Kunal Sharma1 , Amit Gupta2*
, Amit Gupta2* , Simran Srivastava1
, Simran Srivastava1 and Arsh Singh1
and Arsh Singh1
1Department of Microbiology, Graphic Era Deemed to be University, Dehradun, Uttarakhand India.
2Department of Zoology, University of Jammu, Baba Saheb Ambedkar Road, Tawi, Jammu, Jammu, India.
Corresponding Author E-mail:amit.gupta@jammuuniversity.ac.
DOI : https://dx.doi.org/10.13005/bpj/2921
Abstract
In this study, we prepared three extracts (methanolic, ethyl acetate, and n-hexane) of Zanthoxylum armatum from respective regions of Uttarakhand (Bageshwar, Pithoragarh, and Champawat) for determining the antimicrobial activity of fruits and seed samples using the disc diffusion method. These samples were tested in vitro for their ability to inhibit the growth of three different bacterial strains: Pseudomonas aeruginosa, Staphylococcus aureus, and E. coli, and the zone of inhibition was calculated in mm (millimeters). Against the three test pathogens, however, the fruit extracts demonstrated more potent antibacterial activity, but the antibacterial activity of seed extracts was less evident. Staphylococcus aureus was shown to be more susceptible to each of the extracts than other strains. This plant has the potential to treat a wide range of bacterial conditions, including skin infections, urinary tract infections, dental problems, diarrhoea, and dysentery. Similarly, Zanthoxylum armatum fruit and seed extracts were tested for their antioxidant capacity using 2,2′-diphenyl picrylhydrazyl (DPPH) radical scavengers. These studies revealed that the methanolic fruit and seed extract of Zanthoxylum armatum from Bageshwar showed higher antioxidant and antimicrobial effects as compared to the control. Similar effects were obtained from ethyl acetate and n-hexane extracts, but these had a lower effect than the methanolic extract. In short, Zanthoxylum armatum fruits and seeds have shown exceptional antibacterial properties against several pathogenic microorganisms that cause a number of disorders and have also shown antioxidant properties.
Keywords
Antioxidant; Antimicrobial; Fruit; Seed; Zanthoxylum armatum
Download this article as:| Copy the following to cite this article: Sharma K, Gupta A, Srivastava S, Singh A. Antimicrobial and Antioxidant Potential of Zanthoxylum armatum from Uttarakhand locations. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Sharma K, Gupta A, Srivastava S, Singh A. Antimicrobial and Antioxidant Potential of Zanthoxylum armatum from Uttarakhand locations. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3VykBi8 |
Introduction
The Himalayan region of northern India is particularly rich in medicinal plants. Due to the hard climate and short growing season, the Indian Trans-Himalayan Mountain range sustains a limited plant cover in particular. The Indian Himalayas are home to more than 8000 species of angiosperms, 44 species of gymnosperms, and 600 species of pteridophytes, of which 1748 species are recognised for their medicinal potential. 1 The evolution of the modern system for health care and our daily lives both depend heavily on plants. Plants have served as the basis for the usual traditional medical systems that have been in use for thousands of years. The plants are still here to provide new remedies to humankind. In Japan, demand for herbal pharmaceutical goods is higher than for conventional pharmaceutical items. Most of the significant medications in the last 50 years that have changed contemporary medicine have been isolated from plants. The medicinal qualities of medications made from plants and animals are demonstrated by their chemical constituents. 2
Indigenous herbal methods have employed medicinal plants to treat a variety of ailments since the dawn of time. Despite the increased advances in contemporary drug systems in recent years, herbal medicine still plays a significant role in the health care system even now. Particularly in poorer nations, interest in them has increased because of their lengthy history of usage in folk medicine and their positive effects on human health. New antibacterial compounds could come from plants, and these days, herbal medicine, which employs plant sources to treat a variety of infectious disorders, is also becoming more and more popular. Numerous flavonoids and phenolics have been isolated and identified as potential agents for antibacterial, antioxidant, and anticancer activity from a variety of plants. 3 Allium cepa, Boerhavia diffusa, Berberis aristata, Boenninghausenia albiflora, Datura metel, Hedychium spicatum, Lablab purpureus, Melia azedarach, Plantago major, Polygonatum cirrhifolium, Punica granatum, Terminalia arjuna, and Vitex negundo are examples of medicinal plants that have antimicrobial and antioxidant properties. 4
Zanthoxylum armatum leaves, fruits, seeds, and bark have traditionally been used as carminatives, antipyretics, appetizers, stomachics, dyspepsia treatments, and toothaches. The leaves, fruits, seeds, and bark of Zanthoxylum armatum also contain a variety of therapeutic characteristics. Numerous chemical substances, including secondary metabolites, have been discovered in various plant parts and are thought to be responsible for the plant’s immunopharmacological activities, including its antioxidant, antipyretic, larvicidal, and anti-inflammatory properties.5 The safety of medications made from plant-based ingredients has now been proven to be superior to those made from synthetic materials.6 Reactive oxygen species (ROS) such as superoxide anion radicals, hydroxyl radicals, nitric oxide radicals, singlet oxygen, hypochlorite radicals, hydrogen peroxide, and different lipid peroxides are examples of free radicals. Antioxidants are essential chemical substances that may attach to free radicals and counteract their harmful effects on healthy human cells.7, 8 Zanthoxylum armatum DC., a sub-deciduous shrub in the Rutaceae family, is also known as prickly ash in English, tejphal in Hindi, and Timur in Urdu (Nepal). It can be found all over northeastern India as well as eastern and southern Asia, from Kashmir to Bhutan, at elevations up to 2,500 metres. At an altitude of 1,300–1,500 m, it has also been discovered to occur in China, Pakistan, Japan, the Philippines, Taiwan, Nepal, and Malaysia.9-11 The present study’s objectives were to report the potentials of Zanthoxylum armatum from several Kumaun area districts and to evaluate their phytochemical screening, in vitro antioxidant activity using the DPPH assay, and antibacterial activity.
Materials and methods
Chemicals and Instruments
DPPH, Ascorbic Acid, NaOH, 70% Alcohol, Nutrient Agar (NA), Nutrient Broth (NB), Mueller Hinton Agar (MHA), UV-vis Spectrophotometer, Laminar Air Flow, Weighing Balance, Horizontal Shaker, Incubator, Refrigerator
Collection of plant samples (fruits and seeds)
The fruits and seeds of Zanthoxylum armatum were collected in November from three different locations in the Kumaun region that experienced geographic diversity. Table 1 summarizes information regarding the study area, including its elevation range, latitude, longitude, temperature, and typical rainfall.
Shama Dhura, Bageshwar
Barakot, Champawat
Dharchula, Pithoragarh
Table 1: Details of the selected study locations.
|
Study area |
Elevation range |
Altitude (m) |
Latitude |
Longitude |
Average Temperature (min-max °C) |
Average rainfall (mm) |
|
Dharchula, Pithoragarh |
Sub-tropical |
950 m |
29°.88’N |
80°.54’E |
18-32 |
228.52 |
|
Shama Dhura, Bageshwar |
Temperate |
2530 m |
29° 94’N |
79° 90’E |
12.5-25.6 |
350.49 |
|
Barakot, Champawat |
Sub-tropical |
1600 m |
29° 46’N |
80° 07’E |
18.4-33.5 |
310.67 |
Extraction of fruits and seeds from the plant samples
The extraction of the plant materials was done using the maceration technique. To get rid of the dust particles, the samples were cleaned with 70% alcohol and shade-dried for 4-6 days. The dried materials were then powdered to a coarse consistency. 30 g of each sample were collected and mixed well with 300 ml of various solvents (methanol, ethyl acetate, and n-hexane). After being held on a rotating platform for 6-7 days, samples were then moved into a water bath and heated to 60 °C to allow the solvents to evaporate. The Whatman No. 1 filter paper and syringe filter were then used to filter the samples twice. The extracts were then collected and stored at 4 degrees Celsius for future use.
Plant material
The sample of Zanthoxylum armatum (authenticated from Dr AB Bajpai, Department of Botany, DBS College, Dehradun) has been collected from three different districts, namely Pithoragarh, Bageshwar, and Champawat, in the month of November during the fruiting season. The extraction of the plant materials was done using the maceration technique. To get rid of the dust particles, the samples were cleaned with 70% alcohol and shade-dried for 4-6 days. The dried materials were then powdered to a coarse consistency. 30 g of each sample were collected and mixed well with 300 ml of organic solvent (i.e., methanol, ethyl acetate, and hexane). After being held on a horizontal shaker for 6-7 days, samples were then moved into a water bath and heated to 60 °C to allow the solvent to evaporate. The Whatman No. 1 filter paper and syringe filter were then used to filter the samples twice. The extracts were then collected and stored at 4 degrees Celsius for future use. First, qualitative and quantitative tests were performed using standard methods for the determination of secondary metabolites.
Fourier transform infrared spectrophotometer (FTIR) analysis
FTIR is one of the most formidable instrumental appliances for judging several chemical bonds (functional groups) in compounds. A slurry of different solvent extracts of Zanthoxylum armatum (methanolic, ethyl acetate, and n-hexane) was used for FTIR analysis (Perkin Elmer), with a scan range of 400 to 4000 cm-1 and a resolution of 4 cm-1.
Estimation of Antioxidant Activity
Radical Scavenging Activity—The standard protocol of Brand-Williams et al. was used to determine DPPH inhibition in Zanthoxylum armatum; 200 mg of extracts (methanolic, ethyl acetate, and n-hexane) were taken in a centrifuge tube (in triplicate). In place of the sample, 200 microliters of distilled water were taken. Then 1 mL of DPPH solution (8 mg/100 mL of ethanol) was added to the methanolic/ethyl acetate/n-hexane extract (fruits and seeds) of Zanthoxylum armatum and the blank. This frame was left at room temperature for 45 minutes (with vortexing in between). Samples were centrifuged at 4000 rpm for 8 min, and then 0.5 ml of the supernatant was taken into tubes containing 1 ml of ethanol, and its absorbance was measured at 517 nm against the ethanol by using a UV-vis spectrophotometer.8, 12 Each crude sample was analysed in triplicate. The percentage of inhibition was calculated against a blank.
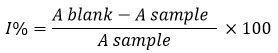
Where, Ablank is the control absorbance (reagents except the test extract) and Asample is the extract absorbance.
Total Phenolic and flavonoid content
The Folin Ciocalteau assay was used to determine the total phenolic content of Zanthoxylum armatum fruits and seeds extract (methanolic, ethyl acetate, and n-hexane). Firstly, an extract (fruits and seeds; 200 µl) of the respective solvent from three different regions of Uttarakhand was pipetted into test tubes. Fill the test tube halfway with distilled water and add the Folin-Ciocalteau reagent (1:10 dilution; 1.8 ml). Incubate samples of the respective extracts for 5 min, and then add sodium carbonate solution (7.5%; 1.2 ml). Incubate the extracts at room temperature and measure their absorbance at 765 nm using a UV-visible spectrophotometer. In order to generate the calibration curve, we used gallic acid (standard dilutions, i.e., 20, 40, 60, 80, and 100 mg/ml) and expressed the result as milligram gallic acid equivalents per gram of sample.7, 13
Similarly, the total flavonoid content of Zanthoxylum armatum fruits and seeds was determined using a colorimetric assay.15 Similarly, we generate a calibration curve using a standard solution of rutin (20, 40, 60, 80, and 100 mg/ml). The determination of total flavonoid content was mainly applied and expressed on the basis of fresh weight as µg of rutin equivalents/g of sample.
Estimation of antimicrobial Activity
Preparing an agar plate and an inoculum: To guarantee precise and consistent outcomes, the Kirby-Bauer technique is standardised in every element. A laboratory must follow these guidelines as a result. Mueller-Hinton agar must be poured into 100-mm or 150-mm Petri plates with a maximum depth of 4 mm for Kirby-Bauer testing. The agar’s pH level needs to range from 7.2 and 7.4. A broth culture is diluted to meet a 0.5 McFarland turbidity level, or roughly 150 million cells per millilitre, to create bacterial inoculum. Broth was then spread on the prepared agar plate through an L-shaped spreader.
At varied doses of 8 µl (1:4), 16 µl (2:3), 24 µl (3:2) and 32 µl (4:1) from a stock solution of extract (1 mg/ml; final volume in each well, i.e., 40 µl), the extract’ antibacterial activity was assessed. The extracts were produced in 80% ethanol in the necessary quantities and kept chilled to prevent evaporation. The extracts were screened for antibacterial activity using the disc diffusion method. With the use of a pipette, a drop of every microbial suspension containing roughly 108 CFU/mL was gently applied to the Mueller-Hinton agar (MHA). Following that, six-millimeter-diameter discs were coated with different concentrations of the extract and sterilised for 15 minutes at 121°C.
As positive controls, streptomycin (10 mg) and ampicillin (2 mg) were utilized. The discs were tagged and incubated for 24 hours at 37 °C after being dried and set in proximity to the bases of the plates containing the organisms. The findings of the studies were reported as the width (mm) of the inhibitory zones in duplicate.14
Statistical analysis
Values were expressed in the form of mean ± S.E. and results were expressed in the form of a one-way ANOVA test.
Results
Phytochemical estimation
These studies revealed a massive amount of secondary metabolites in fruits and seeds, which were qualitatively determined as shown in Table 2. There are numerous variations in phytochemicals extracted from Zanthoxylum armatum fruits and seed extracts. Most importantly, methanolic fruit and seed extract showed higher concentrations of secondary metabolites, but there are some variations in the three districts as compared to ethyl acetate and n-hexane extract.
Table 2: Phytochemical screening of Zanthoxylum armatum fruit and seed extracts
|
S.No. |
Phytochemical |
Location |
FRUITS |
SEEDS |
||||
|
Methanol |
Ethyl acetate |
n-Hexane |
Methanol |
Ethyl acetate |
n-Hexane |
|||
|
1 |
Flavonoids |
BAGESHWAR |
+ |
++ |
+ |
++ |
++ |
+ |
|
CHAMPAWAT |
++ |
– |
– |
+ |
– |
– |
||
|
PITHORAGARH |
++ |
– |
++ |
+ |
– |
+ |
||
|
2 |
Alkaloids |
BAGESHWAR |
– |
+ |
+ |
++ |
+ |
+ |
|
CHAMPAWAT |
– |
– |
+ |
+ |
+ |
+ |
||
|
PITHORAGARH |
++ |
– |
+ |
++ |
+ |
+ |
||
|
3 |
Glycosides |
BAGESHWAR |
++ |
– |
– |
++ |
– |
– |
|
CHAMPAWAT |
++ |
+ |
– |
++ |
+ |
– |
||
|
PITHORAGARH |
+ |
+ |
+ |
+ |
+ |
+ |
||
|
4 |
Steroids |
BAGESHWAR |
+ |
+ |
+ |
++ |
+ |
+ |
|
CHAMPAWAT |
++ |
+ |
– |
++ |
+ |
– |
||
|
PITHORAGARH |
++ |
++ |
+ |
++ |
++ |
+ |
||
|
5 |
Phenols |
BAGESHWAR |
++ |
+ |
++ |
– |
+ |
++ |
|
CHAMPAWAT |
++ |
+ |
++ |
– |
+ |
++ |
||
|
PITHORAGARH |
++ |
++ |
++ |
– |
++ |
++ |
||
|
6 |
Terpenoids |
BAGESHWAR |
+ |
+ |
+ |
++ |
++ |
+ |
|
CHAMPAWAT |
+ |
++ |
– |
+ |
++ |
– |
||
|
PITHORAGARH |
++ |
++ |
++ |
++ |
+ |
++ |
||
|
7 |
Anthraquinone |
BAGESHWAR |
– |
+ |
– |
– |
+ |
– |
|
CHAMPAWAT |
– |
– |
– |
++ |
+ |
– |
||
|
PITHORAGARH |
++ |
+ |
– |
+ |
+ |
+ |
||
|
8 |
Quinone |
BAGESHWAR |
– |
– |
– |
+ |
– |
+ |
|
CHAMPAWAT |
– |
– |
– |
– |
+ |
– |
||
|
PITHORAGARH |
++ |
– |
– |
++ |
– |
+ |
||
+ indicates present, and – indicates absent.
FTIR analysis
The FT-IR spectrum was applied for the identification of active components through functional groups present in the extract, which is totally based on the peak values in the region of IR radiation. In this study, extracts in the form of slurry were passed into the FT-IR, and the functional groups of the components were separated based on their peak ratios. The presence of N-H, O-H, C=C, C-H, C-O, and CH3 functional groups was confirmed by FT-IR analysis (Table 3). FTIR spectroscopy has proven to be a reliable and sensitive method for the detection of biomolecular composition.
Table 3 FTIR analysis of methanolic fruit and extract of Zanthoxylum armatum from different places in Uttarakhand
Table 3A: Pithoragarh (Methanolic Fruit Extract)
|
Absorption (cm-1) |
Appearance |
Group |
Compound Expected |
|
3636.96 |
Medium, sharp |
O-H stretching |
Alcohol |
|
3383.82 |
Medium |
N-H stretching |
Aliphatic primary amine |
|
2990.51 |
Medium |
C-H stretching |
Alkane |
|
2464.53 |
Strong |
O=C=O stretching |
Carbon dioxide |
|
2263.07 |
Strong, broad |
N=C=O stretching |
isocyanate |
|
2016.14 |
Strong |
N=C=S stretching |
Isothiocyanate |
|
1634.14 |
Medium |
C=C stretching |
Alkene |
|
1223.77 |
Strong |
C-O stretching |
Alkyl aryl ether |
|
806.11 |
Medium |
C=C bending |
Alkene |
|
Pithoragarh (Seed Methanolic Seed Extract) |
|||
|
Absorption (cm-1) |
Appearance |
Group |
Compound Expected |
|
3705.32 |
Medium, sharp |
O-H stretching |
Alcohol |
|
3506.80 |
Strong, broad |
O-H stretching |
Alcohol |
|
3165.19 |
Strong, broad |
O-H stretching |
Carboxylic acid |
|
2921.48 |
Strong, broad |
N-H stretching |
Amine salt |
|
2537.59 |
Weak |
S-H stretching |
Thiol |
|
2160.92 |
Strong |
O=C=O stretching |
Carbon dioxide |
|
1801.58 |
Weak |
C@ C stretching |
Alkyne |
|
1428.96 |
Medium |
C-H bending |
Aldehyde |
|
872.61 |
Strong |
C=C bending |
Alkene |
Table 3B: Bageshwar (Methanolic Fruit Extract)
|
Absorption (cm-1) |
Appearance |
Group |
Compound Expected |
|
3607.17 |
Medium, sharp |
O-H stretching |
Alcohol |
|
3260.98 |
Strong, broad |
O-H stretching |
Alcohol |
|
2834.67 |
Medium |
C-H stretching |
Alkane |
|
2606.65 |
Weak |
S-H stretching |
Thiol |
|
2339.62 |
Strong |
O=C=O stretching |
Carbon dioxide |
|
2197.75 |
Weak |
C@ C stretching |
Alkyne |
|
1859.34 |
Weak |
C-H bending |
Aromatic compound |
|
1680.89 |
Strong |
C=O stretching |
Secondary amide |
|
917.80 |
Strong |
C=C bending |
Alkene |
|
Bhageshwar (Methanolic Seed Extract) |
|||
|
Absorption (cm-1) |
Appearance |
Group |
Compound Expected |
|
3501.07 |
Medium, sharp |
O-H stretching |
Alcohol |
|
3057.94 |
Medium |
O-H stretching |
Alcohol |
|
2822.98 |
Medium |
C-H stretching |
Aldehyde |
|
2743.72 |
Medium |
C-H stretching |
Aldehyde |
|
1982.89 |
Weak |
C=C=C stretching |
Alkene |
|
1743.96 |
Strong |
C=O stretching |
Aldehyde |
|
1555.38 |
Medium |
C-H bending |
nitro compound |
Table 3: Champawat (Methanolic Fruit Extract).
|
Absorption (cm-1) |
Appearance |
Group |
Compound Expected |
|
3960.57 |
Medium, sharp |
O-H stretching |
Alcohol |
|
3076.49 |
Strong broad |
O-H stretching |
Carboxylic acid |
|
2750.67 |
Medium |
C-H stretching |
Aldehyde |
|
2525.30 |
Weak |
S-H stretching |
Thiol |
|
2260.75 |
Strong broad |
N=C=O stretching |
Isocyanate |
|
1732.51 |
Strong |
C=O stretching |
Aldehyde |
|
1481.43 |
Medium |
C-H bending |
Alkane |
|
1250.01 |
Strong |
C-O stretching |
Aromatic amines |
|
857.72 |
Strong |
C-Cl stretching |
Halo compound |
|
Champawat (Methanolic Seed Extract) |
|||
|
Absorption (cm-1) |
Appearance |
Group |
Compound Expected |
|
3697.66 |
Medium, sharp |
O-H stretching |
Alcohol |
|
3364.79 |
Medium |
N-H stretching |
Primary amine |
|
2923.50 |
Strong broad |
N-H stretching |
Amino salt |
|
2738.31 |
Medium |
C-H stretching |
Aldehyde |
|
2040.80 |
Strong |
N=C=S stretching |
Isocyanate |
|
1724.80 |
Weak |
C-H bending |
Aromatic compound |
|
1344.54 |
Medium |
O=H bending |
Alcohol |
|
1078.04 |
Strong |
C=O bending |
Primary Alcohol |
|
757.23 |
Strong |
C=H bending |
1,3 disubstitute |
Antioxidant effect
As shown in Fig. 1, it may indicate that methanolic extract has a significant amount of free radical scavenging activity, especially as reported from the samples of Bageshwar, followed by Pithoragarh and Champawat. In comparison to the methanolic extract, ethyl acetate and n-hexane extracts of Zanthoxylum armatum fruit and seeds showed less antioxidant activity.
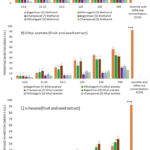 |
Figure 1: Antioxidant activity of fruit and seed extract from Zanthoxylum armatum. |
Total Phenolic and flavonoid content
To estimate the phenolic and flavonoid content of methanolic, ethyl acetate, and n-hexane extracts from fruits and seeds of Zanthoxylum armatum as shown in Table 4. These studies revealed the existence of higher phenolic and flavonoid content in methanolic extract as compared to ethyl acetate and n-hexane extract. Gallic acid (total phenolics) and rutin (total flavonoid) were used as standards for these studies.
Table 4: Estimation of total phenolic and total flavonoid content of Zanthoxylum armatum fruit and seed extracts
|
Extract |
Location |
FRUITS |
SEEDS |
||||
|
Total |
Total |
Extraction |
Total |
Total |
Extraction |
||
|
Methanolic extract |
BAGESHWAR |
142.4 ± 2.78*** |
64.8 |
14.6 ± 1.4** |
84.6 |
38.6 |
6.8 |
|
CHAMPAWAT |
104.6 |
52.4 |
14.1 ± 1.2** |
82.2 |
29.4 |
7.1 |
|
|
PITHORAGARH |
114.2 |
66.2 |
13.8 ± 0.9** |
86.8 |
29.0 |
6.6 |
|
|
Ethyl acetate extract |
BAGESHWAR |
56.8 |
31.4 |
9.78 |
14.2 |
18.0 |
3.62 |
|
CHAMPAWAT |
67.2 |
22.4 |
7.12 |
12.6 |
19.7 |
4.4 |
|
|
PITHORAGARH |
39.6 |
26.6 |
8.16 |
16.7 |
16.4 |
2.7 |
|
|
n-hexane extract |
BAGESHWAR |
46.2 |
18.8 |
7.4 |
14.2 |
6.6 ± |
2.8 |
|
CHAMPAWAT |
52.4 |
16.6 |
9.2 |
10.8 |
8.2 |
3.1 |
|
|
PITHORAGARH |
38.6 |
22.2 |
8.4 |
16.8 |
10.2 |
4.9 |
|
Each value is represented as Mean ± S.E. Statistical analysis were formed using one-way ANOVA test (*P<0.05; **P<0.01 and ***P<0.001)
Antimicrobial activity
The effect of methanolic, ethyl acetate, and n-hexane extracts from fruits and seeds of Zanthoxylum armatum is shown in Fig.2 and Table 5. These studies may suggest that methanol and n-hexane fruit and seed extract showed inhibition in Bageshwar samples, followed by Pithoragarh and Champawat against bacterial (Pseudomonas aeruginosa, Staphylococcus aureus, and E. coli) strains as compared to control. Methanol, ethyl acetate, and n-hexane were used as negative controls for these studies and showed no toxic effect against these bacterial strains.
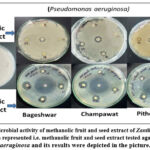 |
Figure 2: Antimicrobial activity of methanolic fruit and seed extract of Zanthoxylum armatum. One set of data is represented i.e. |
Table 5: Antimicrobial activity of Zanthoxylum armatum fruit and seed extracts
|
Micro-organisms |
Location |
FRUITS (32 µl i.e. 4:1 (Inhibition zone |
SEEDS (32 µl i.e. 4:1 (Inhibition zone |
||||
|
Methanol |
Ethyl acetate |
n-Hexane |
Methanol |
Ethyl acetate |
n-Hexane |
||
|
Pseudomonas aeruginosa |
BAGESHWAR |
11.6 |
8.14 |
9.34 |
9.8 |
6.24 |
9.06 |
|
CHAMPAWAT |
9.84 |
7.68 |
8.12 |
7.98 |
5.74 |
7.24 |
|
|
PITHORAGARH |
10.4 |
8.02 |
9.06 |
8.24 |
6.0 |
6.94 |
|
|
Staphylococcus aureus |
BAGESHWAR |
13.4 |
7.98 |
9.56 |
9.22 |
6.24 |
5.4 |
|
CHAMPAWAT |
11.04 |
6.86 |
8.32 |
8.12 |
5.10 |
5.26 |
|
|
PITHORAGARH |
12.2 |
7.64 |
9.14 |
7.94 |
5.78 |
6.12 |
|
|
E. coli |
BAGESHWAR |
8.2 |
7.0 |
7.88 |
8.84 |
5.14 |
5.06 |
|
CHAMPAWAT |
7.64 |
6.54 |
6.24 |
7.56 |
4.86 |
5.34 |
|
|
PITHORAGARH |
7.94 |
6.12 |
6.94 |
7.32 |
5.0 |
4.98 |
|
The discs were tagged and incubated for 24 hours at 37 °C after being dried and set in proximity to the bases of the plates containing the organisms. Each value is represented as Mean ± S.E. *P<0.05; **P<0.01 and ***P<0.001
Discussion
Herbal medicines have been used to treat illness symptoms since very ancient times. Despite the significant advancements in modern medicine over the past few decades, plants continue to play a significant role in healthcare. The lengthy history of medicinal plants’ usage in traditional medicine and their preventive qualities, particularly in poor nations, are what have generated so much interest in them. The antioxidant and antimicrobial activities of a large range of medicinal plants have been studied. Natural antioxidants, whether in the form of unprocessed substances or their chemical components, are particularly powerful at stopping the harmful effects of oxidative stress.8, 12, 15 To reduce the danger of infectious diseases brought on by bacteria, parasites, viruses, and fungi that are pathogenic to humans, one of the most active areas of research has been the quest for compounds with highly antimicrobial action. Plant extracts continue to be the primary source of many medicinal substances, such as antibiotics used to treat infectious disorders. Even though the toxic profile of the majority of medicinal plants has not been thoroughly examined, it is widely agreed that drugs made from plant components are safer than those made from synthetic materials.
The antioxidant activity of 2, 2-diphenyl-1-picrylhydrazylhydrate is measured using DPPH. It is a stable free radical that transforms into something like a steady diamagnetic molecule by accepting an electron or hydrogen. Antioxidants interact with DPPH in the DPPH radical assay, turning it into diphenylpicrylhydrazine, which is yellow in colour. 8, 12 The degree of colour fading indirectly demonstrates the antioxidant’s ability to scavenge free radicals. The reduction of the absorbance at 517 nm is used to measure the antioxidants’ effectiveness in reducing the DPPH radical. The reduction in DPPH radical absorbance brought on by antioxidants is the consequence of a reaction among antioxidant molecules as well as radical production, which scavenges the radical by donating hydrogen. In this study, we worked on one of the medicinal plants, Zanthoxylum armatum, and studied its antioxidant effect (DPPH assay) on fruits and seeds using three different solvent systems from different regions of Uttarakhand, i.e., Bageshwar, Pithoragarh, and Champawat. These studies revealed that methanolic extracts of fruits and seeds showed a higher antioxidant effect, especially in the Bageshwar region as compared to Pithoragarh and Champawat. Similar patterns were also obtained from ethyl acetate and n-hexane extracts, but they showed a comparatively weaker antioxidant effect as compared to the methanolic extract of Bageshwar. In short, these antioxidants are known to be abundant in several plant-based foods. Phytonutrients, sometimes known as plant-based nutrients, include plant-based antioxidants. Antioxidants referred to as endogenous antioxidants are also produced by the body.15, 16 Exogenous antioxidants are those that originate outside the body. Free radicals are byproducts that cells create as they digest food and respond to their surroundings. Oxidative stress can occur if the body is unable to effectively eliminate and process free radicals. Cells and physiological functions may be harmed by this. In other words, antioxidants are supposed to aid in scavenging free radicals from our bodies, which is thought to improve general health.15, 16
The search for antimicrobials derived from plants has accelerated in recent years as bacterial resistance to anti-infection medicines evolves and new, therapeutically useful anti-microbials fail to emerge.14, 17 To assess or screen the in vitro antimicrobial activity of an extract or a pure chemical, a range of laboratory techniques can be applied. The disk-diffusion and broth or agar dilution procedures are the most well-known and fundamental techniques. Other techniques, such as the technique of poisoned food, are used specifically for antifungal testing.14, 17 The disc diffusion technique (DDM) is also referred to as the agar diffusion method (ADM) because the plant concentrate (i.e., Zanthoxylum armatum) using 4 dilutions of methanolic, ethyl acetate, and n-hexane extract that will be tested diffuses through the agar medium that has been grown with the test microorganism as it comes into contact with the supply. A channel-shaped paper circle that is placed on top of an agar surface serves as the repository for the most part. After brooding, a constraint zone develops around the channel paper plate, assuming the tested plant extricates or separates compounds that are microbiologically dynamic. The width of the restraint zone accurately represents the antibacterial effectiveness of plant concentrates or specific mixes. These studies revealed that the methanolic extract of this plant from Bageshwar, followed by Pithoragarh, and then Champawat, showed higher antimicrobial activity as compared to the control. Similar results were also obtained from ethyl acetate and n-hexane extract.
Conclusion
Methanolic fruit and seed extract of Zanthoxylum armatum has some capacity to inhibit the growth of bacteria.It also exhibited good antioxidant activity and has the capability to be effective against many illnesses. Further investigations are needed in order to get an effective remedy against the resistant bacterial strains.Additionally, many phytochemical researchers have neglected bioactivity screening related to ethnopharmacological uses. Thus, there is much additional work that can be carried out to identify phytochemicals associated with biological activities that support traditional uses of medicinal plants.
Acknowledgement
We acknowledge the support of the Department of Microbiology, Graphic Era (Deemed to be) University for providing requisites and all the help in conducting and submitting this research article.
Conflict of Interest
There were no commercial or financial links that may be deemed a potential conflict of interest during the research.
Funding Sources
The authors received no financial support for the research.
References
- White AE, Dey KK, Mohan D, Stephens M, Price TD. Regional influences on community structure across the tropical-temperate divide. Nat Commun 2019; 10: e2646.
CrossRef - Khan M, Kumar S, Hamal IA. Medicinal plants of Sewa River catchment area in the northwest Himalaya and its implication for conservation. Ethnobot Leafl 2009; 13:1113–1139.
- Amarowicz R, Estrella I, Hernandez T, Robredo S, Troszynska A, Kosinska A, Pegg RB. Free radical-scavenging capacity, antioxidant activity, and phenolic composition of green lentil (Lens culinaris). Food Chem 2010; 121:705–711.
CrossRef - Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and praline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 2005; 91:571–577.
CrossRef - Subhan F, Karim N, Ibrar M. Anti-inflammatory activity of methanolic and aqueous extracts of Valeriana wallichii DC rhizome. Pak J Plant Sci 2007; 13:103–108.
- Phuyal N, Jha PK, Raturi PP, Rajbhandary S. Zanthoxylum armatum DC.: current knowledge, gaps and opportunities in Nepal. J Ethnopharmacol 2019; 229:326–341.
CrossRef - Mushtaq MN, Ghimire S, Akhtar MS, Adhikari A, Auger C, Schini-Kerth VB. Tambulin is a major active compound of a methanolic extract of fruits of Zanthoxylum armatum DC causing endothelium-independent relaxations in porcine coronary artery rings via the cyclic AMP and cyclic GMP relaxing pathways. Phytomedicine 2019; 53:163–170.
CrossRef - Kala CP, Farooquee NA, Dhar U. Traditional uses and conservation of timur (Zanthoxylum armatum DC.) through social institutions in Uttaranchal Himalaya, India. Conserv Soc 2005; 3(1): 224.
- Singh TP, Singh OM. Phytochemical and pharmacological profile of Zanthoxylum armatum DC. —an overview. Indian J Nat Prod Res 2011; 2(3): 275–85.
- Rynjah CV, Devi NN, Khongthaw N, Syiem D, Majaw S. Evaluation of the antidiabetic property of aqueous leaves extract of Zanthoxylum armatum DC. Using in vivo and in vitro approaches. J Trad Compl Med 2018; 8 (1):134–40.
CrossRef - Karki H, Upadhayay K, Pal H, Singh R. Antidiabetic potential of Zanthoxylum armatum bark extract on streptozotocin-induced diabetic rats. Intern J Green Pharm 2014;8(2):77.
CrossRef - Joshi SC, Verma AR, Mathela C.S. Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. Food Chem Toxicol 2010; 48:37–40.
CrossRef - Bhatt ID, Dauthal P, Rawat S, Gaira KS, Jugran A, Rawal RS, Dhar U. Characterization of essential oil composition, phenolic content, and antioxidant properties in wild and planted individuals of Valeriana jatamansi. Jones Sci Hortcult 2012; 136:61–68.
CrossRef - Setzer WN, Vogler B, Schmidt JM, Leahy JG, Rives R. Antimicrobial activity of Artemisia douglasiana leaf essential oil. Fitoterapia 2004; 75:192–200.
CrossRef - Loizzo MR, Tundis R, Conforti F, Saab AM, Statti GA, Minichini F. Comparative chemical composition, antioxidant and hypoglycaemic activities of Juniperus oxycedrus ssp. oxycedrus L. berry and wood oils from Lebanon. Food Chem 2007; 105:572–578.
CrossRef - Bora KS, Sharma A. The genus Artemisia: A comprehensive review. Pharm Biol 2011; 49:101–109.
CrossRef - Dorman HJD, Deans SG. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J Appl Microbiol 2000; 88:308–316.
CrossRef








