats' livers from the toxic treatment cisplatin. In virtual medicine, cisplatin is frequently employed. Numerous forms of human cancer, such as advanced cancer, uterine cancer, esophageal cancer, advanced privacy, and ovarian cancer, have been successfully treated with it. infection from other illnesses, like disease. In this experiment, thirty 25–30 g white secretive mice aged 12 weeks were used. 72 hours were spent soaking blood tissue samples in 4% paraformaldehyde before they were paraffin embedded. Hematoxylin-eosin (H, E) staining was applied after the sections were cut into 3-m pieces and embedded in paraffin wax. Establishing with the extraction of Artemisia vulgaris L 400 mg/kg/day of extract is the greatest efficiency technique, as the separated groups revealed. This proved the extract's efficiency. This finding offers thorough and widely reported support for the use of purifications like A. vulgaris in the medical treatment of systemic poisoning with additional benefits like definite therapeutic effects and potent histochemical in the treatment of cancerous tissues.
Keywords
Artemisia vulgaris, Cisplatin; H and E stain; Hepatoprotective; Hepatotoxicity
Download this article as:| Copy the following to cite this article: Abbood A. S, Faisal A. J, Al-Imam M. J. Effect of Artemisia Vulgaris on Liver of Albino Mice Exposed to Cisplatin. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Abbood A. S, Faisal A. J, Al-Imam M. J. Effect of Artemisia Vulgaris on Liver of Albino Mice Exposed to Cisplatin. Biomed Pharmacol J 2024;17(2). |
Introduction
Artemisia vulgaris L. (Artemisia V.), sometimes known as mugwort, and is found all over the world, including Asia, Northern Africa, and Europe. This herb has a storied history in traditional medicine, and it has been dubbed the “mother of herbs” by centenarians 1. A common chemotherapy drug is called cisplatin. Several human malignant tumors have been proven to respond positively to it, including advanced stages of lung, cervical, esophageal, progressive testicular, and ovarian malignancies2. Cisplatin has been associated with an increase in liver enzymes. Additionally, cisplatin use was associated with increases in transaminase, LDH, and bilirubin. These alterations appeared on the initial day of cisplatin administration and vanished after two weeks3.
Cisplatin, which is widely recognized for accumulating in hepatocytes and causing liver injury, is considered to produce reactive oxygen species that stimulate and enhance the activity of intrinsic caspases, leading to the programmed cell death of liver cells.4. Due to the numerous negative effects on hepatocytes that have been linked to the use of cisplatin, the architecture of the liver may be disrupted, and glutathione (GSH) levels may fall. The hepatotoxic side effects of cisplatin are relatively poorly understood compared to its effects on other organs. This is because the treatment strategy, which involves either a more significant dose or repeated low doses, could lead to hepatotoxicity and significantly impact the clinical condition of patients.5. A highly anticancer chemotherapeutic drug, cisplatin is used to treat a variety of cancers6. But it has several harmful consequences on many organs, especially the liver. This chemotherapy drug is used to treat cancers of the testicles, ovaries, bladder, head, and neck. The use of cisplatin has been severely limited because of its serious adverse effects, which include hepatotoxicity, nephrotoxicity, autotoxicity, and ototoxicity. These side effects affect various organs, particularly the liver. Numerous studies and experimental models have connected cisplatin toxicity to reactive oxygen species (ROS), even though the mechanisms underlying cisplatin-induced hepatotoxicity have not yet been thoroughly investigated7,8. The significant increase in ALT, ALP, and AST values is indicative of hepatocyte cell membrane disruption and enzyme leakage caused by cisplatin 9. Cisplatin increases the liver’s sinusoidal diameter and modifies the architecture of the hepatic lobules . Several antioxidants have been used to stop the hepatotoxicity caused by cisplatin10.
Martial and Methods
Materials
All of the compounds were obtained from Sigma and were of analytical quality.
Methods
Preparation of Extract
Artemisia V.’s aerial portions were acquired of an herbalist in Baghdad and verified by a botanist. To prepare the aerial parts for extraction, they have been cleaned, then gently pulverized with an automated blender and weighed. Next, it was dried and concentrated using an Isola rotary evaporator running at 109 rpm and 30 ˚C. It was extracted using 80% ethanol using a Soxhlet (WiseTherm). The filter paper was used for filtering. The extract is stored at room temperature in a dark place after being weighed. The extract may dissolve in both normal saline and distilled water.
Experimentation Design
In this experiment, thirty 12-week-old male Swiss albino mice with a weight range of 25 to 30 grams were used. The mice were kept in a room with a regulated humidity level (54–78%) and a temperature of about 25 °C. The mice were given unrestricted access to water and a normal diet of pellets.
The animals were split up into the following five groups of six
Normal saline (0.01 mL/kg) was given to the first group (control group). The second group was given an intraperitoneal injection of 50 mg/100 ml of Cisplatin (supplied by the Central Pharmacy in Baghdad) at a dose of 10 mg/kg to induce liver injury on day seven. For ten days, the third group was given an oral stomach tube containing 400 mg/kg of Artemisia extract once a day. The fourth group was injected with 10 mg/kg of cisplatin on day seven after receiving the extract using the same protocol for 10 days at a dosage of 200 mg/kg. The fifth group was administered 10 mg/kg of cisplatin on day seven after receiving 400 mg/kg for ten days. On the tenth day, every animal was slaughtered, and a blood.
Biochemical Assay
The activity of aspartate aminotransferase (AST), alanine aminotransferase(ALT) , alkaline phosphatase (ALP) and Serum total serum protein concentration, has been identified by commercial groups according to11,12.
Histopathological study
After being preserved in formalin, tissue samples from mice’s livers were prepared for histological analysis using H&E stain according to 13,14.
Statistical Analysis
Every experiment was run through at least four times. The data were represented as mean ± standard deviation, and a one-way analysis of variance (ANOVA) and post-hoc Tukey HSD test was performed using IBM SPSS v20. P-values below 0.05 are regarded as significant.
Results
The extract collected from the above-ground sections of Iraqi Artemisia V contains alkaloids, polyphenolic compounds, flavonoids, tannins, polysaccharides, and saponins results of first chemical screening tests.
Toxicity Testing
There were no indications of illness or death observed following the administration of Artemisia V. extract at oral doses of up to 400 mg/kg. Consequently, the LD50 value exceeds 400 mg/kg, suggesting that the studied plant extract is considered safe for use at this dosage.
Effect on serum biochemical constituents
Cisplatin greatly improved ALT, ALP, and TSB activity as compared to untreated animals. Cisplatin had no discernible effect on AST serum levels as compared to untreated control rats. There were no obvious variations in liver enzyme levels between animals that were not treated and those who were given Artemesia 400 mg/Kg. A small dose of Artemesia extract (200 mg/kg) significantly (p0.05) lowered serum levels of ALT, ALP, and TSB. Table 1 shows that mice administered with 400 mg/kg Artemesia extract had more significant (p0.001) effects than the Cisplatin Group.
Table 1: The impact of Artemesia Vulgaris on the levels of serum enzymes in mice.
|
Enzymes |
Non-treated Group |
Artemesia 400 mg/kg Group |
Cisplatin Group 10 mg/kg |
Artemesia 200 mg/kg –Cisplatin10mg/kg |
Artemesia 400 mg/kg –Cisplatin10mg/kg |
|
ALT |
**33.67 ± 7.45 |
32.83 ± 5.74 |
58.67 ± 7.66 |
*43.67 ± 12.88 |
**35.16 ± 6.17 |
|
AST |
300.16 ± 67.27 |
337.50 ± 44.58 |
382.50 ± 60.38 |
326.16 ± 60.38 |
314.33 ± 66.26 |
|
ALP |
**66.5 ± 5.36 |
66.83 ± 4.40 |
83.50 ± 6.15 |
*79.00 ± 4.82 |
**71.67 ± 6.45 |
|
TSB |
**0.24 ± 0.05 |
0.21 ± 0.07 |
0.72 ± 0.32 |
*0.35 ± 0.11 |
**0.25 ± 0.10 |
Histopathological study
The current tissue is made up of lobules, including the lobule of the central vein (CV). The central vein occupies and branches from the lobule’s core. Cords of the liver. The hepatic cord is made up of two rows of hepatocytes (HE). Liver cells are distributed radially around, for example. It only restricts capillary blood channels known as sinusoids, as illustrated in Figure 1 A. Significant liver injury was caused by CP. A liver section demonstrating significant congestion on the seventh day, when administered at 10 mg/kg, causes liver damage. B Occurrence of cellular degeneration (D) and cellular necrosis (N), as well as infiltration of mononuclear inflammatory cells (IN) in the liver. C Hemorrhage (H) occurs in the liver when dosed in mice (H&E stain). The four groups got the extract by the same method at a concentration of 200 mg/kg for 10 days before being injected with cisplatin 10mg/kg on day seven. The fifth group was given 400 mg/kg for 10 days before being given 10mg/kg of cisplatin on seven days.
Every animal was killed on the tenth day, and a cardiac incision was used to draw blood. After allowing the blood samples to clot, the liver tissue was prepared by centrifuging them for 20 minutes at 3,000 rpm. Showed a substantial amount of inflammation and tissue necrosis. indicated by arrows, a small amount of tissue necrosis, and mild inflammation. Hepatocytes express themselves and produce both macrovascular and microvascular vacuolation as the liver starts to regenerate.
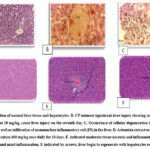 |
Figure 1: A section of normal liver tissue and hepatocytes. B. CP induced significant liver injury showing severe congestion when injected at 10 mg/kg, cause liver injury on the seventh day. C. Occurrence of cellular degeneration |
Discussion
Cisplatin is a common chemotherapeutic medication used to treat various cancer types; however, it is hazardous to several organs, especially the liver 15,16.
Due to the human liver’s quick absorption of cisplatin, excessive dosages of the medication may be hazardous to the liver 17.
The extract of aerial parts of Iraqi Artemisia vulgaris contained alkaloids, polyphenolic compounds, flavonoids, tannins, polysaccharides, and saponins, according to preliminary chemical screening tests. This is in line with studies that have found the compounds listed before 18.
The study found that cisplatin administration resulted in elevated levels of ALT, ALP, and TSB activity in mice, indicating liver injury. This conclusion is consistent with previous research that indicated an increase in these enzymes following cisplatin treatment 19.
In mice, treatment with Artemesia extract at doses up to 400 mg/kg results in no toxicity symptoms in the control non-treated group20.
In the current study, we were able to show that pre-treatment with Artemesia extract decreased blood levels of TSB, ALP, and ALT in a dose-dependent manner, with a dose of 400 mg/kg decreasing enzyme levels more than a dose of 200 mg/kg21. Additional research demonstrating the finding is supported by the hepatoprotective efficacy of Artemisia absinthium L against chemically induced liver injury. This result is also in line with another study that discovered Artemisia absinthium L to be protective against diclofenac-induced liver damage22.
However, no further research provided evidence to substantiate the Artemesia vulgaris effect against the chemotherapeutic medication cisplatin. This study represents the first examination to demonstrate that an extract from Iraqi Artemisia V. protects mice’s livers against cisplatin-induced liver damage23. These changes had a strong association with histological findings, such as an increase in sinusoidal dilatation, vacuolation, and hepatocellular degeneration/necrosis24. These findings are in line with previous research. Other investigations that looked at histological results validated this finding, as evidenced by a decrease in the incidence and severity of cisplatin-induced liver histopathology lesions25.
Histopathology examines the enlargement of organelles such as the endoplasmic reticulum and mitochondria, the rupture of the plasma membrane, and cell lysis characterize necrotic cells26. These modifications cause cells to become more eosinophilic, glassy, and vacuolated27. The first metabolic change observed in injury is ATP depletion or decreased generation, which is exacerbated by the disruption of organelle membranes and loss of cell membrane integrity. In the presence of oxygen, mitochondrial oxidative phosphorylation produces ATP28. Necrosis is characterized by a loss of oxygen supply to cells induced by hypoxia or chemical damage, resulting in decreased ATP generation. The damaging events of hepatocytes caused by CP injection and marked by necrotic centrilobular regions were confirmed29.
Conclusions
Iraqi Artemisia V. ethanolic extract exhibits a notable potential for hepatoprotection against mice’s liver injury induced by cisplatin; initial experiments reveal variant phytochemicals that may be responsible for this protective effect. Based on histological sections, the current study demonstrated that the drug used in chemotherapy for cancer patients is highly toxic to albino mice.
Acknowledgments
The authors would like to thank Mustansiriyah university (www.uomustansiriyah.edu.iq) Baghdad -Iraq for support in the present work .
Conflict of Interest
There are no conflicts of interest for all authors .
Funding source
This study did not receive any grant from any funding agency.
References
- Gaskell H, Ge X, Nieto N. High-mobility group box-1 and liver disease. Hepatol Common. 2018;2(9):1005–1020.
CrossRef - Lee WM. Acute liver failure. S.E.Min. Respir. Crit. Care Med. 2012;33(01):36–45.
CrossRef - Nitzsche B, Gloesenkamp C, Schrader M, et al. Anti-tumour activity of two novel compounds in cisplatin-resistant testicular germ cell cancer. Br J Cancer. 2012;107:1853–1863.
CrossRef - Meng F, Sun G, Zhong M, et al. Anticancer efficacy of cisplatin and trichostatin A or 5-aza-20 -deoxycytidine on ovarian cancer. Br. J. Cancer. 2013;08(3):579–586.
CrossRef - Gonza´lez-Sa´nchez I, Lira-Rocha A, Navarrete A, et al. Synergistic anticancer activity of Thiazolo[5,4-b]quinoline derivative D3CLP in combination with cisplatin in human cervical cancer cells. Anticancer Res. 2012;32:5159–5165.
- Pinto-Leite R, Arantes-Rodrigues R, Ferreira R, et al. Temsirolimus improves the cytotoxic efficacy of cisplatin and gemcitabine against urinary bladder cancer cell lines. Urol Oncol. 2014;32:41.e11–22.
CrossRef - Parlakpinar H, Sahna E, Ozer MK, et al. Physiological and pharmacological concentrations of melatonin protect against cisplatin-induced acute renal injury. J Pineal Res. 2002;33:161–166.
CrossRef - Ezz A. M. M, ALheeti O. N, Hasan A. F, Zaki S, Tabl G. A. Anti-Diabetic Effects of Pomegranate Peel Extract and L- Carnitine on Streptozotocin Induced Diabetes in Rats. Biomed Pharmacol J 2023;16(3).
CrossRef - Gulec M, Oral E, Dursun OB, et al. Mirtazapine protects against cisplatin-induced oxidative stress and DNA damage in the rat brain. Psychiatry Clin. Neurosci. 2013;67(1):50–58.
CrossRef - Lu Y, Cederbaum AI. Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol. Sci. 2006;89(2):515–523.
CrossRef - Alankooshi, A. A., Alankooshi , A. A., Hasan, A. F., Tousson, E., El-Atrsh, A. & Mohamed, T. M. (2023). Impact of Coriander Seeds Extract Against Thyroidectomy Induced Testicular Damage and DNA Replication in Male Rats. OnLine Journal of Biological Sciences, 23(2), 193-201.
CrossRef - Hasan, A. F., Alankooshi, A. A., Abbood, A. S., Dulimi, A. G., Mohammed Al-Khuzaay, H., Elsaedy, E. A. & Tousson, E. (2023). Impact of B-Glucan Against Ehrlich Ascites Carcinoma Induced Renal Toxicity in Mice. OnLine Journal of Biological Sciences, 23(1), 103-108.
CrossRef - Hameed, H. M., Hasan, A. F., Razooki, Z. H., Tousson, E. & Fatoh, S. A. (2023). Orlistat Induce Renal Toxicity, DNA Damage, and Apoptosis in Normal and Obese Female Rats. OnLine Journal of Biological Sciences, 23(1), 25-32.
CrossRef - Hasan A. F, Hameed H. M, Tousson E, Massoud A, Atta F, Youssef H, Hussein Y. Role of Oral Supplementation of Damiana (Turnera diffusa) Reduces the Renal Toxicity, Apoptosis and DNA Damage Associated with Amitriptyline Administration in Rats. Biomed Pharmacol J 2022;15(3).
CrossRef - Naqshbandi A, Khan W, Rizwan S, Khan F. Studies on the protective effect of flaxseed oil on cisplatin-induced hepatotoxicity. Human Exp Toxicol. 2012;31(4):364–375.
CrossRef - Hasan, A. F., Mutar, T. F., Tousson, E. M. & Felemban, S. G. (2021). Therapeutic Effects of Turnera diffusa Extract Against Amitriptyline-Induced Toxic Hepatic Inflammation. OnLine Journal of Biological Sciences, 21(2), 395-408.
CrossRef - Dkhil MA, Al-Quraishy S, Aref AM, et al. The potential role of Azadirachta indica treatment on cisplatin-induced hepatotoxicity and oxidative stress in female rats. Oxid Med Cell Longev. 2013:741817.
CrossRef - Zsengelle´r ZK, Ellezian L, Brown D, et al. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J Histochem Cytochem. 2012;60(7):521–529.
CrossRef - Halliwell B. Free radicals and antioxidants: updating a personal. view. 2012;70:257–265.
CrossRef - Ingawale DK, Mandlik SK, Naik SR. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): a critical discussion. Environ Toxicol Pharmacol. 2014;37,118–133.
CrossRef - Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290(5493):989–992.
CrossRef - Warren C, Wong-Brown M, Bowden N. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019.
CrossRef - Inkaya AC, Demir NA, Kolgelier S, et al. Is serum high-mobility group box 1 (HMGB-1) level correlated with liver fibrosis in chronic hepatitis B? Medicine (Baltimore). 2017;96(36):e7547.
CrossRef - VanPatten S, Al-Abed Y. High Mobility Group Box-1 (HMGb1): current wisdom and advancement as a potential drug target. J. Med. Chem. 2018;61(12):5093–5107.
CrossRef - Ge X, Antoine DJ, Lu Y, et al. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J. Biol. Chem. 2014;289(33):22672–22691.
CrossRef - Vasei N, Shishegar A, Ghalkhani F, Darvishi M. Fat necrosis in the Breast: A systematic review of clinical. Lipids Health Dis. 2019 Jun 11;18(1):139.
CrossRef - Zhang XM, Zhu J. Kainic Acid-induced neurotoxicity: targeting glial responses and glia-derived cytokines. Curr Neuropharmacol. 2011 Jun;9(2):388-98.
CrossRef - Abad MJ, Bedoya LM, Apaza L, Bermejo P. The Artemisia L. Genus, a review of bioactive essential oil. Molecules. 2012;17:2542–2566.
CrossRef - Adekenov SM. Chemical modifications of arglabin and biological activity of its new derivatives. Fitoterapia. 2016;110:196–205.
CrossRef








