Tetiana Gontova1 , Viktoria Mashtaler1
, Viktoria Mashtaler1 , Svitlana Romаnova1
, Svitlana Romаnova1 , Ludmila Maloshtan2
, Ludmila Maloshtan2  and Mariia Shanaida3*
and Mariia Shanaida3*
1Department of Pharmacognosy and Nutriciology, National University of Pharmacy, Ukraine.
2Department of Biologyand Pharmacology, National Technical University "Kharkiv Polytechnic Institute", Ukraine.
3Department of Pharmacognosy and Medical Botany, I. Horbachevsky Ternopil National Medical University, Ukraine.
Corresponding Author E-mail:shanayda@tdmu.edu.ua
DOI : https://dx.doi.org/10.13005/bpj/2900
Abstract
Dahlia Cav. is a genus of ornamental plants that belongs to the Asteraceae family. These plants are visually pleasing and contain biologically active substances such as flavonoids, hydroxycinnamic acids, organic acids, and inulin. Among these substances, anthocyanins are especially noteworthy. These water-soluble vacuolar pigments of a glycoside nature have significant health benefits, including antioxidant, antidiabetic, hypocholesterolemic, anticancer, cardioprotective, and hypotensive properties. The purpose of this study was to evaluate the content of anthocyanins by spectrophotometry and the composition by HPLC in the extracts from flowers of two varieties of dahlias ('La Baron' and 'Colorado Classic') grown in Ukraine, as well as to evaluate the cytotoxic effect of these extracts. According to HPLC analysis, among 18 revealed anthocyanins the fower of ‘La Baron’ cultivar had the highest concentration of cyanidin-3-O-arabinoside (31.85%) and cyanidin-3-O-galactoside (23.01%) while ‘Colorado Classic’ accumulated more delphinidin-3-O-arabinoside (39.80%) and Delphinidin-3-O-galactoside (23.15%). Our study also found that malvidin, peonidin, and petunidin played a minor role in the coloration of the flowers. The total amount of anthocyanins was slightly higher in the flowers of the ‘La Baron’ cultivar (1.250%) compared to the ‘Colorado Classic’ one (1.138%). To determine the toxicity of anthocyanins, it was used an in vitro model of bone marrow cells (BMC) and found that the cytoprotective and cytotoxic activities of the anthocyanins were dose-dependent. Based on these findings, the study concluded that there is potential for developing new herbal medicinal products using dahlia flowers with a significant content of anthocyanins.
Keywords
Anthocyanins; Cytotoxic Effect; Dahlia; Dry Extract; Flowers; In Vitro Study; Spectrophotometry; High-Performance Liquid Chromatography
Download this article as:| Copy the following to cite this article: Gontova T, Mashtaler V, Romаnova S, Maloshtan L, Shanaida M. Phytochemical Analysis of Anthocyanins Extracted from the Flowers of Two Dahlia Cultivars and their Cytotoxic Properties. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Gontova T, Mashtaler V, Romаnova S, Maloshtan L, Shanaida M. Phytochemical Analysis of Anthocyanins Extracted from the Flowers of Two Dahlia Cultivars and their Cytotoxic Properties. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/4bUQYxd |
Introduction
Anthocyanins are part of the flavonoid group of phenolic substances and are synthesized from anthocyanidins1,2. They play a crucial role in determining the color of various plant organs, including flowers, fruits, tubers, and leaves. Anthocyanins are essential for plant survival, as they act as optical filters and protect plant tissues from excessive ultraviolet radiation. Additionally, anthocyanins are effective in inhibiting reactive oxygen species3,4. These compounds are stored in plant cells in the form of glycosides, and their color (rose, red-purple, dark violet, or blue) depends on various factors such as the pH of the medium, temperature, and the structure of the molecule. The color also depends on the number and position of OH groups, methylation, the nature of the sugar, the amounts of molecules and their location.
There are over 1000 natural anthocyanins obtained from the anthocyanidin aglycones with different glycosylations and acylations5. The most common anthocyanidins in nature are cyanidin, pelargonidin, peonidin, delphinidin, petunidin, and malvidin6.
Anthocyanins are compounds that have a wide range of pharmacological effects and are used in medicines and as food additives7. The anthocyanins that have a hydroxyl group at position 3 of the C ring possess high antioxidant properties, which are further enhanced by the acylation of sugar residues8,9. The ability of anthocyanins to enhance the oxidative function of mitochondria in muscle cells and brown adipose tissue affects the increase in the rate of energy metabolism in the body. Anthocyanins, by suppressing the activity of hydrolytic enzymes, slow down the hydrolysis of food, and as a result, after eating, the level of glucose in the blood rises evenly8. Among the delphinidin and cyanidin groups, the representatives exhibit the highest antioxidant activity and can inhibit the inflammatory process8-10. Anthocyanins are also known to restore the integrity of genomic DNA, stabilize collagen molecules, and reduce capillary permeability8,11,12. Numerous studies have demonstrated the antidiabetic, hypocholesterolemic, anticancer, cardioprotective, and hypotensive effects of anthocyanins13-17. Anthocyanins reduce markers of inflammation, therefore they are promising in the treatment of obesity and concomitant or independent diseases such as diabetes, cancer, and dysbiosis. Cardiovascular diseases are directly related to oxidative stress, metabolic syndrome, and chronic inflammation in the human body. Consuming natural products containing anthocyanins on a regular basis helps to reduce the lipid profile and blood sugar levels, which is important in the prevention of diseases of the cardiovascular system8,11,13,14,17. Katsube N, et al conducted a study on blueberry extract and found that among the isolated anthocyanins, delphinidin or malvidin as an aglycone inhibited the growth of HL60 cells by inducing apoptosis, and pure delphinidin and glycoside inhibited the growth of HCT116 cells16. It was established their ability to suppress the growth of Gram-positive (Staphylococcus aureus, Streptococcus faecalis, and Bacillus cereus) and Gram-negative (Pseudomonas aeruginosa, and Escherichia coli) bacteria as well as phytopathogenic (Penicillium spinulosum and Rhizopus stolonifer) and mold (Aspergillus flavus, Aspergillus niger) fungi18.
Curtain plant species are currently being used as an industrial source of anthocyanins. Among them fruits of grapes (Vitis vinifera L.), blackberries (Morus alba L.), blueberries (Vaccinium corymbosum L.), jambul (Syzygium cumini (L.) Skeels), strawberries (Fragaria x ananassa Duchesne ex Rosier) ad purple corn (Zea mays L.) as well as leaves of red cabbage (Brassica oleracea L. var. capitata f. rubra), taproots of carrot (Daucus carota L.) and radish (Raphanus sativus L.) or tubers of purple sweet potato (Ipomoea batatas (L.) Lam.)19. Adding anthocyanins to food products not only imparts different colors, making them visually appealing, but also acts as an antioxidant, protecting the food to which they were added20.
Over the past few years, researchers have taken a keen interest in studying various species of flowers (such as Clitoria ternatea L., Hibiscus sabdariffa L., Dahlia, Viola L., and others) for their anthocyanin content21-25. These anthocyanins can be used in medical treatments, as well as in the food industry. Mishra and his team obtained a purple dye from dahlia flowers that they suggested could be used as an eco-friendly alternative to phenolphthalein26. The anthocyanins in dahlia flowers have also been proposed as an ingredient in yogurt production. In Mexican cuisine, dahlias are used in salads, desserts, and other dishes27.
Scientists are searching for plants with a high concentration of anthocyanins for the development of herbal medicines. Plants of the Dahlia genus, which belong to the Asteraceae family, are of particular interest in this field because they can accumulate anthocyanins in their flower petals. This genus is native to Mexico to Colombia (as shown in Fig. 1), but it is now widely distributed around the world due to its easy cultivation. There are a total of 41 species and 15,000 varieties and cultivars in this genus28.
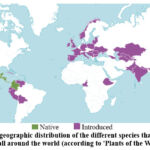 |
Figure 1: The geographic distribution of the different species that belong to the Dahlia genus all around the world (according to ‘Plants of the World online’28). |
The Dahlia species is known to contain inulin, which could be beneficial for patients with type 2 diabetes by preventing high blood glucose levels29. Additionally, these plants can accumulate valuable phenolic substances, amino acids, and organic acids30. The various species and cultivars of Dahlia plants allow for the selection of the most promising ones based on their chemical composition and pharmacological effects30-33. The color of dahlia flowers ranges from white-rose to dark purple, which is related to the level of accumulation of anthocyanins. Due to the diversity of species and cultivars, it is crucial to conduct a phytochemical analysis of plant raw materials for the quality assessment and efficacy of herbal medicines34.
This study aimed to conduct a phytochemical analysis of anthocyanins in the flowers of ‘La Baron’ and ‘Colorado Classic’ Dahlia cultivars and to determine the in vitro cytotoxic activity of their extracts.
Materials and Methods
Plant raw material
The study focused on the flowers of the ‘La Baron’ and ‘Colorado Classic’ Dahlia cultivars (Fig. 2) that belong to a group of ‘Decorative (ornamental) dahlias’ (according to the classification of The International Dahlia Register35). From 2016, we have been conducting research on inulin in underground tubers and phenolic substances in above-ground organs of various dahlias 29, 30. The research objects were selected from the botanical garden at Kharkiv Karazin University in Ukraine, taking into account the tuber weight and flower color. The ‘Colorado Classic’ flowers (Fig. 2, A) are light pink at the base and bright lilac at the tips, up to 15.0 cm long and 0.6-1.3 cm wide, with an elongated oval shape and smooth, pointed, concave edges. The ‘La Baron’ cultivar flowers (Fig. 2, B) are dark pink to purple, 7.0-10.0 cm long and 0.6-1.0 cm wide, with a flat or slightly concave shape, elongated lanceolate form, and smooth edges with a blunt top.
 |
Figure 2: Inflorescences of Dahlia cultivars: ‘Colorado Classic’ (А) and ‘La Baron’ (В) |
The plants’ raw materials were collected during the flowering stage in August 2021 at the collection site of the botanical garden named after V. N. Karazin (Kharkiv, Ukraine). Flowers were air-dried to an air-dry state by the requirements of the State Pharmacopoeia of Ukraine36. The identity of dahlia cultivars has been confirmed by Yuri Gamulya, PhD in Biological Sciences, the curator of the herbarium fund of V. N. Karazin Kharkiv National University.
Spectrophotometric analysis
The total content of anthocyanins was measured using a spectrophotometric method with UV-VIS spectrophotometer Specord 200 (Analytik Jena, Germany)36. Five series of raw materials of each dahlia cultivar were used to prepare the test solution. 5.00 g of raw material was crushed and sifted through a sieve with a hole diameter of 1-2 mm. Then the raw material was placed in a 100 mL conical flask and 95 mL of methanol was added, and stirred evenly for 30 min. The extract was filtered through a paper filter into a 100 mL volumetric flask. The filter was rinsed and the extraction volume was brought to the mark of 100 mL with the same solvent. A 50-fold dilution of the resulting solution was prepared in 0.1% hydrochloric acid in methanol30.
Measurements were carried out at a wavelength of 528±2 nm in terms of cyanidin-3-O-glucoside chloride. A solution of 0.1% hydrochloric acid in methanol was used as a compensation solution.
This formula was used for the calculations of the total content of anthocyanins:
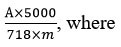
A – optical density of the test solution;
m – weight of the sample of raw material;
718 – specific absorption rate of cyanidin-3-O-glucoside chloride.
Chromatographic analysis using high-performance liquid chromatography (HPLC)
The chromatographic analysis of anthocyanins was carried out in the acidified aqueous extracts of the studied raw materials using reverse-phase HPLC. We used a Shimadzu LC-20 Prominence liquid chromatograph (Japan), equipped with a four-channel pump LC-20AD, a column thermostat STO-20A, an automatic sampler SIL-20A, a diode matrix detector SPDM20A and a ChemStation LC20. An Agilent Technologies liquid chromatograph (model 1200) was also used. The chromatograph is equipped with a G1379A vacuum degasser, a G1313A automatic injector, a G13111A four-channel low-pressure gradient pump, a G1316A column thermostat, and a G1316A diode array detector. The results were calculated using ChemStation LC20 software.
To separate anthocyanins, a Luna C18 (2) chromatographic column (250 mm x 4.6 mm, sorbent size 5 μm); the Phenomenex column was used. The flow rate of the mobile phase during analysis is 1 ml/min. The temperature of the column thermostat is 34º C. The volume of the injected sample is 10 µl. Detection was performed at a wavelength of 520 nm. A mixture of mobile phases was used for the gradient elution of anthocyanins. Mobile phase A consisted of acetonitrile (for HPLC). Mobile phase B comprised a mixture of glacial acetic acid, acetonitrile, trifluoroacetic acid, and highly purified water (MilliQ) (10:0.2:87.8). The resulting solutions were filtered through a membrane filter with a pore diameter of 0.2 μm. The elution mode is presented in Table 1.
Table 1: The elution mode in the HPLC analysis of dahlia’s anthocyanins
|
Time (min) |
Mobile phase A (%) |
Mobile phase Б (%) |
|
0 |
0 |
100 |
|
5 |
0 |
100 |
|
20 |
20 |
80 |
|
25 |
40 |
60 |
|
30 |
0 |
100 |
|
35 |
0 |
100 |
The acidified aqueous extracts were processed to obtain the anthocyanins from plant raw material. 2.00 g of dried and crushed raw material was sifted through a sieve with a hole diameter of 1-2 mm, placed in a 100 mL round-bottom flask, poured with 50 mL of a 4% phosphoric acid solution, and kept in a boiling water bath for 30 minutes using a reflux condenser. The resulting solutions were cooled to room temperature and filtered through a red ribbon paper filter. Before the chromatographic analysis, the test samples were filtered through a membrane filter with a diameter of 0.45 μm37.
The absorption spectra were recorded during HPLC analysis. The spectral measurements were carried out in the wavelength range 200-600 nm and the volume of the injected sample was 10 μL. To determine the total content of anthocyanins, the normalization method was used.
The contents of identified anthocyanins were determined by the area of the peaks. The content of each compound was calculated based on the total amount of anthocyanins36 using the formula:
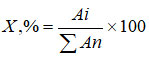
where: Аi – peak area calculated from chromatograms of the test solution;
Аn – the sum of the pigment peak areas, calculated from the chromatograms of the test solution.
Pharmacological in vitro studies
To obtain a dry extract for pharmacological study, the flowers of both Dahlia cultivars were crushed to a particle size of 2-3 mm. 50% ethanol acidified with hydrochloric acid was used as an extractant. The extraction was carried out three times. The raw material-extractant ratio was 1:9 and the time of each extraction was 1 h. The obtained extracts were filtered, combined, and then evaporated in a vacuum evaporator at a temperature of 50º C. The yield of the dry extract from the flowers of the ‘La Baron’ cultivar was 12.06%, and from ‘Colorado Classic’ it was 11.34%.
The quantitative assessment of the basic in vitro cytotoxicity of the test substances was performed using microscopy under SOP /T/001.538,39. The rat BMC were isolated from the diaphysis of the femoral bones of animals according to the generally accepted method37. The resulting suspension contained single cells in an amount of 2.0-2.1∙106 cells/mL. Cells were incubated at 37±2°C (without CO2). To obtain the initial solutions of the studied substances, the dry extracts were dissolved in a physiological solution (the concentration of the initial solutions was 2%). Then the initial solutions were titrated in an immunological plate using the rolling method. The following concentrations of the test substances were studied: 1%, 0.5%, 0.25%, 0.125% and 0.0625%.
Equal volumes of rat MBC suspension were added to each well of the immunological plate with the studied extracts. The native rat MBC in suspension with saline was used as a negative control. A quantitative assessment of the basic cytotoxic effect of the samples was carried out after 15, 45and 90 min of incubation.
The quantitative assessment of included cytotoxicity was conducted by counting the number of viable/non-viable cells in the Goryaev chamber. The results were expressed as the percentage of non-viable cells comparatively to their total number. The 0.1% trypan blue staining method was used to determine cell viability. Trypan blue is an acidic aniline dye that is unable to penetrate cells through an intact cell membrane; it selectively stains dead cells with damaged cell membranes37,38.
Statistical analysis
The statistical processing of the results of quantitative determinations was carried out by the method of dispersion analysis following the requirements of the State Pharmacopoeia of Ukraine under the Microsoft Excel 7.0 program using the “Statistica” application program package36.
Statistical processing of the obtained pharmacological data was processed using the Statistica 11 program using analysis of the variance of the data at a significance level of p<0.05.
Results and Discussion
The profile and content of anthocyanins in the studied dahlia cultivars
According to the results of spectrophotometric analysis (Table 2), the total amount of anthocyanins was slightly higher in the flowers of the ‘La Baron’ cultivar (1.250%) compared to the ‘Colorado Classic’ one (1.138%).
Table 2: Quantitative content of anthocyanins in the flowers of ‘Colorado Classic’ and ‘La Baron’ cultivars of dahlia (n=5)
|
Сultivar |
Content, % |
±ε, % |
|
‘Colorado Classic’ |
1.138 ± 0.002 Хср = 1.138 S2 = 0.0000050 Scp = 0.00100 P = 0.95 |
0.2442 |
|
‘La Baron’ |
1.250 ± 0.002 Хср = 1.250 S2 = 0.0000053 Scp = 0.00102 P = 0.95 |
0.2289 |
The UV spectra of anthocyanins in raw material of both studied cultivars are presented in Fig. 3,4.
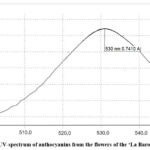 |
Figure 3: UV–spectrum of anthocyanins from the flowers of the ‘La Baron’ cultivar
|
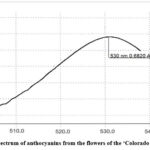 |
Figure 4: UV–spectrum of anthocyanins from the flowers of the ‘Colorado Classic’ cultivar
|
The chromatographic profiles of anthocyanins were analyzed by the HPLC method (Fig. 5,6). The revealed anthocyanins were the derivatives of the delphinidin, cyanidin, petunidin, peonidin, and malvidin groups (Table 3). It was found that the predominant groups of anthocyanins for both studied cultivars were cyanidin and delphinidin derivatives. The content of the petunidin, peonidin, and malvidin groups was significantly lower.
 |
Figure 5: HPLC chromatogram of anthocyanins extracted from the flowers of the ‘La Baron’ cultivar
|
 |
Figure 6: HPLC-chromatogram of anthocyanins extracted from the flowers of the ‘Colorado Classic’ cultivar
|
Table 3: Composition of anthocyanins in the flowers of ‘La Baron’ and ‘Colorado Classic’ dahlia cultivars.
|
Compound |
Cultivars |
|||
|
‘La Baron’ |
‘Сolorado Classic’ |
|||
|
Retention time, min |
Content, % |
Retention time, min |
Content, % |
|
|
Delphinidin-3-O-galactoside |
11.12 |
7.23 |
11.11 |
23.15 |
|
Delphinidin-3-O-glucoside |
12.54 |
0.10 |
12.36 |
0.16 |
|
Cyanidin-3-O-galactoside |
13.46 |
23.01 |
13.46 |
8.42 |
|
Delphinidin-3-O-arabinoside |
14.05 |
12.05 |
14.06 |
39.80 |
|
Cyanidin-3-O-glucoside |
14.50 |
0.73 |
14.50 |
1.26 |
|
Petunidin-3-O-glucoside |
15.07 |
4.44 |
15.12 |
1.70 |
|
Cyanidin-3-O-rutinoside |
15.32 |
1.96 |
15.32 |
2.80 |
|
Cyanidin-3-O- arabinoside |
15.83 |
31.85 |
15.84 |
11.41 |
|
Petunidin-3-O-rutinoside |
16.33 |
0.21 |
16.33 |
0.18 |
|
Peonidin-3-O-galactoside |
16.58 |
3.80 |
16.59 |
1.28 |
|
Petunidin-3-O-arabinoside |
16.96 |
3.50 |
16.96 |
0.57 |
|
Peonidin-3-O-glucoside |
17.48 |
0.67 |
17.50 |
0.11 |
|
Delphinidin-3-O-xyloside |
18.08 |
5.29 |
18.09 |
0.45 |
|
Malvidin-3-O-galactoside |
18.30 |
0.55 |
18.16 |
1.86 |
|
Peonidin-3-O-arabinoside |
19.00 |
0.14 |
19.01 |
0.03 |
|
Cyanidin-3-O-xyloside |
19.60 |
0.16 |
19.60 |
0.24 |
|
Malvidin-3-O-glucoside |
19.99 |
2.36 |
20.04 |
0.47 |
|
Malvidin-3-O-arabinoside |
20.80 |
0.26 |
20.82 |
0.03 |
Table 3 shows data on the percentage of each component among all identified. As can be seen from Table 3, a larger total amount of anthocyanins was identified in the flowers of the ‘La Baron’ cultivar than in the ‘Colorado Classic’. Among the representatives of the delphinidin group, four glycosides were identified in the flowers of both cultivars. It should be noted that anthocyanins of the delphinidin group accumulated in a larger amount in the ‘Colorado Classic’ cultivar (67.7%) compared to ‘La Baron’ one (25.1%). Delphinidin-3-O-arabinoside and delphinidin-3-O-galactoside accumulated in the largest quantities in both cultivars. Delphinidin-3-O-rutinoside, delphinidin-3-O-xyloside and delphinidin-3-O-glucoside also accumulated in insignificant quantities.
In the flowers of both cultivars, five substances of the cyanidin group were identified. The total content of cyanidins in the flowers of the ‘La Baron cultivar was 58.7%, and in the ‘Colorado Classic’ 25.7%. Among the identified cyanidins, cyanidin-3-O-arabinoside and cyanidin-3-O-galactoside accumulated in large quantities in the flowers of both cultivars compared to the other three cyanidin glycosides. The total content of petunidin glycosides was higher in the flowers of the ‘La Baron cultivar (8.3% of the total anthocyanins) compared to the ‘Colorado Classic one (2.6%). The total content of peonidin was 4.7% in the ‘La Baron’ cultivar and 1.5% in the ‘Colorado Classic’ Malvidin anthocyanins were represented by a higher content in the flowers of the ‘La Baron’ cultivar (3.2%) compared to the ‘Colorado Classic’ (1.5%).
Pharmacological studies
As it is known, the in vitro cytotoxicity assays are used for the initial assessment of the toxic potential of chemical or natural substances. It allows to evaluation the effects of plant extracts on cells’ viability, growth, and membrane permeability.
The results of the conducted in vitro studies of the effect of the extracts obtained from ‘La Baron’ and ‘Colorado Classic’ cultivars of dahlia on the viability of rat BMC are presented in Fig. 7,8. It was revealed that both dry extracts showed a cytoprotective effect on cell viability at concentrations of 0.0625 and 0.125 for all three exposures of 15, 45, and 90 min.
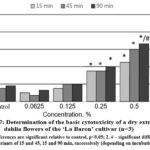 |
Figure 7: Determination of the basic cytotoxicity of a dry extract from dahlia flowers of the ‘La Baron’ cultivar (n=3).
|
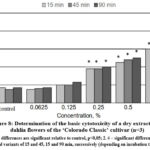 |
Figure 8: Determination of the basic cytotoxicity of a dry extract from dahlia flowers of the ‘Colorado Classic’ cultivar (n=3).
|
It was concluded that the dry extract from the flowers of the ‘La Baron’ cultivar had a significant cytoprotective effect at a concentration of 0.0625%. Under the influence of such a minimum concentration, the number of viable cells increased by 8.0–5.0% after exposure for 45 and 90 min. With an increase in concentration of 0.125% in test samples using the ‘Colorado Classic’ extract, the number of stained cells increased to 23% (Fig. 8). The extract from the ‘La Baron’ flowers showed cytoprotective activity at all exposures at this concentration.
A noticeable increase in cell death by 40% was revealed after the influence of both tested extracts at a concentration of extract 0.25% and higher (with an exposure of 90 min). A reliable cytotoxic effect of extracts from both cultivars was recorded at a concentration of 0.5%. Moreover, for the ‘La Baron’ cultivar (with an exposure of 90 min) the death of BMC corresponded to 67%, while for the Colorado Classic variety, it was 50%. At the maximum concentration (1.0%) of extracts, a cytotoxic effect was observed at all exposures for both cultivars. The percentage of cell death in assays using extracts of ‘Colorado Classic’ and ‘La Baron’ did not differ significantly from each other.
Generally, the conducted studies made it possible to establish the in vitro cytoprotective and cytotoxic activities of dry extracts from the flowers of ‘La Baron’ and ‘Colorado Classic’ cultivars on rats’ BMC. The obtained results indicate that the vital activity of BMC was influenced by both the concentration of the dry extracts studied as well as the time of exposure to the cells. At the lower concentrations of 0.065-0.25%, the ‘La Baron’ and ‘Colorado Classic’ extracts did not have a cytotoxic effect on BMC. On the contrary, they demonstrated cytoprotective activity. Thus, more than 50% of BMC died when it used the concentrations of 0.5-1.0% which revealed the presence of a cytotoxic effect.
Discussion
The most common anthocyanins found in plants are cyanidin, delphinidin, malvidin, petunidin and pelargonidin4,20. In fruits and vegetables, cyanidin accounts for 50% of the distribution, while delphinidin and peonidin account for 12%, and malvidin and petunidin each account for 7%20. Study of black flowers of dahlia cultivars revealed that the dark color of the petals is influenced by a significant amount of compounds from the cyanidin group40. Specifically, the flowers of the ‘Dandy’ cultivar contained cyanidin-3-glucoside-5-arabinoside, while the flowers of Dahlia variabilis accumulated cyanidin-3-monoside, pelargonidin, and delphinidin42.
Pelargonidin glycosides, including diglucoside pelargonin and 3-monoside pelargonin, have been found in Dahlia coecinea and Dahlia coronata43. Granados-Balbuena and co-authors investigated the anthocyanins profile of Dahlia pinnata flowers using UPLC-MS/MS. They identified four major substances, namely delphinidin-3-glucoside, delphinidin-3-rutinoside, pelargonidin-3-sambubioside-5-glucoside, and peonidin-3-sambubioside-5-glucoside21.
The color of anthocyanins and anthocyanidins is determined by how they absorb light in the UV and visible parts of the spectrum. When the pH is low, anthocyanins are in the form of flavylium cations, and at neutral pH, uncharged quinones are formed. Changes in pH can destroy anthocyanins and cause them to lose their color. Generally, adding hydroxyl groups to anthocyanins makes them bluer but less stable, while adding methyl groups makes them redder and more stable45. Berries that are red, blue, violet, or purple are the richest sources of anthocyanins, containing up to 5 mg/g46.
Recent studies have shown that delphinidin and its glycosides represent a promising group of substances for the treatment of lung, skin, liver, prostate, breast, and ovarian cancer47, they have antioxidant and anti-inflammatory properties5,48-51. Cyanidin-3-O-galactoside is a monoglycosylated anthocyanin found in fruit of chokeberries, red-skinned apples, blueberry, elderberry, and blackberry and cranberries which are an important part of the human diet. The amount of Cy3Gal varies depending on the species. The accumulation of Cy3Gal and other anthocyanins is significantly influenced by temperature, light, and air humidity 52. The presence of this substance is linked to the dark red or dark purple coloration of the fruits53.
Rupasinghe and the co-authors discovered that the concentration of cyanidin-3-O-galactoside in apple juice can reach 39 mg/L. This juice exhibited antioxidant activity in in vitro ferric ion reduction antioxidant capacity and oxygen radical absorbance capacity assays. These studies suggested that cyanidin-3-O-galactoside could be used as an effective therapeutic additive54. There is evidence that cyanidin-3-O-galactoside improves human cognitive function55.
Lim and the co-authors conducted experiments on mice and found that an extract from the fruits of Aronia melanocarpa, which was rich in cyanidin-3-O-galactoside, exhibited anti-obesity properties56. The supplementation of this extract was linked with a decrease in insulin resistance, serum triglyceride levels, low-density lipoproteins, and total cholesterol. It also resulted in a reduction in the weight of white adipose tissue. Furthermore, studies have demonstrated that anthocyanins from the purple tubers of Ipomoea batatas are effective in protecting the kidneys against uric acid-related disorders57.
Certain substances act as effective antioxidants, specifically petunidin-3-O-arabinoside and petunidin-3-O-glucoside. These substances are found in high concentrations in blueberries, dark grapes, raspberries, and strawberries56. Additionally, peonidin-3-galactoside was found in red raspberry, mango, blackberry, and blueberry, while peonidin-3-O-glucoside was found in red wine, purple onions, and red corn58.
Malvidin-3-O-galactoside is a compound found in various blueberry varieties and is known for its antitumor activity. Studies have shown that it can inhibit the proliferative ability of Huh-7 cells19. Furthermore, it can promote cell apoptosis and inhibit cell proliferation through the regulation of p38/JNK/ERK MAPK and Akt/PTEN signaling pathways59. Additionally, malvidin and its glycosides have properties that can help regulate blood sugar, prevent cardiovascular diseases, and improve brain function due to their antioxidant and anti-inflammatory effects.
After analyzing data from scientific sources on the content of anthocyanins in the fruits and seeds of various plants, it was found that the black soybean varieties contained anthocyanin content ranging from 1.89-26.33 mg/g60. The anthocyanin content of flesh berries varies significantly, depending on the species. Thus, the amounts of anthocyanins vary from 75.0 mg/g fresh fruit in Ribes rubum up to 46000 mg/g fresh fruit in Aronia melanocarpa61.
There is scientific data that the properties and reactivity of substances are affected by their spatial structure. Stetsenko and the co-authors utilized quantum-chemical methods with modern computer software to investigate how the spatial arrangement of substances impacts their properties and reactivity60. Their research revealed that anthocyanins have a significant ability to counteract free radicals when proton levels are elevated. Anthocyanins are electrophilic compounds, enabling them to readily capture unpaired electrons from free radicals in chemical reactions, which explains their strong antioxidant properties. By scavenging oxygen radicals within cells, anthocyanins can prevent oxidative stress and protect cell membranes from damage. This suggests that the membrane-stabilizing and antioxidant effects of anthocyanins can be anticipated and potentially harnessed in certain doses.
Therefore, derivatives of cyanidin, delphinidin, petunidin, peonidin, and malvidin from plant sources could be considered very promising substances for the prevention of chronic non-infectious diseases such as cardiovascular, metabolic, and neurodegenerative disorders.
Conclusion
In this study, we used HPLC and spectrophotometric methods to examine the anthocyanin profiles of two dahlia cultivars: ‘La Baron’ and ‘Colorado Classic’. The revealed anthocyanins were classified into delphinidins, cyanidins, petunidins, peonidins, and malvidins groups. A total of 18 components were identified and measured. The main coloring substances in ‘Colorado Classic’ flowers were the anthocyanins of the delphinidin group, while the ‘La Baron’ cultivar had a higher concentration of cyanidins. Both cultivars contained small amounts of malvidins, peonidins, and petunidins. Additionally, the study assessed the toxicity of dry extracts from the flowers of the studied cultivars using the BCM in vitro model. The results showed that the cytoprotective and cytotoxic effects of the extracts varied with the dose administered. However, further research is required to fully evaluate the pharmacological properties of the extracts from these two cultivars.
Acknowledgment
We would like to express our gratitude to the employees of the Botanical Garden at Kharkiv National University named after V.N. Karazin (Kharkiv, Ukraine) for providing us with samples of raw materials.
Conflict of interest
The authors declare no conflict of interest
Funding Source
The authors declare that no grants or other support were received during the preparation of the manuscript..
References
- Koss-Mikołajczyk I, and Bartoszek A. Relationship between Chemical Structure and Biological Activity Evaluated In Vitro for Six Anthocyanidins Most Commonly Occurring in Edible Plants. Molecules. 2023;28(16): 6156. https://doi.org/10.3390/molecules 28166156
CrossRef - E.K, Azrina A, Sou T.T, and See M.L. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food & Nutrition Research. 2017; 61(1): 1361779. https://doi.org/10.1080/16546628.2017.1361779
CrossRef - Mannino G, Gentile C, Ertani A, Serio G, and Bertea C.M. Anthocyanins: Biosynthesis, Distribution, Ecological Role, and Use of Biostimulants to Increase Their Content in Plant Foods – A Review. Agriculture. 2021; 11(3): 212. https://doi.org/10.3390/agriculture11030212
CrossRef - Mattioli R, Francioso A, Mosca L, and Silva P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules. 2020; 25: 3809. https://doi:10.3390/molecules25173809
CrossRef - Merecz-Sadowska A, Sitarek P, Kowalczyk T, Zajdel K, Jęcek M, Nowak P, and Zajdel R. Food Anthocyanins: Malvidin and Its Glycosides as Promising Antioxidant and Anti-Inflammatory Agents with Potential Health Benefits. Nutrients. 2023; 15(13): 3016. https://doi: 10.3390/nu15133016
CrossRef - Nassour R, Ayash A, and Al-Tameemi K. Anthocyanin pigments: Structure and biological importance. Journal of Chemical and Pharmaceutical Sciences. 2020; 13(4): 45–57.
- Chen J, Xu B, Sun J, Jiang X, and Bai W. Anthocyanin supplement as a dietary strategy in cancer prevention and management: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021; 1–13. https://doi: 10.1080/10408398.2021.1913092
CrossRef - Tena N, Martín J, and Asuero A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants. 2020; 9(5): 451. https://doi: 10.3390/antiox9050451
CrossRef - Ma Z, Du B, Li J, Yang Yu, and Zhu F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. International Journal of Molecular Science. 2021; 22(20): 11076. https://doi.org/10.3390/ijms222011076
CrossRef - Daveri E, Cremonini E, Mastaloudis A, Hester S.N, Wood S.M, Waterhouse A.L, Anderson M, Fraga, C.G, Oteizaa P.I. Cyanidin, and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biology. 2018; 18: 16–24. https://doi.org/10.1016/j.redox.2018.05.012
CrossRef - P, Headley L, Lizio R, and Hansmann J.A Review of the Properties of Anthocyanins and Their Influence on Factors Affecting Cardiometabolic and Cognitive Health. Nutrients. 2021; 13(8): 2831. https://doi.org/10.3390/nu13082831
CrossRef - Z, Știrbu I, Xiao J, Leopold N, Ayvaz Z, Danciu C, Ayvaz H, Stǎnilǎ A, Nistor M, and Socaciu C. Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention. Biomedicines. 2020; 8(9): 336. https://doi.org/10.3390/biomedicines8090336
CrossRef - Guo H, and Ling W. The update of anthocyanins on obesity and type 2 diabetes: Experimental evidence and clinical perspectives. Rev Endocr Metab Disord. 2015; 16(1): 1–13. https://doi: 10.1007/s11154-014-9302-z
CrossRef - Thornthwaite JT, Thibado SP, and Thornthwaite KA. Bilberry anthocyanins as agents to address oxidative stress. Preedy VR, ed. Pathology. Oxidative stress and dietary antioxidants. London: Academic Press; 2020:179–187. https://doi: 10.1016/B978-0-12-815972-9.00017-2
CrossRef - Norberto S, Silva S, Meireles M, Faria A, Pintado M, and Calhau C. Blueberry anthocyanins in health promotion: A metabolic overview. J Funct Foods. 2013; 5(4): 1518–1528. https://doi:10.1016/j.jff.2013.08.015
CrossRef - Katsube N, Iwashita K, Tsushida T, Yamaki K, and Kobori M. Induction of apoptosis in cancer cells by bilberry (Vaccinium myrtillus) and the anthocyanins. J Agric Food Chem. 2003; 51(1): 68–75. https://doi: 10.1021/jf025781x
CrossRef - Liobikas J, Skemiene K, Trumbeckaite S, and Borutaite V. Anthocyanins in cardioprotection: A path through mitochondria. Pharmacol Res. 2016; 113(B): 808–815. https://doi:10.1016/ j.phrs.2016.03.036
CrossRef - Aly AA, Ali HGM, and Eliwa NER. Phytochemical screening, anthocyanins and antimicrobial activities in some berries fruits. J Food Meas Charact. 2019; 13: 911–920. https://doi:10.1007/s11694-018-0005-0
CrossRef - Zhang J, Celli GB, and Brooks MS. Chapter 1. Natural sources of anthocyanins. In Anthocyanins from Natural Sources: Exploiting Targeted Delivery for Improved Health. 2019; Royal Society of Chemistry: 1–33. https://doi.org/10.1039/9781788012614-00001
CrossRef - Khoo HE, Azlan A, Tang ST, and Lim SM. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017; 61: 1361779. https://doi: 10.1080/16546628.2017.1361779
CrossRef - Granados-Balbuena SYa, Chicatto-Gasperín V, Aztatzi-Rugerio L, Santacruz-Juárez E, Robles-de la Torre R.R, Ocaranza-Sánchez E, and Robles-López M.R. Comparative study of anthocyanin extraction methods in Dahlia pinnata petals. J Appl Botany Food Qual. 2022; 95: 1-5. https://doi.org/10.5073/JABFQ.2022.095.001
- Fernandes L, Casal S, Pereira J.A, Saraiva J.A, and Ramalhosa E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. Journal of Food Composition and Analysis. 2017; 60: 38–50. https://doi.org/10.1016/j.jfca.2017.03.017
CrossRef - Food and Drug Administration. Listing of color additives exempt from certification; butterfly pea flower extract. Federal Register, 2021; 86(168): 49230–49234.
- Pires TCSP, Dias MI, Barros L, Barreira JCM, SantosBuelga C, and Ferreira ICFR. Incorporation of natural colorants obtained from edible flowers in yogurts. LWT. 2018; 97: 668– 675. https://doi.org/ 10.1016/j.lwt.2018.08.013
CrossRef - Mulík S, and Ozuna C. Mexican edible flowers: Cultural background, traditional culinary uses, and potential health benefits. International Journal of Gastronomy and Food Science. 2020; 21: 100235. https://doi.org/10.1016/j.ijgfs.2020.100235
CrossRef - Mishra PK, Singh P, Gupta KK, Tiwari H, and Srivastava P. Extraction of natural dye from Dahlia variabilis using ultrasound. Indian Journal of Fibre & Textile Research. 2011; 37(1): 83-86.
- Lara-Cortés E, Martín-Belloso O, Osorio-Díaz P, Barrera-Necha L.L, Sánchez-López J.A, and Bautista-Baños S. Antioxidant capacity, nutritional and functional composition of edible dahlia flowers. Revista Chapingo Serie Horticultura. 2014; 20(1): 101–116. https://doi.org/10.5154/r.rchsh.2013.07.024
CrossRef - https://powo.science.kew.org/taxon/325940-2 (Date of access: 16.03.2024)
CrossRef - Kriukova Ya, Jakubiak-Augustyn A, Ilyinska N, Krotkiewski H, Gontova T, Evtifeyeva O, Özcelik T, and Matkowski A. Chain length distribution of inulin from dahlia tubers as influenced by the extraction method. International Journal of Food Properties. 2018; 20(3): S3112-S3122. https://doi: 10.1080/10942912.2017.1357043
CrossRef - Gontova T, Ilyinska N, Golembiovska O, and Mashtaler V. Study of the component composition of phenolic compounds obtained from Dahlia varieties Ken’s Flame herb.Der Pharma Chemica. 2016; 8(18): 455-459
- Granados-Balbuena SY, Santacruz-Juárez E, Canseco-González D, Lucía Aztatzi-Rugerio D, Sánchez-Minutti L, Ramírez-López C and Ocaranza-Sánchez E. Identification of anthocyanic profile and determination of antioxidant activity of Dahlia pinnata petals: a potential source of anthocyanins. J. Food Sci. 2022; 87(3): 957-967. https://doi.org/10.1111/1750-3841.16072
CrossRef - Lara-Cortés E, Martín-Belloso O, Osorio-Díaz P, Barrera-Necha L.L, Sánchez-López J.A, and Bautista-Baños S. Antioxidant capacity, nutritional and functional composition of edible dahlia flowers. Revista Chapingo Serie Horticultura. 2014; 20(1): 101–116. https://doi.org/10.5154/r.rchsh.2013.07.024
CrossRef - Espejel EAR, Alvarez OC, Muñoz JMM, Mateos MdRG, León MTBC, Damián MTM Physicochemical quality, antioxidant capacity and nutritional value of edible flowers of some wild dahlia species. Folia Horticulturae. 2019; 31(2): 331–342. https://doi.org/10.2478/fhort-2019-0026
CrossRef - Vlasova I, Gontova T, Grytsyk L, Zhumashova G, Sayakova G, Boshkayeva A, Shanaida M, Kоshovyi O. Determination of standardization parameters of Oxycoccus macrocarpus (Ait.) Pursh and Oxycoccus palustris Pers. leaves. ScienceRise: Pharmaceutical Science. 2022. 3(37): 48–57. https://doi.org/10.15587/2519-4852.2022.260352
CrossRef - International Dahlia Register and Checklist 27th Supplement. The Royal Horticultural Society. 2018: 1-19. (Date of access: 16.03.2024)
- України. Державне підприємство «Український науковий фармакопейний центр якості лікарських засобів». 2-е вид. Державне підприємство «Український науковий фармакопейний центр якості лікарських засобів. 2014 Т. 3. 732 с (Ukrainian)]
- Golembiovska OI, Tsurkan AA. Anthocyanins profiling of Prunella vulgaris L. grown in Ukraine. The Pharma Innovation. 2013; 2(6): 42-48.
- Reddy NM, Panda KK, Subhadra AV, Panda BB. The Allium micronucleus (MNC) assay may be used to distinguish clastogens from aneugens. Biologisches Zentralblatt. 1995; 114(4): 358–368.
- Малоштан ЛМ, Шакіна ЛО, Гонтова ТМ, Романова СВ, Яременко МС. Дослідження цитотоксичної активності сухого екстракту та антоціанового комплексу квіток жоржини сорту Gebu. Український біофармацевтичний журнал. 2021; 1(66): 16-22. https://doi.org/10.24959/ubphj.21.295 (Ukrainian).
CrossRef - Deguchi A, Tatsuzawa F, Hosokawa M, Doi M, and Ohno S. Quantitative evaluation of the contribution of four major anthocyanins to black flower coloring of Dahlia petals. Hort J. 2016; 85(4): 340-350. https://doi.org/10.2503/hortj.MI-121
CrossRef - Lim TK. Dahlia pinnata. Edible Medicinal and Non-Medicinal Plants. 2014; 7: 333-339. https://doi:10.1007/978-94-007-7395-0
CrossRef - Mishra PK, Singh P, Gupta KK, Tiwari H, and Srivastava P. Extraction of natural dye from Dahlia variabilis using ultrasound. Indian Journal of Fibre & Textile Research. 2012; 37(1): 83-86.
- Lawrence WJC, and Scott-Moncrieff R. The genetics and chemistry of flower colour in dahlia: a new theory of specific pigmentation. J. of Genetics 1935; 15(2): 156-228.
CrossRef - Castañeda-Ovando A, de Lourdes Pacheco-Hernández M, Páez-Hernández M.E, Rodríguez J.A, and Galán-Vidal C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009; 113: 859–871. https://doi:10.1016/j.foodchem.2008.09.001
CrossRef - He J, and Giusti M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010; 1: 163–187. https://doi:10.1146/annurev.food.080708.100754
CrossRef - Gasmi A, Mujawdiya PK, Noor S, Lysiuk R, Darmohray R et al. Polyphenols in Metabolic Diseases. Molecules. 2022, 27, 6280. https://doi.org/10.3390/molecules27196280
CrossRef - Sharma A, Choi H-K, Kim Y-K, and Lee H-J. Delphinidin and Its Glycosides’ War on Cancer: Preclinical Perspectives. International Journal of Molecular Sciences. 2021; 22(21):11500. https://doi.org/10.3390/ijms222111500
CrossRef - Bunea A, Rugină D, Sconţa Z, Pop R.M, Pintea A, Socaciu C, Tăbăran F, Grootaert C, Struijs K, and VanCamp J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry. 2013; 95: 436-444. https://doi: 10.1016/j.phytochem.2013.06.018
CrossRef - Dai J, Gupte A, Gates L, and Mumper R. J. A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: extraction methods, stability, anticancer properties and mechanisms. Food and chemical toxicology. 2009; 47(4): 837-847. https://doi: 10.1016/j.fct.2009.01.016
CrossRef - Fimognari C. Antitumor Effects of anthocyanins: focus on apoptosis. Natural compounds as inducers of cell death. 2012. Springer, Dordrecht: 49-68.
CrossRef - Ghafoor K, Choi Y.H, Jeon J.Y, and Jo I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. Journal of agricultural and food chemistry. 2009; 57(11): 4988-4994. https://doi: 10.1021/jf9001439
CrossRef - Liang Z, Liang H, Guo Y, Yang D. Cyanidin 3-O-galactoside: A Natural Compound with Multiple Health Benefits. International Journal of Molecular Sciences. 2021; 22(5): 2261. https://doi.org/10.3390/ ijms22052261
CrossRef - Li X, Wang J, Yin H, Fan Z, and Li J. Variation of flower colors and their relationships with anthocyanins in cultivars of Camellia japonica. J. Ecol. Rural Environ. 2019; 35: 1307–1313.
- Rupasinghe HPV, Huber GM, Embree C, and Forsline PL. Red-fleshed apple as a source for functional beverages. Can. J. Plant Sci. 2010; 90: 95–100. https://doi:10.4141/CJPS09057
CrossRef - Skemiene K, Pampuscenko K, Rekuviene E, and Borutaite V. Protective effects of anthocyanins against brain ischemic damage. J. Bioenerg. Biomembr. 2020; 52: 1–12. https://doi: 10.1007/s10863-020-09825-9
CrossRef - Lim S-M, Lee H.S, Jung J.I, Kim S.M, Kim N.Y, Seo T.S, Bae J-S, and Kim E.J. Cyanidin-3-O-Galactoside-Enriched Aronia melanocarpa Extract Attenuates Weight Gain and Adipogenic Pathways in High-Fat Diet-Induced Obese C57BL/6 Mice. Nutrients. 2019; 11(5):1190. https://doi.org/10.3390/nu11051190
CrossRef - Mahendra AN, Jawi IM, Astawa NM, Astawa P, Yasa IWP. Renoprotective Effects of Anthocyanins Against Uric Acid-Instigated Injury: Mini Review with a Special Emphasis on Purple Sweet Potato (Ipomoea batatas L.) Anthocyanins. Biomed Pharmacol J 2023;16(2).
CrossRef - Zhang W, Shen Y, Li Z, Xie X, Gong E.S, Tian J, Si X, Wang Y, Gao N, Shu C, Meng X, Li B, and Liu R.H. Effects of high hydrostatic pressure and thermal processing on anthocyanin content, polyphenol oxidase and β-glucosidase activities, color, and antioxidant activities of blueberry (Vaccinium Spp.) puree. Food Chem. 2021; 342: 128564. https://doi: 10.1016/j.foodchem.2020.128564
CrossRef - Lin J, Tian J, Shu C, Cheng Z, Liu Yu, Wang W, Liu R, Li and B, Wang Yu. Malvidin-3-galactoside from blueberry suppresses the growth and metastasis potential of hepatocellular carcinoma cell Huh-7 by regulating apoptosis and metastases pathways Food Science and Human Wellness. 2020; 9 (2): 136-145. https://doi.org/10.1016/ j.fshw.2020.02.004
CrossRef - Choi Y. M., Yoon H., Lee S., Ko H. C., Shin M. J., Lee M. C., Hur O. S., Ro N. Y., Desta, K. T. Isoflavones, anthocyanins, phenolic content, and antioxidant activities of black soybeans (Glycine max (L.) Merrill) as affected by seed weight. Scientific Reports. 2020. 10(1), 19960. https://doi.org/10.1038/s41598-020-76985-4
CrossRef - Cisowska A., Wojnicz D., Hendrich A. B. Anthocyanins as antimicrobial agents of natural plant origin. Natural product communications 2011. 6(1), 149–156.
CrossRef - Stetsenko N.O., Goyko I.Yu., Bashta A.O. Study of antioxidant capacity of black elder berries anthocyanins using the method of computer chemistry. Modern engineering and innovative technologies 2023 Issue 30. Part 3 P. 102-107. DOI: 10.30890/2567-5273.2023-30-00-037
CrossRef








