Omonturdiev Sirojiddin Zoirovich1* , Abdullaev Izzatullo Ziyoyiddin ugli1
, Abdullaev Izzatullo Ziyoyiddin ugli1 , Inomjonov Dolimjon Raxmatillayevich2
, Inomjonov Dolimjon Raxmatillayevich2 , Maxmudov Lazizbek Umarjonovich1
, Maxmudov Lazizbek Umarjonovich1 Zaripova Mehrangiz Ravshanovna1, Gayibova Sabina Narimanovna1.2
Zaripova Mehrangiz Ravshanovna1, Gayibova Sabina Narimanovna1.2 , Gayibov Ulugbek Gapparjanovich1,2
, Gayibov Ulugbek Gapparjanovich1,2 and Aripov Takhir Fatikhovich1,2
and Aripov Takhir Fatikhovich1,2
1A. S. Sadykov Institute of Bioorganic Chemistry of the Academy of Sciences of Republic Uzbekistan
2Namangan state University Uzbekistan
Corresponding Author E-mail: siroj.2012@mail.ru
DOI : https://dx.doi.org/10.13005/bpj/2935
Abstract
According to the literature, Ájuga turkestánica extract has an effective impact on diseases of the cardiovascular system. Aortic smooth muscle ion transport systems play an important role in the development of these diseases1. This study investigated the effects of Ájuga turkestánica extract on ion transport systems in rat aortic smooth muscle preparations under isometric conditions in vitro, and it was found that this extract exerts a strong dose-dependent relaxant effect on the contraction induced by KCl (50 mM) From the obtained results, the effect of this extract on potential-dependent Ca2+ channels was estimated, and in order to confirm the assumption, it was compared with verapamil, a specific blocker of this channel, and the effect of Ájuga turkestánica extract on potential-dependent Ca2+ channels was determined. In the course of the studies, it was assumed that the investigated extract affects not only the potential-dependent Ca2+ channels, but also the receptor-dependent Ca2+ channels, the Ca2+ transport system in the sarcoplasmic reticulum, and the endothelium-dependent relaxation mechanisms. According to the results of the study, it was found that Ájuga turkestánica extract has an effective effect on these ion transport systems (the results obtained are described in detail in the article below).
Keywords
Ájuga turkestánica, Ca+2 -ATPase; IP3R, Phenylephrine; Phentolamine; receptor-dependent (TRP) channels; Voltage-gated Ca+ (VGCC) channels
Download this article as:| Copy the following to cite this article: Zoirovich O. S, Ugli A. I. Z, Raxmatillayevich I. D, Umarjonovich M. L, Ravshanovna Z. M, Narimanovna G. S, Gapparjanovich G. U, Fatikhovich A. T. The Effect of Ájuga Turkestánica on the Rat Aortic Smooth Muscle Ion Channels Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Zoirovich O. S, Ugli A. I. Z, Raxmatillayevich I. D, Umarjonovich M. L, Ravshanovna Z. M, Narimanovna G. S, Gapparjanovich G. U, Fatikhovich A. T. The Effect of Ájuga Turkestánica on the Rat Aortic Smooth Muscle Ion Channels Biomed Pharmacol J 2024;17(2). |
Introduction
Control of membrane potential and smooth muscle excitability and intracellular ion concentration is a process dependent on the activity of ion channels, in which voltage-gated Ca+ (VGCC) channels, receptor-dependent (TRP) channels, and Ca2+-activated K+ (BK) channels are the main regulators2. The ability of vascular smooth muscles to contract regulates the work of arteries. Several ion channels, voltage-gated Ca2+ channels, and intracellular Ca2+ channels regulate smooth muscle (SM) contraction by controlling membrane potential3. L-type Ca2+ channels play an important role in the activity of SMCs. The influx of Ca2+ ions and the role of R-type channels are also important for SM contraction4. It has also been shown that these ion channels regulate SM excitability5.
Material and methods
Chemicals
Ajuga turkestanica, a widely utilized medicinal plant in Uzbekistan, has been employed in our research experiments. The extract of Ajuga turkestanica used in these experiments was supplied by “Bioton” LTD, located in Tashkent, Uzbekistan. The chemical agents phenylephrine, phentolamine, and verapamil were procured from Sigma-Aldrich Chemie, a division of Sigma-Aldrich, based in St. Louis, MO, USA.
Tissue Preparation
Our institution’s animal use committee authorized all experimental procedures andpreoperative care guidelines . The animals were kept in standard vivarium conditions (humidity: 55%–65%, temperature: 22°C ± 2°C) with free access to drinking water and laboratory food. All manipulations with the animals complied with the European Directive 2010/63/EU on protecting animals used for scientific purposes. The protocol was approved by the Animal Ethical Committee based on the Institute of Bioorganic Chemistry, AS RUz (Protocol Number: 133/1a/h, dated August 4, 2014). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering6. Experiments were carried out on aortic preparations of white male rats (weight 200-250 g). The experimental animals were euthanized by cervical dislocation, and the chest was opened, the aorta was surgically isolated, and it was placed in a special organ bath (5 ml) perfused Krebs-Henseleit’s physiological solution (mM): NaCl 120,4; КСl 5; NaHCO3 15,5; NaH2PO4 1,2; МgCl2 1,2; СaСl2 2,5; С6Н12О6 11,5, HEPES рН 7.4. Krebs solutions without Ca2+ were also used for some experiments. For this, EGTA (1 mM) was added to the Krebs solution. Physiological solutions were oxygenated with carbogen (95% O2, 5% CO2) and maintained at +37°С using a DAIHAN WATER BATH ultrathermostat. After removing the connective tissue and fat surrounding the aorta, the aorta was segmented into 3-4 mm rings.
Aortic-ring contraction studies
Aortic rings were mounted to a Radnoti (Isometric-Transducer, USA) sensor using platinum wire hooks. In this condition, the aortic rings were held for 60 min until equilibration. Each preparation was subjected to an initial tension corresponding to 1 g (10 mN). The contraction force is transmitted from the trancducer to a signal amplifier and recorded on a computer using a Go-link automated digital converter. The obtained results were statistically processed using special software packages “OriginLab OriginPro v.8.5 SR1 (EULA, Northampton, MA 01060–4401, USA)”. The isometric contraction force (mN) of the rat aortic blood vessel preparation under in vitro conditions was calculated as a percentage (%) in statistical recalculation7.
 |
Figure 1: General manufacturing view of the apparatus for regulating isometric contraction of a rat aortic vascular muscle preparation [Vandier et al., 2002]. |
Results and Discussion
It is known that the contraction of the aortic preparation induced by KCl (50 mM) is caused by the activation of potential-dependent L-type Ca2+-channels located in smooth muscle cells8. In this case, due to the increase in the concentration of K+ ions in the membrane, the value of the membrane potential changes and the potential-dependent L-type Ca2+-channels are activated9. In the conducted experiments, it was observed that Ájuga turkestánica extract significantly relaxes the contraction of aortic preparations pre-contracted with KCl (50 mM). In this case, it was found that Ájuga turkestánica extract attenuated aortic contraction induced by KCl (50 mM) by 16.8±3.4% and 75.2±3.1% compared to the control at dose-dependent concentrations (5-100 μg/ml) (Fig. 2).
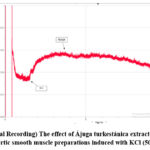 |
Figure 2: (Original Recording) The effect of Ájuga turkestánica extract on contraction of rat aortic smooth muscle preparations induced with KCl (50mM). |
It can be seen that this extract has a significant effect on the activity of potential-dependent Ca2+-channels induced by KCl (50 mM).
In order to verify this prediction, experiments were conducted using calcium-free Krebs solution and potential-dependent Ca2+-channel blocker – verapamil. It is known that increasing the concentration of KCl in Krebs solution with Ca2+ does not cause contraction in aortic preparations, but adding Ca2+ ions to Krebs solution (0 – 2.5 mM) under these conditions causes contraction in aortic preparations10. In our experiments, it was found that Ájuga turkestánica extract (100 μg/ml) in Krebs solution and adding Ca2+ ions in the presence of 50 mM KCl significantly reduced the contraction of aortic preparations compared to the control (Figure 3).
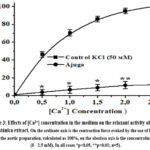 |
Figure 3: Effects of [Ca2+] concentration in the medium on the relaxant activity of Ájuga turkestánica extract. |
Based on the experimental results, the relaxant effect of Ájuga turkestánica (ajuga) extract on aortic contraction induced by KCl is due to the reduction of the entry of Ca2+ ions through potential-dependent Ca2+ channels located in the cell membrane. In our next experiments, in order to prove that the relaxant effect of the examined extract depends on L-type Ca2+ channels, the interaction with the specific blocker of these channels, verapamil, was compared11. For this purpose, the concentration of verapamil 0.1 μM) that causes a half-maximal contraction of aortic preparations evoked with KCl ((50 μM) was used. It was noted that this extract further attenuated aortic contractile activity by 28.1±2.8% when contraction was evoked in aortic preparations evoked with KCl (50 mM) under these conditions, with an IC50 of 41,2 μg/ml ajuga extract(EC50) (Fig. 4).
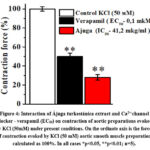 |
Figure 4: Interaction of Ájuga turkestánica extract and Ca2+-channel |
Based on the obtained experimental results, the investigated extract has a strong relaxant effect and significantly reduced the contraction induced by KCl (50 mM). In this case, as a result of the blocking of potential-dependent L-type Ca2+ channels located in the plasmalemma and the entry of Ca2+ ions into them, it leads to muscle relaxation. The results of the experiment showed that the relaxant effect of this extract is related to the blocking of L-type Ca2+ channels, and these results can be evidenced by the results of the experiment conducted with the specific blocker of Ca2+ channels, verapamil12.
It is known that in the contractile activity of vascular smooth muscle cells, in addition to potential-dependent activated Ca2+L-channels, Ca2+ transport systems located in the sarcoplasmic reticulum (SR) are also important13. Therefore, in the next experiments, the effect of ayuga extract on the contraction force induced by the α-adrenoceptor agonist – phenylephrine (1 μM) under the relaxant effect of the aortic vascular smooth muscle blocker was studied. It is known that the force of contraction caused by phenylephrine (1 μM) is related to the increase in the amount of [Ca2+]in at the expense of the Ca2+ ions coming from the SR, and also from the Ca2+-channels controlled by the receptor14. In experiments with phenylephrine (1 μM), it was found that ayuga extract at the maximum concentration (80 μg/ml) reduced the contraction force induced by phenylephrine (1 μM) by 66.6±3.3% compared to the control. (Fig. 5).
 |
Figure 5: (Original Recording) The effect of 80 μg/ml Ájuga turkestánica extract on phenylephrine-induced rat aortic contraction. |
Based on the obtained results, it can be assumed that the relaxant effect of the studied extract occurs with the blockade of receptor-controlled Ca2+-channels. In order to further clarify this prediction, the effects of α-adrenoceptor blocker – phentolamine (PE) and flavonoids were compared.
From the conducted experiments, it was known that in the absence of phentolamine, 1 μM phenylephrine induced reduction of aortic contraction force at the concentration of Ájuga turkestánica (80 μg/ml) as shown in the above experiments. When the effect of phentolamine (10 μM) on the contraction induced by 1 μM phenylephrine was studied, it was found to decrease the force of contraction by 81.7±3.1% compared to the control. When the effect of Ájuga turkestánica extract was studied in this condition, the contracture in the presence of phentolamine was 37.5±4.2%. (Fig. 6).
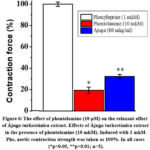 |
Figure 6:The effect of phentolamine (10 μM) on the relaxant effect of Ájuga turkestánica extract. Effects of Ájuga turkestánica extract in the presence of phentolamine (10 mkM). |
From the conducted experiments, it was known that the relaxant activity of Ájuga turkestánica extract is related to the blockade of receptor-controlled Ca2+-channels, the results of the experiments conducted with the blocker of α-adrenoceptors – phentolamine (Phe) can be an example.
The Study the role of endothelium in the relaxant effect of Ájuga turkestánica extract
It is known from the literature that the endothelial layer plays an important role in the functional activity of blood vessels, that is, in maintaining the tone of the vessels. Endothelial cells are located in the inner layer of the blood vessel wall and synthesize locally acting mediators that control blood flow in organs. One of the important active substances of isolated endotheliocytes is nitric oxide (NO), an endothelial vasodilator15. Endothelial function and structural pathology play an important role in the clinical examination and pathogenesis of arterial hypertension, atherosclerosis. In many cardiovascular diseases, endotheliocytes show their initial damage, which causes a cascade of pathological morpho-functional changes and eventually leads to extensive dysfunctions16. These data explain a wide range of functions of endothelial cells: regulation of vascular tone, hemostasis, immune system, migration of blood cells along the vascular wall, synthesis of inflammatory factors and their inhibitors, and carry out barrier functions17. Today, endothelial dysfunction (ED) is a disturbance in the balance between mediators that ensure the correct direction of all endothelium-related processes. Changes in the structure and development of blood vessels associated with vascular diseases, disorders of the ability of blood vessels to respond to external influences, and disorders in the production of endothelial vasoactive factors are observed at the same time18. The presence of many factors affecting NO metabolism causes endothelial dysfunction. Such modifiable risk factors for ED as predictors of cardiovascular disease include: hypokinesia, smoking, high salt intake, poisonings from various causes, estrogen deficiency, disturbances in carbohydrate, lipid, protein metabolism, infection, etc19.
L-arginine amino acid is a substrate for the synthesis of NO in endotheliocytes under the action of NO synthase (eNOS). NO is synthesized as a result of activation of eNOS by calcium-calmodulin and oxidation of L-arginine in a small amount (picomole)20. Normally, eNOS is bound to caveolin and is associated with caveolae. In this case, the activity of eNOS decreases dramatically. Receptor-dependent stimuli (acetylcholine, bradykinin, serotonin, thrombin, ADF, glutamate, substance R) separate eNOS from the caveolin-eNOS complex and release it from the plasma membrane. As a result of inhibition of excess of one of the main blockers of eNOS activation by forming a heterocomplex with caveolin-1, stimulation of NO formation occurs when statins are used in very low concentrations (0,1 mmol). This is one of the mechanisms of the pleiotropic effects of statins, which serve as a basis for correcting ED21.
In the development of atherosclerosis and endothelial dysfunction, oxidative stress (OS), in particular, oxygen free radicals, are the main factors in the process of decomposition of local NO, one of the most effective vasodilators. A decrease in the duration of direct action of nitric oxide at the site of formation leads to the development of ED22. Since NO and oxygen radicals chemically neutralize each other, an increase in local oxygen concentration leads to a decrease in biologically active NO23. NO plays a key role here. It is synthesized by NO synthase in endothelial cells of blood vessels. Thus, the activation of the enzyme guanylate cyclase (GTs) through the activity of NO-synthase (eNOS), which is located in the endothelial layer of smooth muscle cells, increases the concentration of cyclic guanylate monophosphate [cGMP], and the activation of protein kinase G (PKG) causes relaxation through the phosphorylation of myosin light chain24. Endothelial cells play an important role in controlling Ca2+ homeostasis and the contractile activity of SMCs, and they also modulate the functional state of SMCs and maintain vascular tone by producing a number of vasoactive factors25. The main role is played by nitric oxide (NO), which is synthesized by NO synthase (NOS) in endothelial cells and is the main mediator in vascular smooth muscle relaxation. Diffusion of NO in SMCs activates the NO/cGC/cGMP/PKG signaling pathway, causing a decrease in [Ca2+]i in SMCs and their relaxation, along with activation of IP3R and Ca2+-ATP cells in SR26. Therefore, in order to investigate the involvement of the endothelium in providing the relaxant effect of Ájuga turkestánica extract, experiments were performed on aorta preparations with the endothelial layer removed.
In the experiments, a standard method was used to investigate the possibility of modulation of the functional activity of the vascular endothelial layer under the relaxant effect of the extracts. Aortic smooth muscle preparations were elicited contractions using 1 μM Phe under conditions in which the endothelial layer was removed and the endothelial layer was present. An evoked contraction amplitude of 10 mN in the aortic preparation under Phe is the standard condition for the experiment. The absence of the endothelial layer of the aortic preparation is checked using 1 μM acetylcholine. It was noted that the amplitude of contraction of the rat aorta vascular preparation in isometric conditions caused by the α1-adrenoceptor agonist – 1 μM phenylephrine was reduced by 57.8±4.3% under the influence of 1 μM acetylcholine. It was found that 1 μM of acetylcholine has almost no effect on the force of contraction induced by 1 μM of phenylephrine in the aorta preparation, where the endothelial layer was mechanically removed using a cotton swab.
In the experiments, it was determined that the rat aorta blood vessel preparation under the conditions of isometric contraction, under the relaxant effect of the extract, changes to the extent that the vascular endothelial layer is removed. It was found that different concentrations (μg/ml) of ajuga extract reduced the relaxant effect by 57.9±2.8%, respectively, compared to the control. It was found that the endothelial layer was reduced by 23.9±3% compared to the existing conditions (Fig. 7).
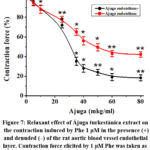 |
Figure 7: Relaxant effect of Ájuga turkestánica extract on the contraction induced by Phe 1 μM in the presence (+) and denuded (–) of the rat aortic blood vessel endothelial layer. |
As can be seen from the results of the experiment, significant changes were observed when the effect of the examined extract on the aorta preparations with the endothelial layer removed was examined. Based on the obtained results, it is possible to estimate the activity of the examined substances on the endothelium. In order to further clarify this assumption, experiments were conducted in the presence of eNOS blocker-L-NAME (100 μM). In experiments, in aortic preparations incubated with L-NAME, it was observed that the relaxant effect of Ájuga turkestánica extract was significantly attenuated. In the presence of 100 μM L-NAME, Ájuga turkestánica extract was found to reduce the contraction force of Phe-induced aortic preparations by 50.9±2.8%. It was found that the endothelial layer was reduced by 30.9±3% compared to the existing conditions (Fig. 8).
 |
Figure 8: Relaxant effect of Ájuga turkestánica extract, eNOS blocker – L–NAME 100 μM on contraction of rat aortic blood vessel preparation under incubation conditions. |
The results of the series of experiments show that the studied extracts have a strong relaxant effect, which is based on endothelium-dependent processes. The relaxation effect of the extract is attenuated in the presence of endothelium removed and L-NAME, indicating the importance of the role of NO synthase. By activating the NO synthase and cGC/cGMP/PKG signaling pathway, they help to reduce the influx of Ca2+ ions through the Ca2+L and Ca2+R channels in the plasmalemma, and also prevent their release from the SR, which leads to a decrease in [Ca2+]I in the SMC and causes a smooth muscle relaxation. During the next experiments, the effect of Ájuga turkestánica extract on the release of Ca2+ ions from SR through IP3R was investigated. In these experiments, the contraction force induced by phenylephrine (1 μM) in the absence of Ca2+ ions in the incubation medium determines the process of Ca2+ ion release from SR through IP3R27. In our studies, the force of contraction caused by phenylephrine (1 μM) was found to be 69±3.1% compared to the normal Krebs solution, and this contraction was taken as 100%. When we studied the effect of the concentration of Ájuga turkestánica extract (80μg/ml) under these conditions, it was found that it reduced the contraction force by 40.5±2.8% compared to the control (Figure 9).
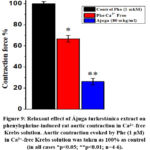 |
Figure 9: Relaxant effect of Ájuga turkestánica extract on phenylephrine-induced rat aortic contraction in Ca2+-free Krebs solution. |
From the obtained results, it was found that Ájuga turkestánica extract significantly reduces the contraction force induced by phenylephrine (1 μM) in the absence of Ca2+ ions. It can be seen that this substance may be caused by its effect on the level of [Ca2+]i, as it inhibits the release of Ca2+ ions from the SR. The obtained results suggest that the relaxant effect of this extract on the contractile activity of the aortic blood vessel in Krebs solution without Ca2+ ions is mainly related to the blockade of Ca2+ ions release process from SR through IP3R. In order to confirm this assumption, experiments were carried out using caffeine. According to the literature, under the influence of caffeine, the release of Ca2+ ions from the SR to the cytosol occurs due to the activity of the ryanodine receptor (RyR) located in the SR in smooth muscle cells13. In this case, the force of contraction caused by caffeine plays an important role as an indicator of the amount of Ca2+ ions in SR. In the conducted experiments, it was determined that caffeine (10 mM) in the medium of normal Krebs solution containing Ca2+ (2.5 mM) causes a reduction of 62.5±2.2% compared to the effect of phenylephrine (1 μM). Under these conditions, Ájuga turkestánica extract was found to reduce the contraction force induced by caffeine by 34.5±2.8% compared to the control. (Figure 10).
 |
Figure 10: Relaxant effect of Ájuga turkestánica extract on caffeine-induced rat aortic contraction in normal Krebs solution. |
The obtained results show that the relaxant effect of this extract on the contraction force induced by caffeine is related to the decrease in the amount of Ca2+ released from the SR. However, the contraction of the smooth muscle cell may be accompanied by Ca2+ ions coming from the external environment through the plasmalemma together with Ca2+ ions coming out of the SR under the influence of caffeine28. Therefore, in order to fully clarify the mechanism of action of the extracts on the contraction caused by caffeine, experiments were performed in the absence of Ca2+ ions in the incubation medium. In this condition, it was noted that the contraction force under the influence of caffeine is 35±2.4% compared to the condition in the presence of Ca2+ ions. In these conditions, experiments were carried out at concentrations of Ájuga turkestánica extract (80 μg/ml). As for the contraction force induced by caffeine, when the extract was studied, it was found that the contraction force decreased by 17.9±2.2% compared to the control (Figure 11).
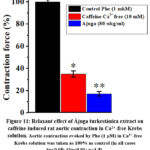 |
Figure 11: Relaxant effect of Ájuga turkestánica extract on caffeine-induced rat aortic contraction in Ca2+-free Krebs solution. |
Conclusion
These obtained results show that the studied extracts effectively reduce potential dependant Ca2+ L type channels and Phe-induced contraction of aortic preparations in Krebs solutions without Ca2+ ions, which is mainly supported by Ca2+ ions released from SR via IP3R. These data suggest that their effect on the release of Ca2+ ions from the SR via IP3R may play an important role in the relaxant effects of Ájuga turkestánica extract. In addition, when the endothelial layer of the aortic blood vessel was removed, the range of effect was not high, which indicates that the role of the endothelium is very important in the regulation of ion channels, so we can assume that Ájuga turkestánica has an effect on smooth muscle ion channels. From the above, we can conclude that Ájuga turkestánica has a significant effect on potential dependent, receptor dependent, sarcoplasmic reticulum Ca+2 ion channels.
Acknowledgement
The work was supported by the Applied Research Program of the Ministry of Higher Education, Science and Innovation Republic of Uzbekistan (project A-FA-2021-372 “Creation of medicine with effective control of the cardiovascular system based on Herba leonuri, Gnaphalii uliginosi herba, Chamomillae recutie flores, Crataegi flores medicinal plants”)
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this article.
Funding Source
Innovative Development Agency under the Ministry of Higher Education, Science and Innovation, grant number is A-FA-2021-372.
References
- Jaouad El-Hilaly a, Badiaˆa Lyoussi a, Maurice Wibo b, Nicole Morel b,∗ Vasorelaxant effect of the aqueous extract of Ajuga iva in rat aorta. J. El-Hilaly et al. / Journal of Ethnopharmacology 93 (2004) 69–74
CrossRef - Du W., McMahon T.J., Zhang Z.Sh., Stiber J.A., Meissner G., Eu J.P. Excitation-contraction coupling in airway smooth muscle // J. Biol. Chem. –2006. –V. 281(40). –P. 30143-30151.
CrossRef - Zhao J., Majewski H. Endothelial nitric oxide attenuates Na+/Ca2+–exchanger–mediated vasoconstriction in rat aorta // British Journal of Pharmacology.– 2008.– V.154(5).– P.982-990.
CrossRef - Ghosh, D., Syed, A. U., Prada, M. P., Nystoriak, M. A., Santana, L. F., Nieves-Cintron, M., et al. (2017). Calcium channels in vascular smooth muscle. Adv. Pharmacol.2016; 78(8), 49–87. doi:10.1016/bs.apha.
CrossRef - Cribbs, L. L. (2006). T-Type Ca2+ channels in vascular smooth muscle: Multiple functions. Cell Calcium. 2006; 40 (2), 221–230. doi:10.1016/j.ceca.
CrossRef - FAO. Directive 2010/63/EU on the protection of animals used for scientific purposes. Off J Eur Union. (2010):33–79.
- Vandier С., Guennec Jean-Yves L., Bedfer G. What are the signaling pathways used by norepinephrine to contract the artery? A demonstration using guinea pig aortic ring segments // Adv. Physiol. Educ. 2002. Vol. 26(1-4). Pp. 195-203.
CrossRef - Plamen I.Z., Kokova V.Yu., Apostolova E.G., Peychev L.P. Possible role of 18-kDa translocator protein (TSPO) in etifoxine-induced reduction of direct twitch responses in isolated rat nerve-skeletal muscle preparations // Tropical Journal of Pharmaceutical Research. 2018. Vol. 17 (7). Pp. 1309-1315.
CrossRef - Hilgers R.H., Webb R. C. Molecular aspects of arterial smooth muscle contraction: Focus on Rho //Experimental Biology and Medicine. –2005. –V. 230. –P. 829-835.
CrossRef - Omonturdiev S.Z., Inomjonov D.R., Abdullaev.A., Komilov.B.J, Gayibov U.G., Eshbakova K.A., Usmanov P.B., Aripov T.F., Studying the influence of quercetin flavonoid on contractility activity of rat aortic smooth muscle cells. Nor J Dev Int Sci. 2023;(112):8-13.
CrossRef - Francis S.H., Busch J.L., Corbin J.D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action // Pharmacol Rev 2010. – V.62(3). – P.525-563 11; – 2001.– V.91 – P.1421-1430.
CrossRef - Ion channels in smooth muscle: regulators of intracellular calcium and contractility Kevin S. Thorneloe and Mark T. Nelson/Can. J. Physiol.Pharmacol. 2005 Vol.83(3) 215-42).
CrossRef - Chalmers S., Olson M.L., Mac M.D., Rainbow R.D., McCarron J.G. Ion channels in smooth muscle: Regulation by the sarcoplasmic reticulum and mitochondria // Cell Calcium.– 2007. – V.42.– P.447-466;
CrossRef - Wray S., Burdyga T. Sarcoplasmic reticulum function in smooth muscle // Physiol. Rev. – 2010. – V.90(1). – P.113-178.
CrossRef - Cribbs L.L. Vascular smooth muscle calcium channels could «T» be a target? // Circ. Res. – 2001. – V.89. – P.560-562.;
CrossRef - Eric A.P, Miguel M.B, Manuel F. Navedo and Madeline N.Cintrón* Ion channel molecular complexes in vascular smooth muscle. Department of Pharmacology, University of California, Davis, CA, United States, 2022; 10(3)/fphys.20-22.
- Halidi N. Calcium dynamics and intercellular communication in arterial smooth muscle cells in vitro // Pour l’obtention du grade de docteur es sciences – Suisse. –2011. –V.2– P.15–32.
- Ghosh, D., Syed, A. U., Prada, M. P., Nystoriak, M. A., Santana, L. F., Nieves-Cintron, M., et al. (2017). Calcium channels in vascular smooth muscle. Adv. Pharmacol. 78, 49–87. doi:10.1016/bs.apha.2016.08.002
CrossRef - Khushmatov Sh.S., Omonturdiev S.Z., Eshbakova K.A., Usmanov P.B., Toshmatov Z.O. Relaxant effect of the flavonoid pulicarin. Med Plant Res. 2012;2(5):21-25. doi:10.5376/mpr.2012.02.0005.
CrossRef - Vandier С., Guennec J.L., Bedfer G. What are the signaling pathways used by norepinephrine to contract the artery? A demonstration using guinea pig aortic ring segments // Adv. Physiol. Educ. 2002. Vol. 26(1-4). Pp. 195-203.
CrossRef - Chitaley K., Weber D.S., Webb R.C. RhoA/Rho-kinase, vascular changes and hypertension // Curr. Hypertension. Rep. –2001. –V. 3. –P. 139-144.
CrossRef - Sobey C. Potassium channel function in vascular disease //Arterioscl. Thromb. and Vasc. Biol. –2001. –V. 21. –P. 28-36.
CrossRef - Stevens T. Is there a role for store-operated calcium entry in vascontraction? // Am. J. Physiol. lung сell мol. physiol –2001. –V. 280(5). –P. 866-869.
CrossRef - Francis S.H., Busch J.L., Corbin J.D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action // Pharmacol Rev 2010. – V.62(3). – P.525-563 11; – 2001.– V.91 – P.1421-1430.
CrossRef - Ion channels in smooth muscle: regulators of intracellular calcium and contractility Kevin S. Thorneloe and Mark T. Nelson / Can. J. Physiol.–Pharmacol. Vol.83, 2005.
CrossRef - Fisslthaler B., Hinsch N., Chataigneau T., Popp R., Kiss L., Busse R., Fleming I. Nifedipine increases cytochrome P450 2C expression and endothelium-derived hyperpolarizing factor-mediated responses in coronary arteries // Hypertension. 2000. –V. 36. –P. 270-275.
CrossRef - Jackson W.F. Ion channels and vascular tone // Hypertension. –2000. –V. 35. –P. 173-194.
CrossRef - Lincoln T.M, Dey N., Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression // J. Appl Physiol; Wier W.G., Morgan K.G. Alpha-adrenergic signaling mechanism in contraction of resistance arteries // Rev.Physiol.Biochem.Pharmacol – 2003.– V.150.– P.91-139.
- Hall I.P. Second messengers, ion channels and pharmacology of airway smooth muscle // Eur. Respir. J. –2000. –V. 15. –P. 1120-7.
CrossRef








