Nirmala Ganesan 1*, N. Anandhabhairavi 2, S. Arivarasan 3, V. Balamurugan,4 and T. Anitha4
1Department of Biotechnology, School of Bioengineering Vels Institute of Science, Technology and Advanced Studies, Chennai, India.
2Department of Agricultural Entomology, School of Agriculture VELS Institute of Science, Technology and Advanced Studies (VISTAS), Chennai,
3Department of Agricultural Economics, Agricultural College and Research Institute Madurai, Tamil Nadu, India.
4Department of Agricultural Economics, School of Agriculture VELS Institute of Science, Technology and Advanced Studies (VISTAS), Chennai, Tamil Nadu, India.
5Department of Postharvest Technology Horticultural College and Research Institute, Tamil Nadu Agricultural University, Periyakulam, Tamil Nadu, India.
Corresponding Author E-mail: gnnirmala.se@velsuniv.ac.in
DOI : https://dx.doi.org/10.13005/bpj/2902
Abstract
Liver plays a vital role in the elimination of xenobiotics that can induce hepatotoxicity in living organisms. Polymeric nanoparticles have evolved recently as an alternative in various industries and are used for their biomedical applications. Astragalin is a least studied flavonoid that has been used in the traditional medicine of Southeast Asia for its healing properties. Hence, in this study we used carbon tetrachloride as a hepatotoxin to induce liver damage. The protective effects of astragalin loaded polymeric nanoparticles on hepatotoxin-induced liver damage in experimental rats were assessed. The results of the assessment indicate that astragalin nanoparticles were effective in protecting the liver from damages induced by carbon tetrachloride. Astragalin nanoparticles formulation is not available in the market. Among existing literature, this is the first ever approach for hepatoprotective effect of astragalin nanoparticles studied.
Keywords
Astragalin; Carbon Tetrachloride; Nanoparticles; Hepatotoxicity PLA
Download this article as:| Copy the following to cite this article: Ganesan N, Anandhabhairavi N, Arivarasan S, Balamurugan V, Anitha T. Astragalin Nanoparticles Ameliorates CCl4 -Induced Liver Fibrosis in Rats. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Ganesan N, Anandhabhairavi N, Arivarasan S, Balamurugan V, Anitha T. Astragalin Nanoparticles Ameliorates CCl4 -Induced Liver Fibrosis in Rats. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/4amznfW |
Introduction
The liver performs a vital function in metabolism and detoxing of compounds which input the frame and can purpose hepatic injury, main to life-threatening illnesses 1. Therefore, predominant toxicological issues related to numerous illnesses were concentrated across the consequences at the liver 2. Usually, liver cells are suffering from hepatotoxic retailers via the induction of oxidative damage 3. Drugs of each artificial and herbal beginning are to be had for remedy of liver illnesses 4. Natural treatments have lengthy been used for remedy of liver illnesses. Based on this, protecting consequences of plant-primarily based totally natural drug treatments in opposition to drug-triggered toxicity have reached paramount significance recently 5. Astragalin (kaempferol-3-O-glucoside) is a flavonoid this is extracted from leaves of persimmon, Rosa agrestic, or inexperienced tea seeds. Numerous preclinical research has proven that astragalin has a huge variety of pharmacological activities, together with antioxidative, anti-inflammatory, and antitumor activities; astragalin can ameliorate apoptosis consequences 6,7,8. It changed into even hypothesized that the antioxidative, anti-inflammatory, and antiapoptotic consequences of astragalin will also be concerned withinside the prevention of Myocardial ischemia/reperfusion (I/R) injury. In this have a look at, we aimed to assess the hepatoprotective consequences of astragalin. Carbon tetrachloride (CCl4) is a xenobiotic launched into the water as waste from numerous industries, thereby main to hepatotoxicity while dwelling organisms are uncovered to it9. It is regularly used to result in liver issues in diverse fashions for the screening of hepatoprotective retailers 10. In the research that contain animal fashions, in particular people who have a look at the consequences at the liver, CCl4 changed into converted into different sorts of unfastened radicals, main to lipid peroxidation. This may also consequently bring about mobile necrosis 11. Hence, on this have a look at, we used CCl4 because the hepatotoxin to research the consequences at the liver. Polymeric nanoparticles were exploited for numerous healing functions due to its residences which includes confined toxicity, multiplied biodegradability, and bioavailability 12,13. They are recognized to have interaction without difficulty with organic structures due to their small size 14. Synthesis of polymeric nanoparticles were done the use of numerous strategies and such nanoparticles were utilized in diverse industries 15. Astragalin was recognized to own hepatoprotective residences. However, there aren’t any reviews on hepatoprotective interest of Astragalin or its nano formulation. Taking this as an initiative, we supposed to have a look at the hepatoprotective interest of astragalin loaded polymeric nanoparticles in CCl4 triggered rat model.
Material and methods
Chemicals
Astragalin, Poly lactic acid (PLA), Dimethyl sulfoxide (DMSO) had been bought from Sigma Aldrich, India. Swiss albino rats had been bought from the Central animal residence facility, Tamil Nādu Veterinary and Animal Sciences University, Chennai, India. All animal experimentation protocol became reviewed and accepted with the aid of using the Institutional Animal Ethics Committee, K.L.R Pharmacy college, Paloncha India and Animal moral committee approval variety 12/2019.
Formulation of Astragalin loaded polymeric nanoparticles
The astragalin loaded nanoparticles had been organized with the aid of using a dialysis method. Astragalin (five mg) and PLA (50 mg) had been dissolved in DMSO (1 mL) and introduced dropwise to twenty-five mL of water beneath Neath stirring. The combination became stirred for every other 30 min at room temperature and dialyzed in opposition to distilled water the usage of a 7 kDa dialysis bag for twenty-four h. The unentrapped astragalin became eliminated with the aid of using filtration via a 0. forty-five μm clear out and freeze-dried 16,17.
Acute oral toxicity study
Acute toxicity research of astragalin nanoparticle had been finished in lady rats with the aid of using the usage of Organization for Economic Co-operation and Development (OECD) tenet 425. Healthy nonpregnant younger lady Wistar rats (200-250 gm) divided in corporations of six animals every had been housed in polypropylene cages in corporations of 5 for five days previous to the experimentation. Standard pellet eating regimen became given with water advert libitum. An unmarried dose of one thousand mg/kg of the astragalin nanoparticle became administered to rats orally. The manage organization obtained identical extent of water orally. Both the manage and experimental rats had been determined often for 1, 2, four and 24 hours. Mortality and symptoms and symptoms of toxicity had been determined, and the statement became endured for 15 days. Changes in hair, skin, eyes, mucus membrane, meals and water consumption, frame weight, behavioral and respiration charge had been determined. At the very last level of experiment, all of the animals had been sacrificed 18.
Subacute toxicity testing
The animals had been divided into 3 corporations of six animals every. Group 1 served as manage wherein water became administered orally. Group 2 and three had been administered with astragalin nanoparticle on the doses of fifty and one hundred mg/kg frame weight primarily based totally at the LD50 (Lethal Dose) dose received from acute toxicity study. The dosing became endured for the subsequent 28 days upon statement 19.
Clinical observations and frame weight
Morbidity and Mortality became determined in all of the animals. Physical and behavioural modifications had been examined. The observations included alternate withinside the skin, fur, eyes, mucus membranes, secretions, excretion, and autonomic activity. Body weight of all animals had been measured on day 0, 7, 14, 21, and 28.
Hematological parameters
The hematological exam that consists of crimson blood cell (RBC), White blood cell (WBC), Lymphocytes, Neutrophils, platelets and hemoglobin (Hb) had been envisioned 20.
Serum chemistry Glucose, cholesterol, triglycerides, urea, creatinine, aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphate, general bilirubin, protein, albumin, globulin, sodium, phosphorus, calcium, chloride, blood urea nitrogen, and cholinesterase had been envisioned the usage of a trendy diagnostic kit 21-26.
Histopathology
The liver, kidney, spleen, brain, and coronary heart of all animals had been dissected, and their moist weight became recorded. For histopathology examinations, tissues had been processed into paraffin blocks; ultra-skinny sections had been dewaxed and stained with hematoxylin and eosin 27,28.
Pharmacokinetic studies
The have a look at changed into achieved in organizations of six rats each. Group 1 acquired astragalin answer containing 10 mg/kg orally. Group 2 acquired astragalin nanoparticles equal to ten mg/kg of drug and administered orally. 0.five ml blood pattern changed into gathered at one-of-a-kind time durations 0, 0.25, 0. five, 0.75, 1, 1. five, 2, 4, 6, 8, 12, and 24 h thru unfashionable orbital puncture. The gathered plasma changed into centrifuged for 10 min at 6000 rpm and saved at -20ºC earlier than evaluation. Drug tiers withinside the plasma samples changed into evaluated through a HPLC method. The following pharmacokinetic parameters region beneath Neath the plasma concentration “time curve (AUC), maximal concentration (Cmax) and the time for maximal concentration (Tmax) have been decided the usage of WinNonlin pharmacokinetic statistics evaluation software 29-31.
Evaluation of hepatoprotective activity
Carbon tetrachloride (CCl4) prompted liver harm version become used withinside the assessment of hepatoprotective hobby. Wistar albino rats have been divided in to 4 corporations of 6 animals each. Group1 acquired everyday saline (1 ml) every day for nine days and served as everyday control. Group 2 acquired CCl4 (dissolved in three instances its quantity of olive oil) at a dose of 0.7 ml/kg intraperitoneally on days three, 6, nine and 12 serving as poisonous control. Group three acquired astragalin drug solution (one hundred mg/kg) orally every day for a length of weeks. Group four acquired the equal dose of astragalin nanoparticle orally every day for a length of weeks. All the corporations acquired CCl4 at days 1, three, 6, nine and 12 of the observe besides everyday control. The animals have been anaesthetized at the ultimate day of the observe and blood become accumulated through cardiac puncture. Plasma become separated from the blood samples through centrifugation at 3000 rpm for 15 min. Hepato-defensive hobby become quantified through serum glutamate oxaloacetate transaminase (SGOT) and serum glutamate pyruvic trans-aminase (SGPT) degrees withinside the plasma. Subsequently, their livers have been subjected to histopathological examination. First, the rats have been sacrificed on the ultimate day of the observe, the liver become separated cautiously and preserved in formalin solution, and liver sections have been prepared. The frame weight of the rats become additionally be monitored 32.
Myeloperoxidase (MPO) analysis
To degree the Myeloperoxidase (MPO) interest, the tissue samples had been accumulated, homogenized, and centrifuged to attain the supernatant. The MPO interest changed into measured via way of means of a MPO dedication package in keeping with the manufacture’s protocol33.
Cytokine analysis
The tissue samples had been homogenized in phosphate-buffered saline (PBS) (1:9, w/v) and centrifuged at 2000 ×g for forty min at 4 °C. Then the supernatant changed into accumulated and the expression of cytokine protein ranges for TNF-α, IL-1β and IL-6 in it changed into decided via way of means of enzyme-related immunosorbent assay (ELISA)34.
Results and Discussion
Acute toxicity study
In acute toxicity studies, oral LD50 of astragalin nanoparticle in Wistar rats was reported at 1000 mg/kg body weight. The day 14 observation in acute oral toxicity study and weekly body weight measurement did not show any toxic effects in rats. There was no abnormal behaviour during the first 30 min (after dosing) and periodically for first 24 h and daily thereafter for 14 days.
Sub-acute toxicity
All animals survived until the scheduled necropsy in 28 days. Physical and behavioural examination did not show any adverse effects in any of the groups receiving 50 mg/kg and 100 mg/kg of astragalin nanoparticle. As compared to control group, no significant changes were noted on body weight gain. These results suggest that administration of astragalin nanoparticle up to the dose of 100/mg/kg/day to rats for 28 days has no adverse effect on the clinical observations and body weights.
Hematology
There was no adverse effect of astragalin nanoparticle on hematological parameters in rats. No significant differences were noted on the hematological parameters when control and treatment groups were compared (Table 1).
Table 1: Effect of astragalin nanoparticle on hematological parameters of rat (sub-acute)
|
Parameters |
Unit |
Control |
50mg/kg |
100mg/kg |
|
RBC |
106/cm |
6.92±0.78 |
7.36±0.76 |
7.72±0.24* |
|
WBC |
103/cm |
8.12±1.22 |
9.56±0.22 |
9.04±0.28* |
|
Lymphocytes |
% |
72.9±2.10 |
86.2±1.20 |
79.2±2.2* |
|
Neutrophils |
% |
20.26±0.87 |
16.30±2.10 |
19.8±2.8* |
|
Platelets |
103/cm |
798.6±15.2 |
812.2±22.24 |
891.2±21.07* |
|
Hemoglobin |
g/dl |
15.02±0.12 |
13.89±0.29 |
14.22±0.32* |
All values are expressed as mean ± SD (n = 6). The data were statistically analyzed by one-way ANOVA followed by Dunnett test. *P < 0.05, statistically significant as compared to normal control. RBC: Red blood cell, WBC: White blood cell, SD: Standard deviation.
Serum chemistry
There was no treatment related biologically significant adverse effects of astragalin nanoparticle on serum chemistry of rats. There is a significant decreased in mean values of glucose, cholesterol, triglyceride in rats. All these variations were marginal and within the normal laboratory ranges. The results of serum chemistry analysis from the test and control groups show that administration of astragalin nanoparticle doses up to 100 mg/kg to rats for 28 days did not cause toxicologically significant adverse effects (Table 2).
Table 2: Effect of astragalin nanoparticle on serum biochemistry parameters of rats.
|
Parameters |
Unit |
Control |
50mg/kg |
100mg/kg |
|
Glucose |
mg/dl |
79.1 ± 3.7 |
83 ± 3.27 |
92.7 ± 3.87* |
|
Cholesterol |
mg/dl |
82 ± 1.34 |
80.3 ± 3.03 |
92.3 ± 2.62* |
|
Triglyceride |
mg/dl |
54.2 ± 1.24 |
57.2 ± 1.18 |
62.2 ± 1.03 |
|
Urea |
mg/dl |
32.8 ± 2.01 |
3.8 ± 2.16 |
40.2 ± 0.08 |
|
Creatinine |
mg/dl |
0.78 ± 0.26 |
0.71 ± 0.02 |
0.74 ± 0.08 |
|
AST |
IU/L |
122 ± 2.12 |
120 ± 2.24 |
129.9 ± 3.12 |
|
ALT |
IU/L |
45.4 ± 2.05 |
44.2 ± 1.29 |
47.04 ± 2.12 |
|
ALP |
IU/L |
82.02 ± 3.12 |
78.2 ± 2.18 |
81.4 ± 1.03 |
|
Total bilirubin |
mg/dl |
0.24 ± 0.003 |
0.22 ± 0.004 |
0.30 ± 0.002 |
|
Protein |
g/dl |
8.02 ± 0.02 |
7.87 ± 0.06 |
8.29 ± 0.12 |
|
Albumin |
g/dl |
4.12 ± 0.06 |
3.82 ± 0.02 |
4.00 ± 0.08 |
|
Globulin |
g/dl |
3.97 ± 0.02 |
3.52 ± 0.22 |
4.22 ± 0.18 |
|
Blood urea nitrogen |
mg/dl |
19.16 ± 0.36 |
18.09 ± 0.62 |
18.58 ± 0.98 |
The values are expressed as mean ± SD(n=6). The data were statistically analyzed by one-way ANOVA. *P < 0.05, statistically significant as compared to normal control. ALP: Alkaline phosphate, ALT: Alanine transaminase, AST: Aspartate aminotransferase, BUN: Blood urea nitrogen, SD: Standard deviation.
Pharmacokinetics
The method was used to investigate the pharmacokinetics of astragalin solution and its nano formulation after oral administration. The pharmacokinetic profiles of the two substances were represented in a one-compartment model. The mean plasma concentration time-curve was illustrated in Figure 1. As shown in Table 3, the AUC(0–24h) of astragalin nanoparticle (2614.12±261.14 µg/L) was high among the two processed substances, which indicated that it possessed abundant plasma exposure. The AUC(0–24h) and Cmax of astragalin solution was lower, which demonstrated that absorption of astragalin solution was low in vivo.
Table 3: Pharmacokinetic parameters of astragalin after oral administration (n=6, mean ±SD)
|
Parameter |
Units |
Astragalin solution |
Astragalin nanoparticle |
|
Cmax |
μg/mL |
84.86±13.23 |
151.24±11.89** |
|
Tmax |
Hour |
0.54±0.12 |
0.33±0.26** |
|
AUC0-24 |
μg/mL |
938.75±275.77 |
2614.12±261.14** |
|
MRT0-24 |
Hour |
18.0±10.2 |
20.86±8.6* |
|
CL |
mL/kg |
135.84±8.64 |
78.94±6.82* |
The values are expressed as mean ± SD (n=6). The data were statistically analyzed by T test Note: * P < 0.05, ** P < 0.01 compared with the astragalin solution group
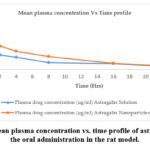 |
Figure 1: The mean plasma concentration vs. time profile of astragalin following the oral administration in the rat model. |
Hepatoprotective activity
Table 4 shows hepatoprotective activity data. The administration of CCl4 to the animals resulted in a marked increase in SGPT and SGOT activities, indicating increased toxicity, but this was mitigated in the animals treated with astragalin nanoparticles. The reduction in toxicity was statistically significant at p < 0.001 for both astragalin nanoparticle and astragalin solution. However, the astragalin nanoparticles completely reversed the elevated levels of SGOT and SGPT.
Table 4: Effect of astragalin nano formulation on enzyme levels in rats with carbon tetrachloride (CCl4) induced hepatotoxicity
|
Treatment group |
Initial body weight (g) |
Body weight after 9 days (g) |
SGPT (U/L) |
SGOT (U/L) |
|
Control |
159±6 |
175±3 |
10.6±1.8 |
29.9±2.2 |
|
CCl4 |
164±8 |
144±9 |
72.7±2.6 |
89.4±1.9 |
|
Astragalin solution |
168±5 |
176±4*** |
41.2±6.2*** |
61.1±2.6*** |
|
Astragalin nanoparticle |
169±3 |
180±2*** |
15.69±2.8*** |
37.8±2.4*** |
The values are expressed as mean ± SD(n=6). The data were statistically analyzed by one-way ANOVA. ***P<0.001, **P<0.01, *P<0.05 statistically significant as compared to CCl4 group.
The photomicrographs in Figure 2 display the histological changes in the liver of the animal’s following administration of the astragalin-loaded nanoparticles. The histological profile of the control animals showed normal hepatic architecture with distinct hepatic cells, well presented cytoplasm sinusoidal spaces and central vein. However, there was disorganization of normal cells with intense centrilobular necrosis following CCl4 intoxication. Moderate accumulation of fatty lobules and cellular necrosis were observed in the animals treated with astragalin solution. However, the nanoparticle formulation exhibited strong protection against CCl4 -induced liver damage, as evidenced by the presence of normal hepatic cords, well-defined cytoplasm and absence of necrosis. Furthermore, the body weights of the rats which fell significantly after CCl4 treatment were restored to normal following administration of the astragalin nanoparticles.
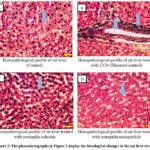 |
Figure 2: The photomicrographs in Figure 2 display the histological changes in the rat liver tissues. |
The histological profile of the control animals showed normal hepatic architecture with distinct hepatic cells, well presented cytoplasm sinusoidal spaces and central vein (Figure- A). Disorganization of normal cells with intense centrilobular necrosis following CCl4 intoxication (Figure- B). Moderate accumulation of fatty lobules and cellular necrosis were observed in the animals treated with astragalin solution (Figure-C). Presence of normal hepatic cords, well-defined cytoplasm and absence of necrosis observed in rats treated with nano particle formulation (Figure- D). The original microscopic magnification was 100X.
Effects of astragalin on MPO activity
The MPO activity, which is a biomarker of neutrophil infiltration, was measured in this study. The results showed that the MPO activity in the rat tissue samples was significantly augmented after CCl4 treatment compared with the control group, and treatment with astragalin or astragalin nanoparticles attenuated the MPO activity obviously (Figure 3).
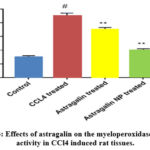 |
Figure 3: Effects of astragalin on the myeloperoxidase (MPO) activity in CCl4 induced rat tissues. |
Each column shows the mean of triplicates mean ± SEM of three independent experiments, and differences between mean values were assessed by Student’s t-test. # p < 0.01 significantly different from the control group, *p < 0.05 and **p < 0.01 significantly different from the CCl4 group.
Effects of astragalin on inflammatory cytokines release
TNF-α, IL-1β, and IL-6 are the major pro-inflammatory cytokines in the inflammatory response. To measure the potential anti-inflammatory effects of astragalin on CCl4 exposed rat tissues, the levels of these three cytokines were detected by ELISA. These results showed that TNF-α, IL-1β, and IL-6 levels in CCl4 treated group was markedly increased compared to those in the control group; while pre-treatment with astragalin or astragalin nanoparticle inhibited the expression of TNF-α, IL-1β and IL-6 compared to that in the CCl4 treated group (Figure. 4A–C).
 |
Figure 4A: Effects of astragalin on the production of TNF-α in CCl4 induced rat tissues. |
Each column shows the mean of triplicates mean ± SEM of three independent experiments, and differences between mean values were assessed by Student’s t test. # p < 0.01 significantly different from the control group, *p < 0.05 and **p < 0.01 significantly different from the CCl4 group.
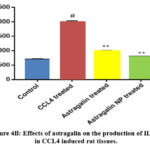 |
Figure 4B: Effects of astragalin on the production of IL-1β in CCL4 induced rat tissues. |
Each column shows the mean of triplicates mean ± SEM of three independent experiments, and differences between mean values were assessed by Student’s t test. # p < 0.01 significantly different from the control group, *p < 0.05 and **p < 0.01 significantly different from the CCl4 group.
 |
Figure 4C: Effects of astragalin on the production of IL-6 in CCl4 induced rat tissues. |
Each column shows the mean of triplicates mean ± SEM of three independent experiments, and differences between mean values were assessed by Student’s t test. # p < 0.01 significantly different from the control group, *p < 0.05 and **p < 0.01 significantly different from the CCl4 group
When comparing the astragalin nano formulation’s pharmacokinetic properties, it was found that the astragalin Tmax (0.54±0.12 h) increased with oral administration, whereas the nanoparticle Tmax (0.33±0.26 h) decreased. These results suggested that the compound’s absorption was enhanced by the nanoparticles35. Additionally, the astragalin nanoparticles’ Cmax, AUC0–24, MRT0–24, and CL significantly vary (p<0.05) from the astragalin solution group, indicating that astragalin absorption can be enhanced in nanodrug delivery. It was shown that astragalin’s plasma exposure increased following the introduction of nanoparticles35. The current study suggested that astragalin’s bioavailability might be considerably raised via nanomedicine delivery. This might be the result of astragalin’s improved solubility due to nanoparticles36. Here, CCl4 was used to test the anti-fibrotic and fibrolytic effects of remedial therapy containing astragalin, either alone or in combination, in rats with developed liver fibrosis. Additionally, we examined the possibility of additive or improved mechanistic effects of combining both treatments on a panel of mediators that promote and inhibit fibrogenesis36. There are numerous cellular and molecular processes involved in hepatic fibrogenesis. Stimulated Kupffer cells release TGF-β1 after liver damage, which then triggers HSC activation and the subsequent deposition of extra ECM. Little is currently known about the possibility that astragalin nanoparticles have direct fibrolytic activity or hepatoprotective effect against CCl4.Regarding this issue, every study that has been conducted to date on the effects of TQ began the treatment regimen either prior to or concurrently with the induction of fibrogenesis; none of these research examined the drug after liver fibrosis had been established[38-39].Hepatotoxicity is still a major barrier to the successful treatment of tuberculosis because it increases the likelihood of noncompliance, which in turn leads to treatment failure, a relapse, or the emergence of drug resistance37.
In summary, the measure of a drug’s hepatoprotective impact is its ability to reduce adverse events or maintain the liver’s normal physiological characteristics following toxicity induction38,39. When plant extracts are
utilized to create polymeric nanoparticles, there may be synergistic effects as well as antioxidant benefits [39]. Based on biochemical and histological characteristics, the results show that astragalin-loaded polymeric nanoparticles were highly protective against CCl4 intoxication. This explains why astragalin is a highly valued option for a variety of therapeutic uses, as well as protective effects on the liver. 40,41 In conclusion, we successfully established a rat model of liver fibrosis induced by CCl4. According to our research, astragalin nanoparticles can reduce rat liver fibrosis. Further large-scale research is necessary because the previous study demonstrated that astragalin nanoparticles have a hepatoprotective activity against CCl4-induced liver damage. It is unlikely, nonetheless, that the drug’s antifibrotic action’s mechanism will be thoroughly investigated in the near future. The study’s scope may also be expanded to examine the mechanism of action of astragalin in hepatoprotective activities and other sports in order to introduce the astragalin nanoparticle system into the market for human use.
Conclusion
Astragalin nanoparticles can lessen liver fibrosis in rats. The gift has a look at confirmed that astragalin nanoparticles own hepatoprotective pastime towards CCl4-brought about liver toxicity, which calls for in addition full-size studies. However, it’s far doubtful whether or not the mechanism in the back of the antifibrotic pastime of the drug can be in addition studied substantially withinside the future. The in-addition scope of the studies paintings may be prolonged to have a look at the Astragalin mechanism of motion in hepatoprotective pastime and extra sports to release the Astragalin nanoparticle system withinside the marketplace for human use.
Acknowledgement
Nirmala Ganesan would like to thank School of Bioengineering, Vels Institute of Science, Technology and Advanced Studies for continuous support and encouragement
Conflict of Interest
All authors are requested to disclose any conflict of interest including honorarium, grants, membership, employment, ownership of stock or any other interest or non‐financial interest such as personal or professional relation, affiliation and knowledge of the research topic.
Funding Source
This research received no external funding Sources
Reference
- Ansari M. A., Alzohairy M. A. One-pot facile green synthesis of silver nanoparticles using seed extract of Phoenix dactylifera and their bactericidal potential against MRSA. Evid Based Complex Alt Med. 2018: Article ID 1860280, 9 pages
CrossRef - Datkhile K. D, Patil S. R, Durgavale P. P, Patil M. N, Jagdale N. J, Deshmukh V. N. Studies on Antioxidant and Antimicrobial Potential of Biogenic Silver Nanoparticles Synthesized Using Nothapodytes foetida Leaf Extract (Wight) Sleumer. Biomed Pharmacol ,2019 13(1).
CrossRef - Baran, A.; Hatipo ˘glu, A.; Yildiztekin, M.; Küçükaydin, S.; Kurt, K.; Ho¸sgören, H.; Sarker, M.M.R.; Sufianov, A.; et al. Green Synthesis of Silver Nanoparticles from Allium cepa L. Peel Extract, Their Antioxidant, Antipathogenic, and Anticholinesterase Activity. Molecules, 2023, 28, 2310. https://doi.org/10.3390/ molecules28052310.
CrossRef - Rane J, Jadhao R, Bakal RL. Liver diseases and herbal drugs: -A review. J innov pharm biol Sci. 2016;3(2):24-36.
- Ramappa V, Aithal GP. Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. Journal of clinical and experimental hepatology. 2013 Mar 1;3(1):37-49.
CrossRef - Singh D, Cho WC, Upadhyay G. Drug-induced liver toxicity and prevention by herbal antioxidants: an overview, Front. Physiol. 6 (2016).
CrossRef - Hong M, Li S, Tan HY, Wang N, Tsao SW, Feng Y. Current status of herbal medicines in chronic liver disease therapy: the biological effects, molecular targets and future prospects. International journal of molecular sciences. 2015 Dec 2;16(12):28705-45.
CrossRef - Luo JY, Niu CY, Wang XQ, Zhu YL, Gong J. Effect of a single oral dose of rabeprazole on nocturnal acid breakthrough and nocturnal alkaline amplitude. World journal of gastroenterology. 2003 Nov 11;9(11):2583.
CrossRef - Kim MS, Kim SH. Inhibitory effect of astragalin on expression of lipopolysaccharide-induced inflammatory mediators through NF-κB in macrophages. Archives of pharmacal research. 2011 Dec;34(12):2101-7
CrossRef - Burmistrova O, Quintana J, Díaz JG, Estévez F. Astragalin heptaacetate-induced cell death in human leukemia cells is dependent on caspases and activates the MAPK pathway. Cancer Letters. 2011 Oct 1;309(1):71-7.
CrossRef - Cho IH, Gong JH, Kang MK, Lee EJ, Park JH, Park SJ, Kang YH. Astragalin inhibits airway eotaxin-1 induction and epithelial apoptosis through modulating oxidative stress-responsive MAPK signaling. BMC Pulmonary Medicine. 2014 Dec;14(1):1-1.
CrossRef - Acharya KR, Chatterjee SO, Biswas GU, Chatterjee AN, Saha GK. Hepatoprotective effect of a wild edible mushroom on carbon tetrachloride-induced hepatotoxicity in mice. Int J Pharm Pharm Sci. 2012;4(3):285-8.
- Mahmoodzadeh Y, Mazani M, Rezagholizadeh L. Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicology reports. 2017 Jan 1; 4:455-62.
CrossRef - Rahmat AA, Dar FA, Choudhary IM. Protection of CCl4-induced liver and kidney damage by phenolic compounds in leaf extracts of Cnestis ferruginea (de Candolle). Pharmacognosy Research. 2014 Jan;6(1):19
CrossRef - Shanmuganathan R, MubarakAli D, Prabakar D, Muthukumar H, Thajuddin N, Kumar SS, Pugazhendhi A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environmental Science and Pollution Research. 2018 Apr;25(11):10362-70.
CrossRef - Saravanan M, Barik SK, MubarakAli D, Prakash P, Pugazhendhi A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microbial pathogenesis. 2018 Mar 1; 116:221-6
CrossRef - Pugazhendhi A, Edison TN, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. International journal of pharmaceutics. 2018 Mar 25;539(1-2):104-11.
CrossRef - Zhang XF. Zhi-Guo liu, Wei shen, Sangiliyandi Gurunathan. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. International Journal of Molecular Sciences. 2016; 17:1534.
CrossRef - Bohrey S, Chourasiya V, Pandey A. Polymeric nanoparticles containing diazepam: preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Convergence. 2016 Dec;3(1):1-7.
CrossRef - Nair KG, Velmurugan R, Sukumaran SK. Formulation and optimization of ansamycin-loaded polymeric nanoparticles using response surface methodology for bacterial meningitis. BioNanoScience. 2020 Mar;10(1):279-91.
CrossRef - Gandhare B, Kavimani S, Rajkapoor B. Acute and subacute toxicity study of methanolic extract of Ceiba pentandra (Linn.) Gaertn. on rats. Journal of Scientific Research. 2013 Apr 22;5(2):315-24.
CrossRef - Docie JV. Practical hematology. London. Churchill Ltd. 1958:38-42.
- Hugget AG, Nixon DA. Use of glucose-peroxidase in determination of blood and urinary glucose. Lancet. 1957;2(368):70.
CrossRef - Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American journal of clinical pathology. 1957 Jul 1;28(1):56-63.
CrossRef - Palanivel MG, Rajkapoor B, Kumar RS, Einstein JW, KUMAR EP, KUMAR MR, KAVITHA K, KUMAR MP, JAYAKAR B. Hepatoprotective and antioxidant effect of Pisonia aculeata L. against CCl4-induced hepatic damage in rats. Scientia pharmaceutica. 2008 Jun;76(2):203-16.
CrossRef - John E. Payne, Harold M. Kaplan, Modified method for quantitative determination of cholesterol and cholesterol esters, Steroids, Volume 1, Issue 3, 1963, Pages 341-344, ISSN 0039-128X.
CrossRef - LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265-75. PMID: 14907713.
CrossRef - Sylvan M Sax, Clinical Chemistry—Principles and Technics, 2nd ed. R. J. Henry, J. W. Winkelman, and D. C. Cannon, Eds. Harper & Row, Publishers, New York, N. Y., 1974, xii + 1629 pp. 267 illustrations. $37.50, Clinical Chemistry, Volume 21, Issue 2, 1 February 1975, Pages 273–274.
CrossRef - Zaccone G. Mucosaccharide histochemistry and histoenzymorphologic observations on the epidermis of Ariosoma balearicum de la Roche (Anguilliformes, Pisces). Acta Histochemical. 1979 Jan 1;65(2):191-IN1.
CrossRef - Velmurugan R, Selvamuthukumar S. In vivo antitumor activity of a novel orally bioavailable ifosfamide nanostructured lipid carrier against Dalton’s ascitic lymphoma. Journal of Pharmaceutical Innovation. 2014 Sep;9(3):203-11.
CrossRef - Chandrashekhar VM, Muchandi AA, Sudi SV, Ganpati S. Hepatoprotective activity of Stereospermum suaveolens against CCl4-induced liver damage in albino rats. Pharmaceutical biology. 2010 May 1;48(5):524-8.
CrossRef - Karthikeyan R, Anantharaman P, Chidambaram N, Balasubramanian T, Somasundaram ST. Padina boergessenii ameliorates carbon tetrachloride induced nephrotoxicity in Wistar rats. Journal of King Saud University-Science. 2012 Jul 1;24(3):227-32.
CrossRef - Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. Journal of pharmacological methods. 1990 Dec 1;24(4):285-95.
CrossRef - Hermenean A, Mariasiu T, Navarro‑González I, Vegara‑Meseguer J, Miuțescu E, Chakraborty S, Pérez‑Sánchez H. Hepatoprotective activity of chrysin is mediated through TNF-α in chemically-induced acute liver damage: An in vivo study and molecular modeling. Experimental and therapeutic medicine. 2017 May 1;13(5):1671-80.
CrossRef - Yadav NP, Dixit VK. Hepatoprotective activity of leaves of Kalanchoe pinnata Pers. Journal of Ethnopharmacology. 2003 Jun 1;86(2-3):197-202.
CrossRef - Nagaich U, Gulati N, Chauhan S. Antioxidant and antibacterial potential of silver nanoparticles: biogenic synthesis utilizing apple extract. J Pharm. 2016; 7141523. doi:10.1155/2016/7141523
CrossRef - Chandan BK, Sharma AK, Anand KK. Boerhaavia diffusa: a study of its hepatoprotective activity. Journal of Ethnopharmacology. 1991 Mar 1;31(3):299-307.
CrossRef - Lin YC, Cheng KM, Huang HY, Chao PY, Hwang JM, Lee HH, Lu CY, Chiu YW, Liu JY. Hepatoprotective activity of Chhit-Chan-Than extract powder against carbon tetrachloride-induced liver injury in rats. journal of food and drug analysis. 2014 Jun 1;22(2):220-9.
CrossRef - Zhang H, Jacob JA, Jiang Z, Xu S, Sun K, Zhong Z, Varadharaju N, Shanmugam A. Hepatoprotective effect of silver nanoparticles synthesized using aqueous leaf extract of Rhizophora apiculata. International journal of nanomedicine. 2019; 14:3517.
CrossRef - Baravalia Y, Vaghasiya Y, Chanda S. Hepatoprotective effect of Woodfordia fruticosa Kurz flowers on diclofenac sodium induced liver toxicity in rats. Asian Pacific Journal of Tropical Medicine. 2011 May 1;4(5):342-6.
CrossRef - Arthur F, Woode E, Terlabi E, Larbie C. Evaluation of hepatoprotective effect of aqueous extract of Annona muricata (Linn.) leaf against carbon tetrachloride and acetaminophen-induced liver damage. Journal of Natural Pharmaceuticals. 2012 Jan 1;3(1):25.
CrossRef








