Ni Putu Widya Astuti1,2*  , Ida Bagus Putra Manuaba3
, Ida Bagus Putra Manuaba3 , I Made Jawi4
, I Made Jawi4 , Anak Agung Bawa Putra3
, Anak Agung Bawa Putra3 , Putu Angga Wiradana5
, Putu Angga Wiradana5 , I Gede Widhiantara5
, I Gede Widhiantara5 , Anak Agung Ayu Putri Permatasari5
, Anak Agung Ayu Putri Permatasari5 , Arif Nur Muhammad Ansori6
, Arif Nur Muhammad Ansori6 , and Viol Dhea Kharisma7
, and Viol Dhea Kharisma7
1Doctoral Study Program, Faculty of Medicine, Universitas Udayana, Denpasar City, Bali Province (80232), Indonesia
2Study Program of Public Health, Faculty of Health, Science, and Technology, Universitas Dhyana Pura, Jalan Raya Padangluwih, Dalung, North Kuta, Badung Regency, Bali Province (80351), Indonesia
3Department of Chemistry, Faculty of Mathematical and Natural Sciences, Universitas Udayana, Badung Regency, Bali Province (80361), Indonesia
4Department of Pharmacology, Faculty of Medicine, Universitas Udayana, Jalan P.B. Sudirman, Dangin Puri Klod, Denpasar City, Bali Province (80232), Indonesia
5Research Group of Biological Health, Study Program of Biology, Faculty of Health, Science, and Technology, Universitas Dhyana Pura, Jalan Raya Padangluwih, Dalung, North Kuta, Badung Regency, Bali Province (80351), Indonesia
6Postgraduate School, Universitas Airlangga, Kampus B, Jalan Airlangga, Surabaya, East Java (60286), Indonesia
7Study Program of Biology, Faculty of Science and Technology, Universitas Airlangga, Kampus C, Jln. Dr. Ir. H. Soekarno, Mulyorejo, Surabaya, East Java (60115), Indonesia
Corresponding Author E-mail: widyaastuti@undhirabali.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2929
Abstract
Traditional herbal remedies have an important role in human health. Empirically, Blumea balsamifera is often used as a traditional beverage to alleviate fever symptoms, lower cholesterol levels, and maintain body immunity. The purpose of this study was to discover the phytoconstituent profile that contributes to the anti-diabetic properties of B. balsamifera leaf extract (BBLE) using in silico approaches.LCMS/MS was used to identify the constituent profile of BBLE, and the ability of these compounds against diabetes-related proteins was analyzed computationally.Three proteins related to diabetes are NF-KB p65, GLP-1, and DPP-4. A total of 18 compounds were successfully identified through LCMS/MS, including 4 compounds known to be flavonoid derivatives and can be used as markers of BBLE. Pheophorbide A and 1,1-Cyclopentanediacetic acid were reported for the first time to inhibit the NF-KB p65, GLP-1, and DPP-4 proteins in docking simulation studies. Based on these findings, it can be confirmed that the bioactive compounds in BBLE show strong inhibitory potential against anti-diabetic proteins.
Keywords
Antidiabetic activity; Blumea balsamifera; In Silico; LCMS/MS; Phytoconstituent
Download this article as:| Copy the following to cite this article: Astuti N. P. W, Manuaba I. B. P, Jawi I. M, Putra A. A. B, Wiradana P. A, Widhiantara I. G, Permatasari A. A. A. P, Ansori A. N. M, Kharisma V. D. Phytoconstituents Analysis and Anti-Diabetic Potential of Sembung Leaf Extract (Blumea balsamifera L. DC.) through Inhibition of NF-KB p65, GLP-1, and DPP-4 Proteins with In-Silico Approaches. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Astuti N. P. W, Manuaba I. B. P, Jawi I. M, Putra A. A. B, Wiradana P. A, Widhiantara I. G, Permatasari A. A. A. P, Ansori A. N. M, Kharisma V. D. Phytoconstituents Analysis and Anti-Diabetic Potential of Sembung Leaf Extract (Blumea balsamifera L. DC.) through Inhibition of NF-KB p65, GLP-1, and DPP-4 Proteins with In-Silico Approaches. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3XqVZcv |
Introduction
Diabetes mellitus is a widespread metabolic condition in which glucose levels in the blood rise due to beta cells’ inability of producing enough insulin (Type-1) or ineffective release of insulin (Type-2). Both are caused by insulin resistance, production issues, or both, and alter the way proteins, lipids, and carbohydrates are synthesized1. Diabetes raises the risk of amputation, renal failure, stroke, cardiovascular disease, and lifelong blindness.Diabetes is influenced by genetic and environmental variables, and the incidence rate varies among ethnic groups and specific communities2. According to estimates, diabetes affected about 463 million people globally in 20193.
Interestingly, urban areas have a higher incidence rate of 10.8% compared to rural areas at 7.2%. Similarly, high-income nations have a greater frequency of 10.4% than nations with low incomes at 4% 3. Despite the fact that extensive research has been conducted to control diabetes and a number of oral medications, gene therapies, and stem cell therapies have been successfully implemented, the development of new diabetes medications through the search for chemical compounds with specific bioactive features that help manage glucose homeostasis and improve insulin sensitivity is necessary4.
Antioxidants have an important function in the treatment of disorders like diabetes mellitus 5. This is because the formation of free radicals in cells due to oxidative stress exposure has been linked to the occurrence of diabetes, especially Type-26. Natural compounds have attracted considerable attention over the past few decades for their biological and pharmacological properties as sources of antioxidants7. Natural compounds are widely known to provide anti-inflammatory, anti-cancer, anti-bacterial, anti-fungal, and anti-viral activities8. Since they have been scientifically proven to exhibit hypoglycemic activity, these active compounds are of great interest for exploration in the development of new diabetes medications.
Indonesia is a center of mega-biodiversity for medicinal plants in the Asia region, and their traditional use is employed by many ethnic community societies across various regions in Indonesia to support their health needs9. The Sembung plant (Blumea balsamifera) is one of the plants used in traditional medicine practices as an anti-hypercholesterol10, anti-bacterial11, anti-cancer12, anti-neuroinflammation13, gastroprotectant14, and as a source of natural antioxidants15. Empirically, in the Bali Province, B. balsamifera can be processed into a health drink known as “Loloh,” which is considered capable of maintaining immune function. Previous reports revealed that the extract of B. balsamifera (BBLE) contains a profile of secondary metabolites such as flavonoids, saponins, phenols, tannins, and steroids16. Apart from Indonesia, several regions around the world also utilize plants from the Blumea species as traditional medicine. For example, in China, dried preparations of Blumea riparia have been standardized and used to treat irregular menstruation, postpartum hemorrhage, infertility, and vulva wounds. These plants are also commercialized because their phenolic compounds, flavonoids, acetylenes, and sesquiterpenes are considered to play a crucial role in health17,18.
However, so far, reports on the metabolite profile of BBLE aimed at anti-diabetic purposes are very limited. Phytochemical screening of Blumea spp. is crucial, given their wide abundance in the wild, and the morphological similarity of the B. balsamifera species with other Blumea spp. such as B. megacephala and B. riparia19. As a result, the aim of this research is to identify the secondary metabolites of BBLE using High Performance Liquid Chromatography/Mass Spectrometry (HPLC/MS) investigations and discover its anti-diabetic mechanism by in silico analysis. This study is valuable for developing new biologically active molecules, especially for controlling diabetes through the inhibition of related proteins.
Materials and methods
Preparation of crude extract
Fresh Sembung (B. balsamifera) leaves were collected from a plantation in Luwus Village, Tabanan Regency, Bali Province. Types of B. balsamifera L. (DC.) leaf samples were determined at the Bali Botanical Gardens, National Research and Innovation Agency (BRIN) with Sample Registration Number B. 206/IPH.7/AP/VIII/2020. The same kind of extract had been used in our previous publication16. Sembung leaves were then cleaned of organic material or contaminants using running water several times. The samples were then air-dried at room temperature to reduce moisture content and subsequently dried for 24 hours at 50℃ using an oven, resulting in dried samples or simplicia for further processing. The simplicia were then ground using a blender and sieved through a 20 mesh screen to obtain a powdered sample.
A total of 250 grams of sembung leaf powder preparation was weighed and moistened using 70% ethanol solvent, then left for 4 hours in a glass container covered with sterile gauze and wrapped in plastic. The maceration process was carried out for 24 hours, and during this process, stirring was performed to evenly extract the metabolites. The macerate was then separated by filtration method using sterile flannel cloth. The filtration process was performed three times with the same type and amount of solvent.The collected macerate was evaporated using a vacuum rotary evaporator at 40℃ and 100 rpm until a viscous extract was produced.This viscous extract of sembung, referred to as BBLE in this study, was adjusted according to the standards of the Indonesian Herbal Pharmacopoeia of 2017, which includes a yield of not less than 10.6%, flavonoid/quercetin identity compound (+), moisture content of no more than 14%, and total ash of no more than 6.7%10,20.
Identification of the phytochemical from BBLE
The chemical profile of BBLE was identified and quantified using the pure compound as a reference by High Performance Liquid Chromatography (HPLC) (LC: ACQUITY UPLC® H-Class System, Waters, USA) and mass spectrometry (MS) (Xevo G2-S QTof, Waters, USA). Briefly, an AC18 column measures 1.8 μm 2.1 × 100 mm at 50°C (column) and 25°C (ambient temperature). HPLC analysis used a mobile phase of water + 5 mM ammonium formate and acetonitrile + 0.05% formic acid, with a flow rate of 0.2 mL/min running for 23 minutes (mobile phase) and an injection volume of 5 μL. Mass spectrometer (MS) investigations were carried out using electrospray ionization in positive mode with a mass range of 50–1200 m/z and source and dissolution temperatures of 100 and 350°C. The conical gas and dissolution flows were 0 L/h and 793 L/h, respectively, with collision energies ranging from 4 to 60 eV. MassLynx software version 4.1 was used for data collection, analysis, and instrument control21–23.
In silico analysis
Molecular docking analysis
The phytochemical profile, consisting of 18 compounds detected by BBLE extract analysis with HPLC/MS, was used in molecular docking studies. The PubChem database was utilized to obtain compound names, PubChem IDs, molecular weights, and structures of the obtained compounds24. ADMET profiling was carried out for assessing the pharmacokinetic characteristics of the compounds utilizing online pkCSM and Swiss ADME 25–27.The 3D structures of each compound were downloaded in .sdf format, and the respective proteins were obtained from the RCSB PDB website. NF-kB p65, Glucagon-like peptide (GLP-1), and Dipeptidyl peptidase-4 (DPP-IV) were identified as potential target receptors.Analysis was performed using PyRx 0.8 software with specific coordinates corresponding to the active site of each protein. The strength of the bond between the ligand and protein was measured based on binding energy and RMSD from the molecular docking results. The smaller/negative the binding energy value, the more stable the bond between the ligand and protein.
Ligand-Protein Interaction Analysis
The types of chemical bond interactions formed in the ligand-protein complex were further analyzed using Discovery Studio software. This analysis aims to identify the position of the active site and the amino acids that bond with the compounds through hydrogen bonding.
Druglikeness and Toxicity Analysis
The druglikeness analysis of compounds in sembung leaves was conducted using the websitehttp://www.swissadme.ch/index.php by copying the Simplified Molecular Input Line Entry System (SMILES) of each compound. Subsequently, the toxicity (LD50) of the compounds was assessed using the websitehttps://pubchem.ncbi.nlm.nih.gov/compound/.
Bioactivity Analysis with PASS Online Server
The bioactivity analysis of compounds contained in sembung leaves was performed by copying the Simplified Molecular Input Line Entry System (SMILES) notation of each compound onto the website https://www.way2drug.com/PassOnline/predict.php. The Pa (probability of activity) and Pi (probability of inactivity) values were determined for each ligand. Finally, only activities involved in diabetes were considered28.
Results and Discussion
Chemical profiling
The phytochemical profiling results of BBLE using HPLC/MS revealed the presence of 18 chemical compounds at various retention times (RT) (Table 1). The compound 2,3-Dihydroxypropyl (9Z,12Z,15Z) – 9,12,15-octadecatrienoate (C21H36O4) with an RT of 11.61 had the highest peak area percentage of 20.89%. Similarly, the compound 2-Hexyl-3,5-dipentylpyridine (C21H37N) with an RT of 11.80 had a peak area percentage of 20.89%. The BBLE chromatogram in this study is shown in Figure 1 below.
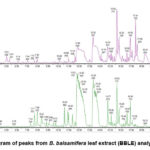 |
Figure 1: Chromatogram of peaks from B. balsamifera leaf extract (BBLE) analyzed using LCMS/MS. |
The chemical 2,3-Dihydroxypropyl, also called Octadecatrienoic Acid, is an a link in the production of arachidonic acid and an important component of essential oils in plants 29. Reports indicate that conjugated Octadecatrienoic Acid can cause a decrease in the viability of LNCaP and PV-3 (human prostate cancer cells) cells, depending on the concentration, but is not toxic to normal human prostate epithelial cells RWPE-1, which are normal epithelial cells30.
Table 1: Phytoconstituent content of B. balsamifera leaf extract (BBLE) characterized by LCMS/MS.
|
Retention Time |
m/z Result (M+H) |
Compound Prediction |
%IFIT |
Peak Area |
||
|
Precursor Ion |
Product Ion |
Area |
% |
|||
|
7,71 |
361,0927 |
346,0688 |
3′,4′,5-Trihydroxy-6,7,8-trimethoxyflavone (C18H16O8) |
99 |
3702571 |
2.08 |
|
7,85 |
132,0817 |
105,0707, 91,0553 |
3-Methyl-1H-indol (C9H9N) |
97 |
5378427 |
3.02 |
|
8,86 |
375,1090 |
342,1925 233,1551 |
3′,5-Dihydroxy-3,4′,6,7-tetramethoxyflavone (C19H18O8) |
100 |
4368431 |
2.45 |
|
10,59 |
389,1252 |
353,2695, 261,2227, 243,2121 |
5-Hydroxy-3′,4′,6,7,8-pentamethoxyflavone (C20H20O8) |
99 |
4655888 |
2.61 |
|
11,34 |
359,1133 |
343,0828, 316,2856, 283,0616 |
3,3′,4′,7-Tetramethylquercetin (C19H18O7) |
99 |
3238175 |
1.82 |
|
11,61 |
353,2695 |
335,2593, 261,2227, 243,2121 |
2,3-Dihydroxypropyl (9Z,12Z,15Z)-9,12,15-octadecatrienoate (C21H36O4) |
99 |
37204892 |
20.89 |
|
11,80 |
304,3012 |
212,2384 |
2-Hexyl-3,5-dipentylpyridine (C21H37N) |
99 |
37204892 |
20.89 |
|
12,06 |
408,2378 |
318,3161, 304,3003, 231,1388 |
2-(2,6-Dimethyl-1-piperidinyl)-2-oxoethyl 3,4,5-triethoxybenzoate (C22H33NO6) |
90 |
|
17.58 |
|
13.36 |
332,3322 |
240,2699 |
N,N-Dimethylpregnan-3-amine (C23H41N) |
100 |
31302042 |
4.31 |
|
14.10 |
607,2572 |
91,0554 |
(3S)-3-[(2E)-3-Carboxy-2-buten-1-yl]-7-hydroxy-4-methoxy-1,1,8,8,9-pentamethyl-11-(3-methyl-2-buten-1-yl)-6-oxo-3,6,8,9-tetrahydro-1H-difuro[3,2-b:3′,4′-h]xanthene – 3-carboxylic acid (C34H38O10) |
71 |
7671267 |
4.31 |
|
14,74 |
360,3631 |
304,3004 |
n-benzyloctadecylamine (C25H45N) |
100 |
7671762 |
1.63 |
|
15,07 |
609,2714 |
360,3630 |
2-[4-(Diphenylmethyl)-1-piperazinyl]ethyl methyl 2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate (C35H36N4O6) |
99 |
2894592 |
1.63 |
|
15.14 |
625,2670 |
609,2716 |
(1S,5S)-1,5-Anhydro-2,3-di-O-benzyl-4-deoxy-5-(2-methoxy-2-oxoethyl)-1-[(6R,9R)-6-methyl-4,11-dioxo-3,5,10,12-tetraoxapentadec-14-en-7-yn-9-yl]-L-threo-pentitol (C34H40O11) |
99 |
2895590 |
6.80 |
|
15,62 |
593,2755 |
368,4253 |
Pheophorbide A (C35H36N4O5) |
99 |
12109066 |
2.30 |
|
16,13 |
593,2759 |
535,2703, 332,3317, 304,3007 |
Bis[2-(4-butoxyphenoxy)ethyl] (4-hydroxybenzylidene)malonate (C34H40O9) |
95 |
4089161 |
2.30 |
|
16,28 |
611,4679 |
458,4736, |
1,1-Cyclopentanediacetic acid(C39H62O5) |
86 |
4089110 |
2.29 |
|
16,62 |
637,3012 |
– |
N’1,N’9-Bis[(4-biphenylyloxy)acetyl]nonanedihydrazide (C37H40N4O6) |
99 |
4076512 |
3.11 |
|
16,92 |
621,3037 |
486,5033 |
N-{[(3S)-2-(L-Tyrosyl)-1,2,3,4-tetrahydro-3-isochinolinyl]methyl}-L-phenylalanyl-L-phenylalanin (C37H40N4O5) |
99 |
5534532 |
2.08 |
The compound 2-Hexyl-3,5-dipentylpyridine was also previously found in mango peel waste extract analyzed using LC-MS with a retention time (RT) of 19.266 minutes and a molecular weight of 303.29137 g/mol31. This compound is capable of inhibiting the ACE2 receptor, which is a membrane protein on alveolar cells acting as an entry point for viruses into the human body32–34. The compound 2-Hexyl-3,5-dipentylpyridine was successfully identified in the extract of the tuber of Merremia mammosa with bioactivities as an antiviral, antioxidant, anti-inflammatory, and anti-tuberculosis agent35.
In addition to those three compounds, there are phytochemical compounds identified in BBLE that belong to the flavonoid group, such as 3′,4′,5-Trihydroxy-6,7,8-trimethoxyflavone (TTF); 3′,5-Dihydroxy-3,4′,6,7-tetramethoxyflavone (TMF); 5-Hydroxy-3′,4′,6,7,8-pentamethoxyflavone (PMF); and 3,3′,4′,7-Tetramethylquercetin (TMQ). Compounds from the flavonoid group have been reported as standardization markers for BBLE phytochemistry and are known for their role as sources of natural antioxidants with various important bioactivities10,36. Similar research revealed that the compound 3,5,4′-trihydroxy-6,7,3′-trimethoxyflavone (TTF) isolated from Achillea fragrantissima extract could prevent cell damage due to oxidative stress and inhibit protein phosphorylation that signals cells, including the mitogen-activated protein kinase (MAPK) family37. Compounds from this flavonoid group were also found in Loranthus acutifolius extract with antityrosinase bioactivity38.
The compound 3′,5-Dihydroxy-3,4′,6,7-tetramethoxyflavone (TMF), also known as Casticin, is a bioactive compound that can be found in various parts of plants. Casticin’s therapeutic properties include anti-tumor, anti-inflammation, neuroprotective activity, and natural analgesic39. Casticin isolated from Larrea tridentata has shown antibacterial activity against Mycobacterium tuberculosis , including sensitive and multidrug-resistant strains40. The compound 5′ – Hydroxy -6, 7,8,3′,4′-pentamethoxyflavone (PMF), isolated from the mandarin orange Citrus reticulata, has anti-inflammatory properties and modulates immune function. Recent studies also report the effect of this compound in preventing psoriasis, a chronic and benign proliferative skin disease, through the regulation of several gene expressions related to immunity and inflammation41.
The compound 3,3′,4′,7-Tetramethylquercetin (TMQ) is one of the parent compounds expected to be found in the phenolic hydroxyl groups within quercetin. The bioactivity demonstrated by TMQ includes acting as an anti-prostate cancer agent through the activation of apoptosis in PC-3 cells42. On the other hand, this quercetin derivative is capable of multi-drug resistance as well as human breast cancer cells (MCF-7) by inhibiting the activity of TrxR, which activates cell death through apoptosis43. TMQ has also been reported in extracts from Cissus quadrangularis extracted with various solvents44.
Ligand Compounds
Phytochemical compounds acting as ligands must meet inclusion criteria that fulfill both pharmacological and pharmacodynamic criteria. Based on their similarity as drug candidate materials, each compound successfully identified using HPLC/MS is used as a ligand compound in this study (Table 2).
Table 2: Samples of ligand compounds from BBLE accessed from the PubChem database and SMILES notation
|
No. |
Compounds |
PubChem ID |
SMILE |
|
1 |
3′,4′,5′-Trihidroxy-6,7,8-trimethoxyflavone |
6453535 |
COC1=C(C=CC(=C1) |
|
2 |
3-Methyl-1H-indole |
6736 |
CC1=CNC2= |
|
3 |
3′,5-Dihidroxy-3,4′,6,7-tetramethoxyflavone |
5459184 |
COC1=C(C=CC(=C1) |
|
4 |
5-Hidroxy-3′,4′,6,7,8-pentamethoxyflavone |
183466 |
COC1=C(C=C(C=C1) |
|
5 |
3,3′,4′,7-tetramethylquercetin |
5352005 |
COC1=C(C=C(C=C1) |
|
6 |
2,3-Dihydroxypropyl)9Z,12Z,15Z)-9,12,15-octadecatrienoate |
5367328 |
CCC=CCC=CCC |
|
7 |
2-Hexyl-3,5-dipentylpyridine |
6430301 |
CCCCCCC1=C |
|
8 |
2-(2,6-Dimethyl-1-piperidinyl)-2-oxoethyl 3,4,5-triethoxybenzoate |
– |
|
|
9 |
N,N-Dimethylpregnan-3-amine |
22214757 |
CCC1CCC2C1 |
|
10 |
(3S)-3-[(2E)-3-Carboxy-2-buten-1-yl]-7-hydroxy-4-methoxy-1,1,8,8,9-pentamethyl-11-(3-methyl-2-buten-1-yl)-6-oxo-3,6,8,9-tetrahydro-1H-difuro[3,2-b:3′,4′-h)xanthene-3-carboxylic acid |
162879284 |
CC1C(C2=C(O1)C |
|
11 |
n-benzyloctadecylamine |
88404 |
CCCCCCCCCCCCC |
|
12 |
2-[4-(Diphenylmethyl)-1-piperazinyl]ethyl methyl 2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate |
128529 |
CC1=C(C(=C(C(=N1)C) |
|
13 |
(1S,5S)-1,5-Anhydro-2,3-di-O-benzyl-4-deoxy-5-(2-methoxy-2-oxoethyl)-1-[(6R,9R)-6-methyl-4, 11-dioxo- 3,5,10,12-tetraoxapentadec-14-en-7-yn-9-yl]-L-threo-pentitol |
10675413 |
CCOC(=O)OC(C)C#CC |
|
14 |
Pheophorbide A |
253193 |
CCC1=C(C2=NC1=CC3= |
|
15 |
Bis[2-(4-butoxyphenoxy)ethyl] (4-hydroxybenzylidene)malonate |
43836111 |
CCCCOC1=CC=C(C=C1) |
|
16 |
1,1-Cyclopentanediacetic acid |
473107 |
CC(=C)C1CCC2 |
|
17 |
N’1,N’9-Bis |
66554672 |
CC1=C(N=C(C(=N1)C) |
|
18 |
N-{[(3S)-2-(L-Tyrosyl)-1,2,3,4-tetrahydro-3-isochinolinyl]methyl}-L-phenylalanyl-L-phenylalanin |
5311481 |
C1C(N(CC2=CC=CC=C21) |
The results of the molecular docking analysis of each BBLE compound against the target protein NF KB-p65 (1LE5) show that the best binding energy was obtained by the compound Pheophorbide-A, with a binding energy value of -8.1 kcal/mol and an RMSD of 0 Å. Pheophorbide A is a breakdown product of chlorophyll A, reported to be used as a photosensitizer and utilized in photodynamic therapy to reduce tumor growth45,46. The inhibition of Pheophorbide A detected in BBLE against the target protein NF KB-p65 (1LE5) is the first report of its kind (Table 3).
Table 3: Molecular Docking analysis of the compounds contained in BBLE against NF protein KB-p65 (1LE5).
|
No. |
Compounds |
Protein |
Binding Affinity (Kcal/mol) |
RMSD (Å) |
|
1 |
3′,4′,5′-Trihidroxy-6,7,8-trimethoxyflavone |
NF KB-p65 (1LE5) |
-6.7 |
0 |
|
2 |
3-Methyl-1H-indole |
-4.8 |
0 |
|
|
3 |
3′,5-Dihidroxy-3,4′,6,7-tetramethoxyflavone |
-6.4 |
0 |
|
|
4 |
5-Hidroxy-3′,4′,6,7,8-pentamethoxyflavone |
-5.9 |
0 |
|
|
5 |
3,3′,4′,7-tetramethylquercetin |
-6.3 |
0 |
|
|
6 |
2,3-Dihydroxypropyl)9Z,12Z,15Z)-9,12,15-octadecatrienoate |
-5.1 |
0 |
|
|
7 |
2-Hexyl-3,5-dipentylpyridine |
-4.8 |
0 |
|
|
8 |
2-(2,6-Dimethyl-1-piperidinyl)-2-oxoethyl 3,4,5-triethoxybenzoate |
|||
|
9 |
N,N-Dimethylpregnan-3-amine |
-6.9 |
0 |
|
|
10 |
(3S)-3-[(2E)-3-Carboxy-2-buten-1-yl]-7-hydroxy-4-methoxy-1,1,8,8,9-pentamethyl-11-(3-methyl-2-buten-1-yl)-6-oxo-3,6,8,9-tetrahydro-1H-difuro[3,2-b:3′,4′-h)xanthene-3-carboxylic acid |
|||
|
11 |
n-benzyloctadecylamine |
-3.7 |
0 |
|
|
12 |
2-[4-(Diphenylmethyl)-1-piperazinyl]ethyl methyl 2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate |
-7.5 |
0 |
|
|
13 |
(1S,5S)-1,5-Anhydro-2,3-di-O-benzyl-4-deoxy-5-(2-methoxy-2-oxoethyl)-1-[(6R,9R)-6-methyl-4, 11-dioxo- 3,5,10,12-tetraoxapentadec-14-en-7-yn-9-yl]-L-threo-pentitol |
|||
|
14 |
Pheophorbide A |
-8.1 |
0 |
|
|
15 |
Bis[2-(4-butoxyphenoxy)ethyl] (4-hydroxybenzylidene)malonate |
-5.9 |
0 |
|
|
16 |
1,1-Cyclopentanediacetic acid |
-7.1 |
0 |
|
|
17 |
N’1,N’9-Bis[(4-biphenylyloxy)acetyl]nonanedihydrazide |
-6.8 |
||
|
18 |
N-{[(3S)-2-(L-Tyrosyl)-1,2,3,4-tetrahydro-3-isochinolinyl]methyl}-L-phenylalanyl-L-phenylalanin |
-6.0 |
0 |
In several cell-based and animal experimental systems, it has been proven that NF-KB activation has a role in the early pathobiology of diabetes47. NF-KB is activated by increased oxidative stress, which in diabetes patients is caused by high glucose levels and advanced glycation end products48. Inhibition of this protein can offer new opportunities in the treatment process of diabetes played by BBLE through the compound Pheophorbide-A. The 2D interaction and 3D visualization of the Pheophorbide A compound against the NF KB-p65 protein (ILE5) are shown in Figure 2 below.
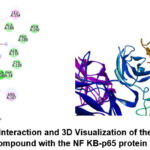 |
Figure 2: 2D Interaction and 3D Visualization of the Pheophorbide A Compound with the NF KB-p65 protein (1LE5) |
The Glucagon-like peptide-1 (GLP-1) protein is one of the important incretin hormones for preventing postprandial hyperglycemia49. The results of the molecular docking analysis of the compounds contained in BBLE against the GLP1 protein show that the best binding energy was obtained by the compound Cyclopentanediacetic acid with a binding energy value of -7.2 kcal/mol and an RMSD of 0 Å (Table 4).
Table 4: Molecular Docking analysis of the compounds contained in BBLE against GLP 1 (5NIQ) protein.
|
No. |
Compounds |
Protein |
Binding Affinity (Kcal/mol) |
RMSD (Å) |
|
1 |
3′,4′,5′-Trihidroxy-6,7,8-trimethoxyflavone |
GLP 1 (5NIQ) |
-5.6 |
0 |
|
2 |
3-Methyl-1H-indole |
-4.8 |
0 |
|
|
3 |
3′,5-Dihidroxy-3,4′,6,7-tetramethoxyflavone |
-5.5 |
0 |
|
|
4 |
5-Hidroxy-3′,4′,6,7,8-pentamethoxyflavone |
-5.3 |
0 |
|
|
5 |
3,3′,4′,7-tetramethylquercetin |
-5.6 |
0 |
|
|
6 |
2,3-Dihydroxypropyl)9Z,12Z,15Z)-9,12,15-octadecatrienoate |
-4.6 |
0 |
|
|
7 |
2-Hexyl-3,5-dipentylpyridine |
-4.7 |
0 |
|
|
8 |
2-(2,6-Dimethyl-1-piperidinyl)-2-oxoethyl 3,4,5-triethoxybenzoate |
|||
|
9 |
N,N-Dimethylpregnan-3-amine |
-6.8 |
0 |
|
|
10 |
(3S)-3-[(2E)-3-Carboxy-2-buten-1-yl]-7-hydroxy-4-methoxy-1,1,8,8,9-pentamethyl-11-(3-methyl-2-buten-1-yl)-6-oxo-3,6,8,9-tetrahydro-1H-difuro[3,2-b:3′,4′-h)xanthene-3-carboxylic acid |
|||
|
11 |
n-benzyloctadecylamine |
-3.7 |
0 |
|
|
12 |
2-[4-(Diphenylmethyl)-1-piperazinyl]ethyl methyl 2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate |
-6.7 |
0 |
|
|
13 |
(1S,5S)-1,5-Anhydro-2,3-di-O-benzyl-4-deoxy-5-(2-methoxy-2-oxoethyl)-1-[(6R,9R)-6-methyl-4, 11-dioxo- 3,5,10,12-tetraoxapentadec-14-en-7-yn-9-yl]-L-threo-pentitol |
|||
|
14 |
Pheophorbide A |
-6.3 |
0 |
|
|
15 |
Bis[2-(4-butoxyphenoxy)ethyl] (4-hydroxybenzylidene)malonate |
-4.8 |
0 |
|
|
16 |
1,1-Cyclopentanediacetic acid |
-7.2 |
0 |
|
|
17 |
N’1,N’9-Bis[(4-biphenylyloxy)acetyl]nonanedihydrazide |
-6.6 |
0 |
|
|
18 |
N-{[(3S)-2-(L-Tyrosyl)-1,2,3,4-tetrahydro-3-isochinolinyl]methyl}-L-phenylalanyl-L-phenylalanin |
-6.7 |
0 |
This study is the first to report the inhibition of the GLP-1 protein by the active compound 1,1-Cyclopentanediacetic acid in an in silico manner. There are not many reports explaining the bioactivity of this compound against degenerative diseases like diabetes. However, similar compounds that are derivatives of diacetic acid have been reported to have bioactivity as anti-inflammatory agents. Interestingly, the compound regulated several pro-inflammatory cytokines in microglial BV-2 cells induced with lipopolysaccharide (LPS)50. On a molecular level, the compound 1,1-Cyclopentanediacetic acid contained in BBLE, associated with anti-diabetic effects through the activation of GLP1 protein and its analogs, can be linked to the pancreatic GLP1R signaling that activates insulin production51,52. Pre-clinical scale research is still needed to prove the activation of BBLE compounds on the expression of GLP-1 and GLP1R genes in the ileum of diabetic rats. The 2D interaction and 3D visualization of the compound 1,1-Cyclopentanediacetic acid binding with the GLP-1 protein are displayed in Figure 3 below.
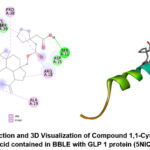 |
Figure 3: 2D Interaction and 3D Visualization of Compound 1,1- Cyclopentanediacetic acid contained in BBLE with GLP 1 protein (5NIQ) |
The molecular docking analysis of the compound contained in BBLE against the DPP-IV protein (5YP1) shows the highest binding energy by the compound 1,1-Cyclopentanediacetic acid with a binding energy value of -10.5 kcal/mol and an RMSD of 0 Å (Table 5). DPPIV inhibitors have been proven to benefit various organs, including renal and cardiovascular health53. DPP-IV inhibitors are widely contained in incretin-based oral hypoglycemic drugs intended for diabetes patients, which have been commercially available for nearly a decade. Several types of DPP-IV inhibitors, such as Litagliptin and Saxagliptin, are capable of controlling glycemia and reducing the risk of renal and cardiovascular complications in diabetic patients and have been approved by the US FDA 54–56.
Table 5: Molecular docking analysis of the compound contained in BBLE with the DPP-IV protein (5YP1)
|
No |
Compounds |
Protein |
Binding Affinity (Kcal/mol) |
RMSD (Å) |
|
1 |
3′,4′,5′-Trihidroxy-6,7,8-trimethoxyflavone |
DPP IV (5YP1) |
-8.3 |
0 |
|
2 |
3-Methyl-1H-indole |
-6.2 |
0 |
|
|
3 |
3′,5-Dihidroxy-3,4′,6,7-tetramethoxyflavone |
-8.2 |
0 |
|
|
4 |
5-Hidroxy-3′,4′,6,7,8-pentamethoxyflavone |
-7.9 |
0 |
|
|
5 |
3,3′,4′,7-tetramethylquercetin |
-8.1 |
0 |
|
|
6 |
2,3-Dihydroxypropyl)9Z,12Z,15Z)-9,12,15-octadecatrienoate |
-5.4 |
0 |
|
|
7 |
2-Hexyl-3,5-dipentylpyridine |
-5.4 |
0 |
|
|
8 |
2-(2,6-Dimethyl-1-piperidinyl)-2-oxoethyl 3,4,5-triethoxybenzoate |
|
0 |
|
|
9 |
N,N-Dimethylpregnan-3-amine |
-8.0 |
0 |
|
|
10 |
(3S)-3-[(2E)-3-Carboxy-2-buten-1-yl]-7-hydroxy-4-methoxy-1,1,8,8,9-pentamethyl-11-(3-methyl-2-buten-1-yl)-6-oxo-3,6,8,9-tetrahydro-1H-difuro[3,2-b:3′,4′-h)xanthene-3-carboxylic acid |
|
|
|
|
11 |
n-benzyloctadecylamine |
-5.4 |
0 |
|
|
12 |
2-[4-(Diphenylmethyl)-1-piperazinyl]ethyl methyl 2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate |
-8.7 |
0 |
|
|
13 |
(1S,5S)-1,5-Anhydro-2,3-di-O-benzyl-4-deoxy-5-(2-methoxy-2-oxoethyl)-1-[(6R,9R)-6-methyl-4, 11-dioxo- 3,5,10,12-tetraoxapentadec-14-en-7-yn-9-yl]-L-threo-pentitol |
|
|
|
|
14 |
Pheophorbide A |
-9.1 |
0 |
|
|
15 |
Bis[2-(4-butoxyphenoxy)ethyl] (4-hydroxybenzylidene)malonate |
-7.5 |
0 |
|
|
16 |
1,1-Cyclopentanediacetic acid |
-10.5 |
0 |
|
|
17 |
N’1,N’9-Bis[(4-biphenylyloxy)acetyl]nonanedihydrazide |
-9.5 |
0 |
|
|
18 |
N-{[(3S)-2-(L-Tyrosyl)-1,2,3,4-tetrahydro-3-isochinolinyl]methyl}-L-phenylalanyl-L-phenylalanin |
-9.3 |
0 |
This study reveals that the compound 1,1-Cyclopentanediacetic acid from BBLE has good potential as a DPP-IV inhibitor in silico. The 2D interactions and 3D visualization of the compound 1,1-Cyclopentanediacetic acid with the DPP-IV protein are displayed in Figure 4. Several related studies have confirmed the role of herbal plant extracts and their constituents as DPP-IV inhibitors. Procyanidin compounds isolated from Vitis vinifera seed extracts were able to reduce DPP-IV levels and down-regulate its gene expression in the human intestinal cell model Caco-2.In vivo, procyanidin compounds also decrease the regulation of DPP-IV in the intestines of obese Wistar rats 57. Similarly, the compound Emodin from the extract of Rheum palmatum L. shows inhibition against the DPP-IV enzyme in vitro and a dose-dependent decrease in DPP-IV levels in the plasma of Balb/c mice 58. However, in vivo scale research is still needed to determine the effectiveness of the BBLE compound in reducing DPP-IV levels in diabetic rats, thereby clarifying the results from in silico studies.
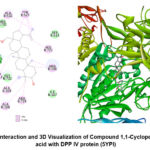 |
Figure 4: 2D Interaction and 3D Visualization of Compound 1,1-Cyclopentanediacetic acid with DPP IV protein (5YPI) |
Conclusion
In this study, LCMS/MS and molecular docking methods were used to analyze the phytoconstituent profile of BBLE with potential anti-diabetic properties. A total of 18 active compounds were identified in BBLE, including several that are flavonoid derivatives. Docking studies showed that Pheophorbide A could inhibit the NF KB-p65 protein (1LE5), and the compound 1,1-Cyclopentanediacetic acid inhibited the GLP 1 (5NIQ) and DPP-IV (5YP1) proteins. This study reveals the anti-diabetic effects of BBLE and further research is needed to determine the effectiveness of BBLE’s active compounds and to identify their molecular mechanisms of action on diabetic animal models.
Acknowledgement
The authors would like to thank the Institute for Research and Community Service (LPPM) at Universitas Dhyana Pura for supporting the implementation of this research. The authors also thank those who have helped carry out the research, such as students, workers at the Science and Health Laboratory, Universitas Dhyana Pura, and ASCAdemia who have provided proofreading services for this manuscript.
Conflict of Interest
The authors reported no declarations of interest.
Funding Source
This research was funded by the Institute for Research and Community Service (LPPM) Universitas Dhyana Pura through the Higher Education Excellence Research Scheme Research Funding Grant Program on 2023 with Contract Number: 011/UNDHIRA-LPPM/Lit./IX/2023.
References
- Ahmed S, Al-Rehaily AJ, Alam P, Alqahtani AS, Hidayatullah S, Rehman MT, Mothana RA, Abbas SS, Khan MU, Khalid JM, Siddiqui NA. Antidiabetic, antioxidant, molecular docking and HPTLC analysis of miquelianin isolated from Euphorbia schimperi. C. Presl. Saudi Pharm J., 2019; 27(5): 655–63.
CrossRef - Adeghate E, Schattner P, Dunn E. An Update on the Etiology and Epidemiology of Diabetes Mellitus. Ann N Y Acad Sci., 2006;1084(1):1–29.
CrossRef - Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract., 2019; 157: 107843.
CrossRef - Bhutta ZA, Salam RA, Gomber A, Lewis-Watts L, Narang T, Mbanya JC, Alleyne G. A century past the discovery of insulin: global progress and challenges for type 1 diabetes among children and adolescents in low-income and middle-income countries. Lancet., 2021; 398(10313): 1837–50.
CrossRef - Fatima MT, Bhat AA, Nisar S, Fakhro KA, Al-Shabeeb AAS. The role of dietary antioxidants in type 2 diabetes and neurodegenerative disorders: An assessment of the benefit profile. Heliyon., 2023; 9(1):1 2698.
CrossRef - Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes., 2015; 6(3): 456.
CrossRef - Widhiantara I. G, Permatasari A. A. A. P, Rosiana I. W, Sari N. K. Y, Sudyadnyana I. M. G. S, Wiradana P. A, Jawi I.M. The role of biopolymers as candidates for promoting health agents: A review. J Appl Pharm Sci., 2022; 13(1): 042–55.
CrossRef - Parham S, Kharazi A. Z, Bakhsheshi-Rad H. R, Nur H, Ismail A. F, Sharif S, RamaKrishna S, Berto F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants., 2020; 9(12): 1309.
CrossRef - Permatasari A. A. A. P, Rosiana I. W, Wiradana P. A, Lestari M. D, Widiastuti N. K, Kurniawan S. B, Widhiantara I.G. Extraction and characterization of sodium alginate from three brown algae collected from Sanur Coastal Waters, Bali as biopolymer agent. Biodiversitas J Biol Divers., 2022; 23(3): 1655–63.
CrossRef - Widhiantara I. G, Wiradana P. A, Permatasari A. A. A. P, Sari N. K. Y, Rosiana I. W, Astuti N. P. W, Widiastini L.P, Jawi I.M, Yasa I.W.P.S. Blumea balsamifera Leaf Extract Maintain Testosterone Levels in Hypercholesterolemic Rats Through Antioxidant Mechanism and Upregulation of StAR Gene Expression. Biomed Pharmacol J., 2023; 16(3): 1463–72.
CrossRef - Yogeswara I. B. A, Kusumawati I. G. A. W, Nursini N. W. Antibacterial activity and cytotoxicity of sequentially extracted medicinal plant Blumea balsamifera Lin. (DC). Biocatal Agric Biotechnol., 2022; 43: 102395.
CrossRef - Hasegawa H. Dihydroflavonol BB-1, an extract of natural plant Blumea balsamifera, abrogates TRAIL resistance in leukemia cells. Blood., 2006; 107(2): 679–88.
CrossRef - Ma J, Ren Q, Dong B, Shi Z, Zhang J, Jin D. Q, Xu, J, Ohizumi Y, Lee D, Guo Y. NO inhibitory constituents as potential anti-neuroinflammatory agents for AD from Blumea balsamifera. Bioorg Chem., 2018; 76: 449–57.
CrossRef - Mutmainah S.R, Rahmawati N, Nugroho A. E. Gastroprotective effects of combination of hot water extracts of turmeric (Curcuma domestica L.), cardamom pods (Ammomum compactum S.) and sembung leaf (Blumea balsamifera DC.) against aspirin–induced gastric ulcer model in rats. Asian Pac J Trop Biomed., 2014; 4: 500–4.
CrossRef - Ginting B, Maulana I, Karnila I. Biosynthesis Copper Nanoparticles using Blumea balsamifera Leaf Extracts: Characterization of its Antioxidant and Cytotoxicity Activities. Surfaces and Interfaces., 2020; 21: 100799.
CrossRef - Widhiantara I. G, Permatasari A. A. A. P, Rosiana I. W, Wiradana P. A, Widiastini L. P, Jawi I. M. Antihypercholesterolemic and Antioxidant Effects of Blumea balsamifera L. Leaf Extracts to Maintain Luteinizing Hormone Secretion in Rats Induced by High-Cholesterol Diets. Indones Biomed J., 2021; 13(4): 396–402.
CrossRef - Huang L, Lei T, Lin C, Kuang X, Chen H, Zhou H. Blumeaxanthene II, a novel xanthene from Blumea riparia DC. Fitoterapia., 2010; 81(5): 389–92.
CrossRef - Cao J, Zhu B, Xu Y, Li J, Chen C. Preparation and Characterization of PVDF‐HFP Membrane. J Macromol Sci Part A., 2008; 45(6): 449–55.
CrossRef - Su H, Li X, Li Y, Kong Y, Lan J, Huang Y, Liu Y. Chemical profiling and rapid discrimination of Blumea riparia and Blumea megacephala by UPLC-Q-Exactive-MS/MS and HPLC. Chinese Herb Med. 2023; 15(2): 317–28.
CrossRef - Ministry of Health Republic of Indonesia. Farmakope Herbal Indonesia Edisi II. 2017; 213–218.
- Ismed F, Desti W. N, Arifa N, Rustini R, Putra D. P. TLC-Bioautographic and LC-MS/MS Detection of Antimicrobial Compounds from Four Semipolar Extracts of Cladonia Species. In: 2nd International Conference on Contemporary Science and Clinical Pharmacy 2021 (ICCSCP 2021). Atlantis Press., 2021; 1–11.
CrossRef - Devnath H. S, Ahmed M. I, Medha M. M, Islam M. N, Biswas R. P, Islam M. A, Sadhu, S. K. HPLC Analysis and Antimicrobial, Antidiarrheal and Antihyperglycemic Properties of Eurya acuminata along with in silico Profiles. Phytomedicine Plus., 2022; 2(3): 100291.
CrossRef - Ahmed K. S, Jahan I. A, Jahan F, Hosain H. Antioxidant activities and simultaneous HPLC-DAD profiling of polyphenolic compounds from Moringa oleifera Lam. Leaves grown in Bangladesh. Food Res., 2021; 5(1): 401–8.
CrossRef - Wahyuni D. K, Wacharasindhu S, Bankeeree W, Punnapayak H, Purnobasuki H, Junairiah J, Ansori, A. N. M, Kharisma V. D, Parikesit A. A, Suhargo L, Prasongsuk, S. Molecular simulation of compounds from n-hexane fraction of Sonchus arvensis L. leaves as SARS-CoV-2 antiviral through inhibitor activity targeting strategic viral protein. J Pharm Pharmacogn Res., 2022; 10(6): 1126–38.
CrossRef - Nahar N, Nazmul Hasan Zilani M, Biswas P, Morsaline Billah M, Bibi S, Albekairi N. A, Alshammari, A, Nazmul H. M. Profiling of secondary metabolite and evaluation of anti-diabetic potency of Crotalaria quinquefolia (L): In-vitro, in-vivo, and in-silico approaches. Saudi Pharm J., 2024; 32(1): 101887.
CrossRef - Madeswaran A, Mohan S. Neuroprotective effects of terpenoids against streptozotocin-nicotinamide-induced diabetic rats: An in silico, in vitro and in vivo study. Int J Biol Macromol. 2023; 247: 125817.
CrossRef - Xavier T. F, Sabitha R, Balavivekananthan S. In vitro pharmacological investigations of Oxystelma esculentum R.Br. and in silico molecular docking analysis of its leaf constituents on diabetic related target. South African J Bot., 2022; 149: 320–38.
CrossRef - Filimonov D. A, Lagunin A. A, Gloriozova T. A, Rudik A. V, Druzhilovskii D. S, Pogodin P. V, Poroikov V. V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem Heterocycl Compd., 2014; 50(3): 444–57.
CrossRef - Christie WW, Han X. Lipids: their structures and occurrence. In: Lipid Analysis. Elsevier., 2012; 3–19.
CrossRef - Gasmi J, Thomas S. J. Jacaric acid and its octadecatrienoic acid geoisomers induce apoptosis selectively in cancerous human prostate cells: a mechanistic and 3-D structure–activity study. Phytomedicine., 2013; 20(8–9): 734–42.
CrossRef - Luthfia M, Eryandini A, Geraldi D, Narita C, Jannah C. M, Ambarsari L. Potency of Bioactive Compounds in Indramayu Mango Peel Waste to Inhibit ACE2. Curr Biochem., 2021; 8(2): 51–62.
CrossRef - Pratama H, Sumantri N.I, Fauziyah R.S, Kharisma V.D, Ansori A.N.M. Epitope-based Vaccine Design from Alpha and Beta Variant of SARS-CoV-2: An Immunoinformatics Approach. Res J Pharm Technol. 2023; 4617–25.
CrossRef - Kharisma V. D, Ansori A. N. M, Antonius Y, Rosadi I, Murtadlo A. A. A, Jakhmola V, et al. Garcinoxanthones from Garcinia mangostana L. against SARS-CoV-2 infection and cytokine storm pathway inhibition: A viroinformatics study. J Pharm Pharmacogn Res., 2023; 11(5): 743–56.
CrossRef - Nidom A. N, Madyawati S. P, Rachmawati K, Rahmahani J, Santoso K. P, Ansori A. N. M, Nidom R. V, Afifah B, Kusala M. K. J, Prakoso D. Protection and profile of immune response against SARS-CoV-2 among the COVID-19 vaccinated and unvaccinated individuals. Int J Health Sci (Qassim)., 2022; 29; 6011–9.
CrossRef - Purwitasari N, Agil M. Metabolite Profiling of Extract and Fractions of Bidara Upas (Merremia Mammosa (Lour.) Hallier F.) Tuber Using UPLCQToF-MS/MS. Biomed Pharmacol J. 2022; 15(4): 2025–41.
CrossRef - Widhiantara I. G, Jawi I. M. Phytochemical composition and health properties of Sembung plant (Blumea balsamifera): A review. Vet World., 2021; 14(5): 1185–96.
CrossRef - Elmann A, Telerman A, Mordechay S, Erlank H, Rindner M, Ofir R, Kashman Y. 3,5,4′-Trihydroxy-6,7,3′-trimethoxyflavone protects astrocytes against oxidative stress via interference with cell signaling and by reducing the levels of intracellular reactive oxygen species. Neurochem Int., 2014; 78: 67–75.
CrossRef - Wang N, Hebert D. N. Tyrosinase maturation through the mammalian secretory pathway: bringing color to life. Pigment Cell Res., 2006; 19(1): 3–18.
CrossRef - Rasul A, Zhao B-J, Liu J, Liu B, Sun J-X, Li J, Li X-M. Molecular Mechanisms of Casticin Action: an Update on its Antitumor Functions. Asian Pacific J Cancer Prev., 2014; 15(21): 9049–58.
CrossRef - Boniface P. K, Ferreira E. I. Opportunities and challenges for flavonoids as potential leads for the treatment of tuberculosis. 2020; 85–124.
CrossRef - Yang G, Li S, Yang Y, Yuan L, Wang P, Zhao H, Ho C-T, Lin C-C. Nobiletin and 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone Ameliorate 12- O -Tetradecanoylphorbol-13-acetate-Induced Psoriasis-Like Mouse Skin Lesions by Regulating the Expression of Ki-67 and Proliferating Cell Nuclear Antigen and the Differentiation of CD4. J Agric Food Chem., 2018; 66(31): 8299–306.
CrossRef - Rajaram P, Jiang Z, Chen G, Rivera A, Phasakda A, Zhang Q, Zheng S, Wang G, Chen Q-H. Nitrogen-containing derivatives of O-tetramethylquercetin: Synthesis and biological profiles in prostate cancer cell models. Bioorg Chem., 2019; 87: 227–39.
CrossRef - Martins I. L, Charneira C, Gandin V, Ferreira da Silva J. L, Justino G. C, Telo J. P, Vieira A. J. S. C., Marzano C. A, Alexandra M. M. Selenium-Containing Chrysin and Quercetin Derivatives: Attractive Scaffolds for Cancer Therapy. J Med Chem. 2015; 58(10): 4250–65.
CrossRef - Kaur J, Dhiman V, Bhadada S, Katare O, Ghoshal G. LC/MS guided identification of metabolites of different extracts of Cissus quadrangularis. Food Chem Adv [Internet]. 2022; 1: 100084.
CrossRef - Lim D-S, Ko S. H, Lee W. Y. Silkworm-pheophorbide a mediated photodynamic therapy against B16F10 pigmented melanoma. J Photochem Photobiol B Biol., 2004; 74(1): 1–6.
CrossRef - Rapozzi V, Miculan M, Xodo L. Evidence that photoactivated pheophorbide a causes in human cancer cells a photodynamic effect involving lipid peroxidation. Cancer Biol Ther., 2009; 8(14): 1318–27.
CrossRef - Patel S, Santani D. Role of NF-κB in the pathogenesis of diabetes and its associated complications. Pharmacol Reports., 2009; 61(4): 595–603.
CrossRef - Romeo G, Liu W-H, Asnaghi V, Kern T. S, Lorenzi M. Activation of Nuclear Factor-κB Induced by Diabetes and High Glucose Regulates a Proapoptotic Program in Retinal Pericytes. Diabetes., 2002; 51(7): 2241–8.
CrossRef - Yabe D, Seino Y. Two incretin hormones GLP-1 and GIP: Comparison of their actions in insulin secretion and β cell preservation. Prog Biophys Mol Biol., 2011; 107(2): 248–56.
CrossRef - Abed D. A, Lee S, Wen X, Ali A. R, Mangipudy V, Aleksunes L. M, Hu, L. Optimization of 1,4-bis(arylsulfonamido)naphthalene-N,N’-diacetic acids as inhibitors of Keap1-Nrf2 protein-protein interaction to suppress neuroinflammation. Bioorg Med Chem., 2021; 44: 116300.
CrossRef - Müller T. D, Finan B, Bloom S. R, D’Alessio D, Drucker D. J, Flatt P. R, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab., 2019; 30: 72–130.
CrossRef - Knudsen L. B, Nielsen P. F, Huusfeldt P. O, Johansen N. L, Madsen K, Pedersen F. Z, Thøgersen H, Wilken M, Agersø H. Potent Derivatives of Glucagon-like Peptide-1 with Pharmacokinetic Properties Suitable for Once Daily Administration. J Med Chem., 2000; 43(9): 1664
CrossRef - El Mouhayyar C, Riachy R, Khalil A. B, Eid A, Azar S. SGLT2 Inhibitors, GLP-1 Agonists, and DPP-4 Inhibitors in Diabetes and Microvascular Complications: A Review. Int J Endocrinol., 2020; 2020: 1–11.
CrossRef - Bhat G. A, Khan H. A, Alhomida A. S, Sharma P, Singh R, Paray B. A. GLP-I secretion in healthy and diabetic Wistar rats in response to aqueous extract of Momordica charantia. BMC Complement Altern Med., 2018 Dec; 18(1): 162.
CrossRef - Cahn A, Cernea S, Raz I. An update on DPP-4 inhibitors in the management of type 2 diabetes. Expert Opin Emerg Drugs. 2016 Oct 18;21(4):409–19. Available from: https://www.tandfonline.com/ doi/full/10.1080/14728214.2016.1257608
CrossRef - Hussein G. M. E, Matsuda H, Nakamura S, Hamao M, Akiyama T, Tamura K, Yoshikawa M. Mate Tea (Ilex paraguariensis) Promotes Satiety and Body Weight Lowering in Mice: Involvement of Glucagon-Like Peptide-1. Biol Pharm Bull., 2011; 34(12): 1849–55.
CrossRef - González-Abuín N, Martínez-Micaelo N, Blay M, Pujadas G, Garcia-Vallvé S, Pinent M, Ardévol A. Grape Seed-Derived Procyanidins Decrease Dipeptidyl-peptidase 4 Activity and Expression. J Agric Food Chem., 2012; 60(36): 9055–61.
CrossRef - Wang Z, Yang L, Fan H, Wu P, Zhang F, Zhang C, Liu W, Li M. Screening of a natural compound library identifies emodin, a natural compound from Rheum palmatum Linn that inhibits DPP4. PeerJ., 2017; 5: 3283.
CrossRef







