Sadhni Induar1 , Debasmita Dubey2
, Debasmita Dubey2 , Shakti Rath3*
, Shakti Rath3* , Rajesh Kumar Meher4
, Rajesh Kumar Meher4 , Santosh Kumar Swain5
, Santosh Kumar Swain5 and Subrat Kumar Tripathy6
and Subrat Kumar Tripathy6
1Food Sciences and Technology, Kalinga Institute of Social Sciences, Bhubaneswar, India.
2Medical Research Laboratory, IMS and SUM Hospital, Siksha ‘O’ Anusandhan Deemed to be University, K8, Kalinga Nagar, Bhubaneswar, Odisha, India.
3Microbiology and Research, Central Research Laboratory, Institute of Dental Sciences, Siksha ‘O’Anusandhan Deemed to be University, K8, Kalinga Nagar, Bhubaneswar, Odisha, India
4Centre of Excellence in Natural Products and Therapeutics, Department of Biotechnology and Bioinformatics, Sambalpur University, Jyoti Vihar, Burla, Sambalpur, Odisha, India.
5Department of Otorhinolaryngology, IMS and SUM Hospital, Siksha ‘O’ Anusandhan Deemed to be University, K8, Kalinga Nagar, Bhubaneswar, Odisha, India.
6Department of Biochemistry, IMS and SUM Hospital, Siksha ‘O’ Anusandhan Deemed to be University, K8, Kalinga Nagar, Bhubaneswar, Odisha, India.
Corresponding Author E-mail: dr.shaktirath@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2940
Abstract
Dioscorea alata belongs to Dioscoreaceae or the yam family. Around 600 Dioscorea species are consumed in various regions of the world. Dioscorea alata is well known cultivated tuber consumed by both rural and urban people. In this study, we have investigated the nutrient composition, phytochemicals, and antioxidant and antimicrobial activity of the underground and aerial tubers of Dioscorea alata. The result of the analysis showed that the aerial tuber of D. alata contained a higher amount of moisture (68.51%), ash (4.64%), starch (5.61%), reducing sugar (0.029%), fat (0.33%) and protein (1.39%) than underground tuber of D. alata. At the same time, carbohydrates, free amino acids, vitamin C, sodium, potassium and iron contents were superior in the underground tuber than in the aerial tuber. Further, both underground and aerial tuber was a good source of phenols, flavonoids, tannins and diosgenin. The underground tuber exhibited better DPPH scavenging potential compared to the aerial tuber. Six solvents extract of D. alata showed significant to moderate antibacterial activity toward seven tested clinical stains. Thus, the tuber of D. alata could be used as a better food supplement to meet the calorie requirement and a rich source of relevant antimicrobial agents to treat microbial infections.
Keywords
Antimicrobial agent; Diosgenin; DPPH; Flavonoid; TPC
Download this article as:| Copy the following to cite this article: Induar S, Dubey D, Rath S, Meher R. K, Swain S. K, Tripathy S. K. Evaluation of the Antioxidant and Antimicrobial Activity of the Nutritionally Rich Plant, Dioscorea alata L. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Induar S, Dubey D, Rath S, Meher R. K, Swain S. K, Tripathy S. K. Evaluation of the Antioxidant and Antimicrobial Activity of the Nutritionally Rich Plant, Dioscorea alata L. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3JGXMSD |
Introduction
Tubers are the storage organ of plants that store edible starch material as well as other nutrients and play a significant role in the contribution of dietary energy. Dioscorea, commonly known as yam, is a vital tuber-yielding crop that produces about 10% of the total global production of roots and tubers.1Genus Dioscorea of the family Dioscorea constitutes about 600 species throughout the world.2 There are about 12 species found in Odisha, among which Dioscorea alata is cultivated and the other eleven are wild species.2 Dioscorea alata produces two types of tuber. One type produces under the ground, known as an underground tuber while another type produces above the ground on the axils of the stem, known as an aerial tuber. These are found in Tropical areas, North America, Africa, Nepal, Indonesia, India, Japan, China, Mexico, Australia, South pacific islands, South America, West Africa, and East Africa.2-4 In India, D. alata is largely found the in the Eastern Ghats, Northeastern Himalayas and Western Ghats.2 In Odisha, it is distributed all over the state. It is rich in Similipal Biosphere Reserve, Phulbani district, Koraput, and Malkangiri districts of Odisha state.5 D. alata is the third most major tuberous crop after cassava and sweet potato.4 D. alata L. is documented as Greater yam, Water yam, purple yam and Winged yam.3,6 In different local languages identified as Kath also, Banra, Bandrara, Maati Aalu, Desia Aalu, Mate alu, Raja ala, Bebaru.2,3,6-10 D. alata is an annual and perennial climber plant of about 20-30 feet in height.3 Its purple colour stem has long petioles, bright green colour leaves and yellow-white colour flowers.3 The tuber of D. alata is white in colour and watery in texture.3 The tubers of D. alata are sources of many essential nutrients such as carbohydrates, protein, vitamins and other nutrients.1,4,10This nutritional richer is used as a chief ingredient of traditional Odia food dalma2. Besides nutrient components,Dioscorea alata also contains secondary metabolites such as phenolic acid, flavonoids, coumarins, quinines, alkaloids, amines, terpenoids, phytosterols, tannin, diosgenin, and saponins.2, 3, 8-11 D. alata has been reported to exhibit antifungal, antidiabetic, antibacterial and antioxidant activities.2, 9,12-15 Further D. alata helps to cure piles, and stomach worm and demonstrate anti-diarrhoea, anti-inflammatory, antihypertensive, hypolipidemic and hypocholesteric activity3. Juice of D. alata is used as a cooling agent during summer.2 Tuber pastes of D. alata are applied on cancerous wounds, leprosy, gonorrhoea and skin disease.8 In this present study nutritional values, DPPH scavenging potential and antimicrobial activity of both underground and aerial tuber of D. alata were investigated.
Materials and Methods
Collection and preparation of sample
Freshly harvested elongated spherical shape, D. alata tuber of good quality purchased from the local market of Burla, Odisha, India, in November 2018. The tubers were thoroughly washed three to four times to remove adhering soil. Cleaned tubers were peeled and sliced about 1-2mm in thickness. Thinly sliced tubers were oven dried at 80°C until a steady weight was obtained. Then pulverized by a food processor (Usha FP 3811 Food Processor) and screened through a 1mm sieve to get the powder form of the sample. Tuber powder was kept in an airtight glass bottle for further analysis.
Methods
Determination of moisture
The A.O.A.C method was used to determine the moisture content (1970).16 The samples were weighed carefully and dried at 80°C until they attained a consistent weight. The moisture content was calculated using the following relationship after the estimation was done in triplicate, and the mean values of both were recorded.
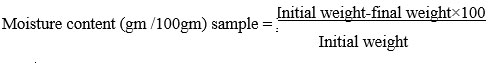
Ash
The ash content was determined by heating the food sample in a muffle furnace at 5500C.17
Carbohydrate
The Anthrone reagent method was used to estimate carbohydrates. 100mg of material was hydrolyzed in 5 ml of 2.5N HCL for three hours. Then sodium carbonate was added to this sample solution until the formation of effervescences ceased for neutralization and the volume was made up to 100ml before centrifugation. Anthrone reagent of 4ml was added to the 0.5ml of supernatant. Afterwards, the reaction mixture was heated for eight minutes in a hot water bath and the intensity of the developed green colour was measured spectrophotometrically at 630nm.18
Starch
100mg of the sample was washed with hot 80% ethanol till the washing didn’t give in green colour with Anthrone reagent to eliminate sugar from the sample. The residue was dried over a water bath before being mixed with 5 mL of distilled water and 52 % perchloric acid. Anthrone reagent of 4ml was added to 0.2 ml of supernatant and the reaction mixture was heated for eight minutes in a boiling water bath. After that, the intensity of colour was measured at 630nm. The sample’s glucose concentration was then multiplied by 0.9 to get the starch content.18
Protein estimation
Protein content was estimated by the Lowery et al., 1951 method. 19 Reagent C was a mixture of 50ml of reagent A (2 % sodium carbonate in 0.1N sodium hydroxide) and 1ml of reagent B (0.5 % copper sulphate in 1 % potassium sodium tartrate). Reagent D contained 1 mL Folin-Ciocalteau reagent and 1 mL distilled water. 1gm of dry powder sample was homogenized with 10ml cold phosphate buffer (Ph 7.5, 0.1M). After centrifugation, 5 ml of reagent C was added to 0.2 ml of sample extract, brought up to 1 ml with water, and left for 10 minutes. Then 0.5ml of reagent D was added to the reaction mixture and incubated for 30 minutes at room temperature. At 660nm, the developed blue colour was measured.
Fat
A powder sample of 5gm was transferred to a thimble plugged with a wad of fat-free cotton and dropped into the bottom of the extraction tube. The bottom of the extraction tube was connected to the Soxhlet flask and the top to the condenser. Before joining the flask weight of the empty flask was taken. In the extraction flask, 200ml of petroleum ether (Boiling point 400-500C) was poured. The extractions were continued for up to 16 hours. At the end of the extraction period, all the petroleum ether was evaporated and dried at 100°C for 1 hour, and it was cooled in desiccators and weighed.18
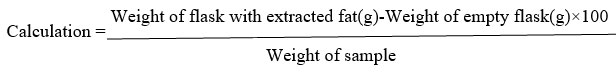
Ascorbic acid
The ascorbic acid solution was prepared by dissolving 5mg of ascorbic acid in 50 ml of4 % oxalic acid. The ascorbic acid solution was again mixed with 10 mL of 4 % oxalic acid. The prepared solution was titrated against 2, 6-dichlorophenolindophenol dye in sodium bicarbonate until the pink colour developed, which lasted a few minutes (V1). A sample of 5gm was extracted in 4 % oxalic acid and diluted to a volume of 100 mL before centrifugation. The supernatant of 5 ml was mixed with 10 mL of 4 % oxalic acid and titrated against the dye (V2 ml). 18
Calculation
Amount of ascorbic acid (mg/100g of sample)
=0.5mg/V1 ml ×V2 ml/5ml×100ml/ weight. of sample ×100
Sodium and potassium estimation
Flame photometry was used to determine sodium and potassium levels. KCL and NaCl were used to make standard solutions at different levels (0,5, and 10 ppm) of K and Na. A sample obtained by dry ashing was used to determine the total K and Na. First, the instrument was calibrated using a standard solution, and a standard curve was created. The digest was diluted to the appropriate concentration range, resulting in a final concentration of 0 to 5 mg/kg. The samples were then examined at 768 nm in a flame photometer. 17
Phosphorus
The phosphomolybdate technique was used to calculate phosphorus. 1ml of molybdate reagent (6.0g of ammonium molybdate was dissolved in 40ml of water, then 50ml of 10N H2SO4 was added, bringing the total amount to 100 ml) was added to 1ml of ash solution prepared by dry ashing. After that, 0.4ml of amino naphthol sulphonic acid solution was added, increasing the volume to 10ml. A blank was made the same way but using water instead of the sample. It was allowed to stand for 10 minutes before being tested at 650nm.17
Iron
The amount of iron in the sample was assessed by oxidizing it with potassium persulphate and then treating it with potassium thiocyanate to produce red ferric thiocyanate, which was quantified calorimetrically at 480nm.17
Phytochemicals estimation
Total phenolic content
Total phenolic content was determined by a slightly modified method of Oueslati et al., 2012.20 Distilled water (0.5ml) and Folin-Ciocalteu reagent (0.2ml) were added to the 0.5ml sample extract. The mixture was mixed properly and left for 6min. After that 0.2ml of 7% Na2CO3 was added to it. The final volume was made up to 3ml and incubated for 90 minutes in a dark place. Absorbance was measured at 760nm. The total phenolic content was calculated as mg of gallic acid equivalents per 100g of dry mass through a calibration curve with gallic acid.
Flavonoid estimation
The flavonoid content of the sample was determined using the method of Kamtekar et al., 2014 with slight changes.21 In a test tube, 1 mL of aliquots and 1 ml of quercetin solution received 4 mL of distilled water and 0.3 mL of 5% sodium nitrite solution. After 5mintues 0.3 mL of 10% aluminium chloride was added. Further 2 mL of 1M sodium hydroxide was added at 6 minutes. At 510 nm, the intensity of the yellowish-orange colour was measured. The flavonoid content was calculated as mg of quercetin equivalents per 100g of dry mass.
Tannin estimation
Tannin was calculated using Schanderl’s techniques (1970).22 The powder sample (0.5g) was boiled in 75ml water for 30 minutes and centrifuged at 2,000rpm for 20 minutes, the supernatant was collected, and a final volume of 100ml was obtained. From this, 0.1ml extract was taken with 7.5ml water, 0.5ml Folin-Denis reagent, and 1ml sodium carbonate solution was added, and a final volume of 10ml was obtained. After 30 minutes, the absorbance was measured at 700nm. Tannic acid equivalents are used to express the sample’s tannin content.
Diosgenin estimation
One gram of material was mixed with 30 ml of methanol and agitated overnight. The supernatant was obtained after centrifuging the extract for 18 minutes at 3500 rpm. The supernatants were obtained after two further extractions. The ultimate capacity was set at 100 milliliters. A tube was filled with 0.1ml of the methanol extract, evaporating under decreased pressure. The residue was diluted in 2 mL ethyl acetate, 1 mL each of reagent A (0.5 mL p-anisaldehyde in 99.5 mL ethyl acetate) and B (50 mL concentrated H2SO4 + 50 mL ethyl acetate) was added and thoroughly mixed. The test tube was inserted into a water bath and kept at 600 degrees for 10 minutes to generate a colour. After that, it was allowed to cool in a 250°F water bath for 10 minutes. A spectrophotometer was used to measure the absorbance at 430nm. 2ml ethyl acetate was put in a tube and tested in the same way as the reagent blank.23, 24
DPPH radical scavenging assay
DPPH free radical scavenging activity of the sample extract was evaluated using the method of Sakthidevi and Mohan, 2013 with slight modification.25 DPPH (1ml of 0.1mm) solution in methanol was added to 3ml of sample extract and ascorbic acid solution in methanol at different concentrations (100, 200, 400, 800 µg/ml). Ascorbic acid was taken as a reference. Absorbance was measured at 517nm. A lower absorbance value refers to higher DPPH scavenging activity. The following formula was used to calculate DPPH scavenging activity.
DPPH scavenging activity (% of inhibition) = (A0-A1)/A0 ×100
Where A0= Absorbance of the control, A1= Absorbance of sample and reference
Antimicrobial activity
Following the conventional procedure outlined, the powder sample was extracted sequentially using six organic solvents (methanol, chloroform, ethyl acetate, acetone, water, and petroleum ether).26 The extracts were centrifuged, filtered, and concentrated further in a vacuum rotary evaporator (model with evaporation condition). The antibacterial activity of the sticky layers was tested by dissolving them in 100 mg/ml DMSO. The antibacterial activities of different solvents and aqueous extracts of D. alata were determined using the agar well diffusion method, slightly modified from Navarro et al. (1966). Each Petri plate received about 25 mL of nutritional agar. Pathogenic multidrug-resistant bacteria (MDR) cultures such as A. baumannii, E. faecalis, K. pneumonia, and P. mirabilis were added to the agar once it had solidified. The antibacterial activity was measured in triplicate and expressed as the mean of inhibition. The sample’s minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) were determined a using previously published method.27
Toxicity evaluation
Methanolic extract of D. alata was evaluated for its acute and sub-acute toxicity following the OECD guidelines 423 and 407, respectively. The animal experiment was conducted at the School of Pharmacy, Siksha ‘O’ Aunsandhan University, and the protocol used was approved by the Animal Ethics Committee (Protocol IAEC/SPS/SOA/17/2018). Twenty-four male albino Wister rats (180-230g), aged 7-8 weeks, were divided into six rats, one control group, and three treated groups. Animals were kept in a temperature-controlled environment (23 ± 20 oC) with a 12-hour light-dark cycle. The control group received water only, and each treated group received a single oral dose of extract. Methanolic extract of D. alata was given in 2000 mg/kg, 4000 mg/kg and 8000 mg/kg body weight. After administering the extract, the animals were sectioned for changes in their general behaviour, physiological activities and survival for 72 hours in acute toxicity evaluation. In subacute toxicity analysis, the t, related group received extracts for 45 days and the animals were anaesthetised with formalin. The animals were sacrificed to collect their blood and organs (liver, kidney) for biochemical and histological analysis. On day 46, the control and treated groups o were given an overdose (0.2 ml) of 3.5% formaldehyde. Then blood was taken from the heart for analysis. The biochemical parameters analysed from serum were glucose (G), total cholesterol (TC), triglycerides (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea (Ur), creatinine (Cr) and total protein. The organs, liver, and kidney were removed and embedded in paraffin, sectioned and stained with hematoxylin and eosin. The tissues were observed under the microscope for histopathological toxicity evaluation.
Statistical Analysis
The results obtained were subjected to statistical analysis as mean and standard deviation.28 The mean values and standard deviations were calculated from the data obtained from three different experiments. The statistical difference at p < 0.05 was considered to be significant. Analysis of variance (ANOVA) was subjected within the animal groups for obtained data of each biomedical parameter in toxicity analysis.
Result
Nutritional components
Nutritional components analysis included moisture, ash, carbohydrate, starch, fat, protein, ascorbic acid and minerals (Figures 1 & 2; Table 1). The underground tuber’s moisture content was high (66.22%) than the areal tuber (58.94%). Areal tuber was significantly higher at p<0.05 ash (4.64%) than underground tuber (2.68%). The Carbohydrate Content of the areal tuber was estimated to be reasonably low (42.08%) compared to the underground tuber (58.96%). The starch content of the underground tuber was higher (5.61%) than the areal tuber (3.26%). The Underground and areal tuber contained the nearly same amount of fat (0.30%-0.33%). The protein content was higher (1.39%) in the aerial tuber compared to the underground tuber (0.78%). The amino acid content of the underground tuber was estimated to be quite high (2.49%) compared to the areal tuber (0.8%). The ascorbic acid content of the areal tuber on a dry weight basis was significantly low at p<0.05 (45mg/100gm) compared to the underground tuber (87.34mg/100gm). Analysis of minerals on a dry weight basis included sodium, potassium, iron and phosphorus. Sodium, potassium, iron and phosphorus contents of underground tuber were found to be 51.38mg/100gm, 206.33mg/100gm, 129.5mg/100gm and 20.21mg/100gm respectively while areal tuber contained 39.08mg/100gm, 195.23mg/100gm, 118.4mg/100gm and 22.02mg/100gm of sodium, potassium, iron and phosphorus respectively.
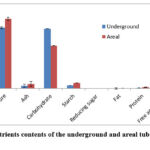 |
Figure 1: Nutrients contents of the underground and areal tuber of D. alata |
Table 1: Nutritional compositions of the underground and aerial tuber of D. alata
|
Parameter |
DA(Underground) |
DA(Aerial) |
|
Moisture |
59.73±0.88 |
68.51±1.5 |
|
Ash |
2.68±1.53 |
4.64±2.4 |
|
Carbohydrate |
58.96±0.02 |
42.08±0.05 |
|
Starch |
3.26±0.01 |
5.61±0.007 |
|
Reducing sugar |
0.014±0.005 |
0.029±0.007 |
|
Fat |
0.30±0.7 |
0.33±0.28 |
|
Protein |
0.78±0.01 |
1.39±0.22 |
|
Free amino acid |
2.49±0.48 |
0.8±0.9 |
|
Ascorbic acid (mg/100gm) |
87.43±1.2 |
45±0.05 |
|
Sodium(mg/100gm) |
51.38±2.56 |
39.08±1.67 |
|
Potassium(mg/100gm) |
206.33±2.51 |
195.23±2.02 |
|
Iron(mg/100gm) |
129.5±3.11 |
118.4±2.13 |
|
Phosphorus(mg/100gm) |
20.21±2.12 |
22.02±1.95 |
Note: Each value is the average of three analyses ± standard deviation.
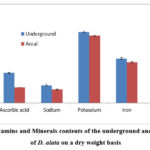 |
Figure 2: Vitamins and Minerals contents of the underground and areal tuber of D. alata on a dry weight basis |
Bioactive components:
The Flavonoid content of underground and areal tuber of D. alata was estimated to be 390mg/100gm and 273mg/100gm, respectively on a dry weight basis (Table 2; Figure 3). The total phenolic content of the underground tuber was found to be significantly high at p<0.05 (248.30mg/100gm) compared to the areal tuber (130mg/100gm) (Figure 3). The Diosgenin content of the underground tuber was found to be relatively low (50.87mg/100gm) compared to the areal tuber (89.67mg/100gm) (Figure 3). Tannin content was 451.23mg/100gm and 721.06mg/100gm for an underground and aerial tuber of D. alata, respectively (Figure 3).
Table 2: Bioactive components of D. alata tubers
|
Parameters |
Underground |
Aerial |
|
Flavonoid(mg/100gm) |
390 ±2.1 |
273±0.8 |
|
Phenol(mg/100gm) |
248.30±1.9 |
130±1.1 |
|
Tannin(mg/100gm) |
451.23±1.7 |
721.06±1.2 |
|
Diosgenin(mg/100gm) |
50.87±2.2 |
89.67±1.3 |
Each value is the average of three analyses ± standard deviation.
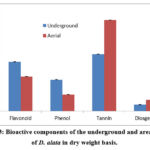 |
Figure 3: Bioactive components of the underground and areal tuber of D. alata in dry weight basis. |
Determination of antioxidant activity by DPPH scavenging activity
The IC50 value is defined as the amount of sample necessary to decrease the absorbance of DPPH by 50 %. The IC50 value of methanolic extract of the underground and areal tuber of D. alata was 121.81 µg/ml and 324.28 µg/ml, respectively. The underground tuber possessed a lower value of IC50 than the areal tuber, which indicated the underground tuber exhibited a higher potential for DPPH scavenging activity than the areal tuber. Figure 4 represents the DPPH scavenging activity of the underground and areal tuber of D. alata.
Table 3: IC50 values of D. alata tubers
|
Concentration (µg/ml) |
Underground |
Aerial |
|
100 |
33.8 |
29.6 |
|
200 |
58.2 |
39.8 |
|
400 |
95.2 |
58.9 |
|
600 |
95.7 |
75.5 |
|
800 |
97.1 |
86.5 |
|
IC50 |
121.81 |
324.28 |
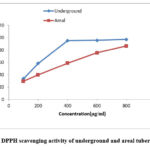 |
Figure 4: DPPH scavenging activity of underground and areal tuber of D. alata |
Antimicrobial property
The antibacterial activity of six solvent extracts was tested using the agar well diffusion method on independent lawn cultures of seven bacterial strains (2 GPs and 5 GNs). Acetone extracts had the most significant inhibitory zones against MRSA (29 mm) and P. mirabilis (29 mm). Similarly, the inhibitory zone against VRE was the largest in methanolic extract (29 mm). The petroleum-ether extract and aqueous tuber extract demonstrated deficient antibacterial activity than the other four solvent extracts. Antibacterial activity was evaluated on all other solvent extracts (Table 4).
The maximal antibacterial activity was evaluated by determining acetone and methanolic extracts’ MIC and MBC values. A MIC value of 3.0 mg/mL of acetone extract was registered against A. baumannii, E. fecalis, S. aureus, S. pyogenes, and P. mirabilis; 6.0 mg/mL as MIC against P. aeruginosa and K. pneumonia was recorded; Similarly, the MIC value of 6.0 mg/mL of methanolic extract was registered against A. baumannii, K. pneumoniae E. fecalis, S. pyogenes; 3.0 mg/mL against S. aureus, P. aeruginosa and P. mirabilis (Table 5). Further, the MBC values of these two active extracts were determined. An MBC value of 15.0 mg/mL of acetone extract wasregistered against A. baumannii and E. fecalis, and a discount of 25 mg/mL against S. aureus, P. mirabilis and K. pneumonia was recorded, and 50 mg/mL against P. aeruginosa was recorded. Similarly, an MBC value of 15.0 mg/mL of methanolic extract was registered against P. mirabilis; a 25 mg/mL discount against A. baumannii, E. faecalis, S. pyogenes, and K. pneumonia was reported; and a value of 50 mg/mL against S. aureus was recorded.
Table 4: Six hot solvents D. alata antimicrobial assays using the agar well diffusion method against MDR bacterial strains (zone of inhibition in mm).
|
Clinical Strain |
Petroleum ether |
Chloro- form |
Ethyl acetate |
Acetone |
Methanol |
Water |
Linezolid/imipenem (30/10 mg/mL) |
|
A. baumannii |
10± 1.33 |
21±1.37 |
13±1.21 |
33±1.13 |
27±1.21 |
15±1.31 |
29±0.89 |
|
E. faecalis |
15±2.37 |
22±1.33 |
23±1.12 |
28±1.61 |
26±0.93 |
22±1.37 |
29±0.37 |
|
K. pneumoniae |
17±1.33 |
15±1.24 |
14±1.59 |
26±0.53 |
29±0.51 |
13±1.15 |
33±1.23 |
|
P. mirabilis |
08±1.78 |
12±1.15 |
12±1.04 |
29±1.87 |
19±1.15 |
13.5±1.83 |
31±1.73 |
|
P. aeruginosa |
11±1.53 |
18±0.57 |
14±1.27 |
27±1.39 |
21±1.27 |
13±0.67 |
26±1.21 |
|
S. aureus (MRSA) |
10±1.01 |
19±1.93 |
14±1.53 |
26±1.73 |
23±1.33 |
14±1.19 |
29±0.73 |
|
S. pyogenes |
18±0.53 |
18±0.89 |
15±1.21 |
29±1.91 |
22±1.97 |
17±0.79 |
26±1.51 |
Each value is the average of three analyses ± standard deviation.
Table 5: MIC and MBC of two bioactive fractions of D. alata against MDR bacterial strains (mg/ml).
|
Strain |
Acetone |
Methanol |
||
|
MIC |
MBC |
MIC |
MBC |
|
|
A. baumannii |
3.0 |
12 |
6.0 |
25 |
|
E. faecalis (VRE) |
3.0 |
15.0 |
6.0 |
25 |
|
K. pneumoniae |
6.0 |
25 |
6.0 |
25 |
|
P. mirabilis |
3.0 |
25 |
1.5 |
15.0 |
|
P. aeruginosa |
6.0 |
50 |
3.0 |
25 |
|
S. aureus (MRSA) |
3.0 |
25 |
6.0 |
50 |
|
S. pyogenes |
3.0 |
25 |
6.0 |
25 |
MIC: Minimal inhibitory concentration, MBC: Minimal bactericidal concentration
Acute toxicity study
Oral administration of the D. alata methanolic extract (2000 to 8000 mg/kg body weight) neither caused any death nor produced significant changes in the spontaneous type, alertness, awareness, good response, touch response, pain response, righting reflex, pinna reflex, grip strength in the experimental rats, during 72 hours of the testing period. All groups of animals showed neither any toxic effect nor any lethal effect. Administration of doses up to 8000 mg/kg body weight of D. alata methanolic extract did not reveal any toxicity or mortality in rats during the entire observation period. Therefore, the LD50 of D. alata methanolic extract may be greater than 8000 mg/kg.
Sub-acute toxicity
Blood biochemical parameters and histopathology of the kidney and liver of the control and experimental animals were observed to investigate any side effects on the animal.
Biochemical parameters
The blood biochemical parameters between treated and untreated animals were analysed to examine whether the D. alata methanolic extract has any side effects on animals. The data were collated in Table 6 as well as Figure 5. Briefly, the parameters examined include glucose, urea, creatinine, protein, cholesterol, triglycerides, aspartate aminotransferase (AST) and alanine aminotransferase (ALT). After 45 days of daily doses of methanolic extract of D. alata failed to reveal any significant difference (using a one-way ANOVA test at p≤0.05) in various blood biochemical parameters between treated and untreated groups, indicating no side effects to animals.
 |
Figure 5: Blood biochemical parameters between treated and untreated groups of animals |
Table 6: Blood biochemical parameters of the control and treated groups of animals with boiled methanolic extracts of D. alata tuber.
|
Parameters |
Group-I |
Group-II |
Group-III |
Group-IV |
Normal range |
|
Glucose(mg/dl) |
78.46±0.46 |
75.24±0.12 |
76.10±0.05 |
75.24±0.08 |
70-110 |
|
Urea(mg/dl) |
35.60±0.62 |
32.37±0.18 |
31.84±0.29 |
34.21±0.28 |
15-45 |
|
Creatinine(mg/dl) |
0.86±0.05 |
0.85±0.03 |
0.83±0.01 |
0.83±0.007 |
0.5-1.5 |
|
Total protein(mg/dl) |
6.56±0.11 |
6.81±0.099 |
6.83±0.05 |
6.92±0.10 |
6.0-8.0 |
|
Total cholesterol (mg/dl) |
165.1±0.99 |
167.5±0.53 |
158.4±0.74 |
161.2±0.88 |
140-250 |
|
Tri glycerides(mg/dl) |
92.12±0.83 |
113.5±1.77 |
113.1±0.83 |
115±1.06 |
25-160 |
|
(AST)(IU/L) |
32.37±0.51 |
37.24±0.13 |
39.08±0.22 |
42.05±0.02 |
Up to 46 |
|
(ALT)(IU/L) |
27.46±0.41 |
26.58±1.42 |
31.53±0.67 |
34.05±0.03 |
Up to 40 |
The values are mean ± standard deviation. The differences in various blood biochemical parameters are statistically insignificant using one away ANOVA test among control and treated groups of the animal at p≤0.05). Group-I (Control), Group-II (2000 mg/kg body weight), Group-III (4000 mg/kg body weight), Group-IV (8000 mg/Kg body weight), AST–Asparateamino transferase, ALT- Alanine aminotransferase.
Histopathological studies
The effect of the methanolic extract of D. alata tuber on the histological changes of kidney and liver tissues after 45 days of the treatment has been shown below. The therapy with daily doses of 2000, 4000 and 8000 mg/kg body weight for 45 days failed to reveal any significant changes compared to the control group. Necrosis, infiltration, oedema and conjunction, which are signs of hepatotoxicity, were not observed in the liver cells of the experimental group. The liver showed standard hepatic lobular architecture. The kidneys revealed normal glomeruli, proximal and distal tubules, interstitium, and blood vessels. The histopathological images of the kidney and liver of control and treated with different doses are shown in Figure 6.
 |
Figure 6: Panels represent H&E staining of paraffin-embedded five-micron-thick sections of the kidney and liver at magnifications 200x of control and treated animals with an increasing dose of D. alata tuber methanolic extract. |
Table 7: Effect of methanolic extract of Dioscorea alata on acute toxicity.
|
Behaviour Type |
Treatments |
|||
|
Control |
2000 mg/kg body weight |
4000 mg/kg body weight |
8000 mg/kg body weight |
|
|
Spontaneous type |
N |
N |
N |
N |
|
Alertness |
N |
N |
N |
N |
|
Awareness |
N |
N |
N |
N |
|
Sound response |
N |
N |
N |
N |
|
Touch response |
N |
N |
N |
N |
|
Pain response |
N |
N |
N |
N |
|
Righting reflex |
N |
N |
N |
N |
|
Pinna reflex |
N |
N |
N |
N |
|
Grip strength |
N |
N |
N |
N |
|
Food intake |
N |
N |
N |
N |
|
Water intake |
N |
N |
N |
N |
|
Mortality |
Ab |
Ab |
Ab |
Ab |
Discussion
Moisture content represents the water present in the tuber. Water physically interacts with protein, polysaccharides, and lipids and influences texture, appearance, and flavour. But high moisture content affects the keeping quality of tubers.29 The moisture content of D. alata was significantly higher in the present study than the values recorded in the available literature.4,30,31 However, another study reported a meagre amount of moisture content for D. alata.32This variation in moisture content between the present study and other reports might be due to the level of maturity of tubers, geographical regions of cultivation, and methods used for estimation. Ash is the inorganic food residue remaining after heating destroys organic matter. Inorganic components within a food represent minerals such as Na, K, Ca, Mg, Mn, P, Fe, Zn, Cu etc. Similar ash content has been reported in a previous study.31 At the same time, the ash value reported in other studies differs remarkably from the present study. 4,33 The ash content of tubers varied due to soil, harvesting time and moisture content.31 Carbohydrates are the primary energy source in the body. Fauziah et al., 2020 reported a low amount (17.10-29.37%) of carbohydrates compared to the present findings4. D. alata tubers are considered energy-giving food crops due to their appreciable amount of carbohydrates. Starch is the most abundant form of carbohydrates which store energy in plants. Another study reported a much higher value of starch (62.94%) than the present study.34 Fat supplies more than twice the energy furnished by carbohydrates or protein per unit weight. Fat in the diet helps with the absorption of fat-soluble vitamins, and it also contributes to the palatability of food.29 Fat is an essential component of the diet. An earlier study reported 1.62% of fat and 8.40% of protein for Dioscorea alata.32 Both areal and underground tuber contained appreciable amounts of vitamin C, which indicates consumption of this tuber could help in the absorption of iron, and decrease atherosclerosis and some kind of cancer.35 Minerals are inorganic nutrients only needed in small quantities.36 Minerals are necessary for the bulk of the body’s metabolic processes—electrolytes like potassium and salt help keep fluid and blood volume in check. Blood pressure rises when people consume too little potassium and too much sodium. Phosphorus is needed for various functions, including ATP generation, signal transmission, and bone mineralisation. Iron is a component of cytochromes and electron transport. Available literature shows that D. alata is a good source of minerals. 4,31,32
Bioactive compounds
Bioactive compounds are biologically active substances which have positive or negative effects on living organisms.37 Flavonoids are a class of polyphenolic chemicals with several benzene rings. Allergies, inflammation, free radicals, platelet aggregation, bacteria, ulcers, hepatotoxins, viruses, and cancers are all protected by flavonoids.38 Phenolic compounds are potent antioxidant and scavenging agents. The present study suggested that D. alata is a rich source of phenols and flavonoid content. This report was corroborated by other studies 9,14,25. Dioscorea species are an essential source of Diosgenin, a commercially vital bioactive sapogenin.39 It is used to manufacture crucial pharmaceutical steroidal drugs, such as precursors, to produce sex hormones and oral contraceptives.39 Tannin is one of the phenolic compounds which give an astringent and bitter taste.38 A previous study reported 0.58mg/100gm of tannin for D. alata.34
DPPH scavenging activity
Antioxidant molecules in food are a vital protective factor for one’s health. Antioxidants protect by these defence methods: the first line of defence prevents excessive formation of reactive oxygen species by inactivating endogenous cations like Fe+ and Cu+. The second line of defence comprises tocopherols, tocotrienols, carotenoids, ascorbic acid, and other phytochemicals that can scavenge reactive oxygen species. The result of the present study showed that the underground tuber has more DPPH scavenging potential than the areal tuber. Similarly, a study from India also recorded underground tuber of D. alata is a more potent DPPH scavenger than the aerial tuber.14 D. alata tuber exhibited DPPH scavenging potential might be due to the presence of phenols, flavonoid, tannin, diosgenin and ascorbic acid in tuber 6, 9, 35, 40.
Antimicrobial activity
Based on susceptibility tests that produce MIC in 100-1000 mg/mL, phytochemicals are classified as to whether they have antimicrobials.41 If the MIC values are observed below 100 µg/mL, the activity is considered significant and moderate when 100<MIC<625 μg/mL.42,43 The agar healthy diffusion test against all tested clinical strains revealed that the crude extracts from D. alata showed significant to moderate antibacterial activity. Therefore, the activity recorded with the natural section on the clinical stress of A. baumannii, E. faecalis, K. pneumonia, P. mirabilis, P. aeruginosa, S. aureus (MRSA), S. pyogenes has similar activity with the earlier report 2,8,9,44. A study reported an inhibition zone of 12mm against Salmonella paratyphi and Shigella dysenteriae for chloroform soluble fraction of D. alata at a concentration of 400µg/disc8. Another study recorded an inhibition zone of 1cm against S. pyogenes at a concentration of 1mg/ml for acetone extract of D. alata.2 At the same time, tuber extract of 500µg/ disc showed a maximum inhibition zone of 17.16mm against Shigella dysenteria9. D. alata tuber exhibited antibacterial activity may be due to the presence of phytochemicalsPhenol, flavonoid and tannin in the tuber.25,45-47 The tannin inhibits the synthesis of cell protein in bacteria because tannin forms irreversible complexes with proline-rich proteins.45 Carbonyl group of flavonoids form complexes with extracellular and soluble proteins within the bacterial cell wall.46,47
Toxicity analysis
Administration of doses up to 8000 mg/kg body weight of D. alata methanolic extract did not reveal any behavioural changes or mortality in rats during the entire observation period. Therefore, LD50 of D. alata methanolic section may be greater than 8000 mg/kg. Daily doses of methanolic extract of D. alata for up to 45 days failed to reveal any significant difference in various blood biochemical parameters between treated and untreated groups, indicating no side effects to animals. Necrosis, infiltration, oedema and conjunction, which are a sign of hepatotoxicity, were not observed in the liver cells of the experimental group. The kidneys revealed normal glomeruli, proximal and distal tubules, interstitium, and blood vessels. The result of the study indicated that the consumption of D. alata is entirely safe.
Conclusion
The nutritional composition, antioxidant and antimicrobial activity of the underground and aerial tuber of D. alata were evaluated in this present study. Results of the analysis suggested that the nutritional composition of the underground tuber is more vibrant than the aerial tubers. Further, the antioxidant activity of the underground tuber was found to be significantly very high compared to the aerial tuber. The present study also emphasizes the phytochemical analysis and antimicrobial potential of D. alata against clinical microbial cultures. The extracts of D. alata tubers have shown excellent activity against A. baumannii, E. faecalis, K. pneumonia, P. mirabilis, P. aeruginosa, S. aureus (MRSA) and S. pyogenes. Hence, the tuber D. alata can be used for functional food and as a potential antimicrobial agent against pathogenic microorganisms. All the biochemical parameters were found to be within the normal range in all the treated groups, and also there were no changes in the structure of the kidney and liver in all the treated groups. The raw and boiled D. alata does not create any injury. There was an absence of acute and sub-acute toxicity in mice.
Acknowledgement
None
Conflicts of Interests
The authors do not have any conflicts of interests
Funding Sources
None
References
- Panda D, Biswas M, Padhan B, Lenka SK. Traditional processing associated changes in chemical parameters of wild Yam (Dioscorea) tubers from Koraput, Odisha, India. Indian. J. Traditional. Knowl. 2020; 19(2): 268-276.
CrossRef - Kumar S, Mahanti P, Sk R, Jk P. Qualitative phytochemical analysis and antibacterial activity of Dioscorea alata L.: A nutraceutical tuber crops of rural Odisha. J. Altern. Med. Res. 2017; 3(1), 1-5.
- Kaur B, Khatun S, Suttee A. Currents highlights on biochemical and pharmacological profile of Dioscorea alata: A review. Plant. Archives. 2021; 21(1):552-559
CrossRef - Fauziah F, Mas’udah, S, Hapsari L, Nurfadilah S. Biochemical composition and nutritional value of fresh tuber of water yam (Dioscorea alata L.) local accessions from East Java, Indonesia. Agrivita. J. Agri. Sci. 2020; 42(2); 255-271.
CrossRef - Kumar S, Behera SP, Jena PK. Validation of tribal claims on Dioscorea pentaphylla L. through phytochemical screening and evaluation of the antibacterial activity. Plant. Sci. Res. 2013; 35(1&2):55-61.
- Amarasekara R, Wickramarachchi SR. (). Antioxidant activity of phenolic compounds in Dioscorea alata L. (Raja Ala) tuber cooking water. Acta chemical IASI. 2021; 29(2), 183-200.
CrossRef - Das A, Chaudhuri D, Ghate NB, Chatterjee A, Mandal N. Phytochemicals analysis, antioxidant and anticancer potential of leaf extracts from edible greater yam, Dioscorea alata L., from North-east India. Int. J. Phytopharmacol. 2014; 5(2):109-119.
- Hossain MA, Haque ME, Kawsar MH, Rana MS. In vitro Bioactivities of aerial parts of Dioscorea alata. Bangladesh. Pharm. J. 2017; 20(2); 200-204.
CrossRef - Anisuzzman Md, Zilani MNH, Khusi SS, Asaduzzman Md, Hossain MG. Antioxidant, antibacterial potential and HPLC analysis of Dioscorea alata bulb. Indonesian. J. Pharm. 2016; 27(1):9-14
CrossRef - Saklani S, Chandra S, Mishra AP. Nutritional profile, Antinutritional profile and Phytochemical screening of Garhwal Himalaya medicinal plant Dioscorea alata Tuber. Int J Pharm Sci Rev Res. 2013; 23(2):42-46.
- Gul MZ, Bhakshu LM, Ahmad F, Kondapi AK, Qureshi IA, Ghazi IA. Evaluation of Abelmoschus moschatus extracts for antioxidant, free radical scavenging, antimicrobial and antiproliferative activities using in vitro assays. BMC. Complement. Altern. Med. 2011; 11:64.
CrossRef - Eleazu CO, Kolawole S, Awa E. Phytochemical composition and antifungal actions of aqueous and ethanolic extracts of the peels of two yam varieties. Medicinal. Arom. Plant. 2013; 2, 1-4.
- Maithili V, Dhanabal SP, Mahendran S, Vadivelan R. Antidiabetic activity of ethanolic extract of Dioscorea alata in alloxan-induced diabetic rats. Indian. J. Pharmacol. 2011; 43(4); 455.
CrossRef - Das A, Chaudhuri D, Mandal N, Chatterjee A. Study of antioxidant and reactive oxygen species scavenging activity of the edible tuber of “greater yam” (Dioscorea alata L.) from north-east India. Asian J Pharm Clin Res. 2012; 5(3:) 74-78.
- Pugazhendhi S, Sathya, P, Palanisamy PK, Gopalakrishnan R. Synthesis of silver nanoparticles through a green approach using Dioscorea alata and their characterization on antibacterial activities and optical limiting behaviour. 2016;159:155-160.
CrossRef - AOAC. Official Methods of analysis. 11th edition Association of Official Analytical Chemists, Washington, DC, EUA, 1970; 997, 129
- Ranganna S. Handbook of analysis and quality control for fruits and vegetable products, 2nd edition. Tata McGraw-Hill Publishing Company Ltd, New York; 2007
- Sadasivam S, Manickam A. Biochemical methods, 3rd edition. New age international (P) Limited, Publishers, New Delhi; 2008.
- Lowery OH, Rosebraugh NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biological Chem. 1951; 193, 265-275.
CrossRef - Oueslati S, Ksouri R, Falleh H, Pichette A, Abdelly C, Legault J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012; 132, 943-947.
CrossRef - Kamtekar S, Keer V, Patil V. Estimation of phenolic content, Flavonoid content, Antioxidant and Alpha-amylase inhibitory activity of marketed polyherbal formulation. J Appl Pharmaceutical Sci. 2014; 4(9), 61-65.
- Schanderl SH. In: Method in Food Analysis, Academic Press, New York, 1970; p.709
- Baccou JC, Lambert F, Sanvaire Y. Spectrophotometric method for the determination of total steroidal sapogenin. Analyst 1977; 102: 458-466.
CrossRef - Uematsu Y, Hirata K, Saito K. Spectrophotometric Determination of saponin in yucca extract used as a food additive. J AOAC Int. 2000; 83: 1451-1454.
CrossRef - Sakthidevi G, Mohan VR. Total phenolic, flavonoid contents and in vitro antioxidant activity of Dioscorea alata L. tuber. J Pharmaceutical Sci Res 2013; 5(5): 115-119.
- Abubakar AR, Haque M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J PharmBioallied Sci. 2020; 12; 1-10.
CrossRef - Kuete V, Betrandteponno R, Mbaveng AT, Tapondjou LA, Meyer JJ, Barboni L, Lall N. Antibacterial activities of the extracts, fractions, and compounds from Dioscorea bulbifera. BMC. Complement. Alternat. Med. 2012;12: 228.
CrossRef - Zar JH. Biostatistical Analysis. Englewood Cliffs NJ, Prentice-Hall. 1984;5: 437-467.
- Manay NS, Shadaksharaswamy. Foods facts and principle, third revised edition. New age international (P) limited, publishers. 2014
- Shanthakumari S, Mohan VR, Britto JD. Nutritional evaluation and eliminating toxic principles in wild yam (Dioscorea spp.). Trop. Subtrop. Agroecosys. 2008; 8: 319-325.
- Baah FD, Maziya-Dixon B, Asiedu R, Oduro I, Ellis WO. (). Nutritional and biochemical composition of D. alata (Dioscorea spp.) tubers. J. Food. Agri. Environment. 2009; 7(2):373-378
- Oko AO, Famurewa AC. Estimation of nutritional and starch characteristics of Dioscorea alata (water yam) varieties commonly cultivated in South-Eastern Nigeria. British. J. Appl. Sci. Technol. 2015; 6(2): 145-152.
CrossRef - Ogidi IA, Wariboko C, Alamene A. Evaluation of some nutritional properties of water-yam (Dioscorea alata) cultivars in Bayelsa State, Nigeria. European. J. Food. Sci. Technol. 2017; 5(3): 1-14.
- Adegunwa MO, Alamu EO, Omitogun LA. Effect of processing on the nutritional contents of yam and cocoyam tubers. J. Appl. Biosci. 2011; 46: 3086-3092.
- Gandhi R, Jagtap T, Kopare N, Shirsat R, Koche D. Nutritional profiling of wild areal tubers of Dioscorea bulbifera L. from Maharashtra, India. Int. J. Botany. Stud.2021; 6(5):458-462.
- Soetan KO, Olaiya CO, Oyewole OE. The importance of mineral elements for humans, domestic animals and, plants-A review. African. J. Food. Sci. 2010; 4(5): 200-222.
- Guaadaoui A, Benaicha S, Elmajdoub N, Bellaout M, Hamal A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J Nutr. Food. Sci. 2014; 3(3):174-179.
CrossRef - Poornima GN, Ravishankar RV. Evaluation of phytonutrients and vitamin contents in wild yam, Dioscorea pentaphylla (Prain) Haines. African. J. Biotech. 2009; 8(6):971-973
- Shah HJ, Lele SS. Extraction of diosgenin, a bioactive compound from natural source Dioscorea alata var purpurea. Analytical. Bioanalyt. Techn. 2012; 3(4), 1-3.
- Jesus M, Martins APJ, Gallardo E, Silvestre S. Diosgenin: Recent highlights on pharmacology and analytical methodology. J. Analytical. Method. Chem. 2016; 2016:1-16
CrossRef - Simoes M, Rocha S, Coimbra MA, Vieira MJ. Enhancement of Escherichia coli and Staphylococcus aureus antibiotic susceptibility using sesquiterpenoids. Med. Chem. 2008;4(6):616-23
CrossRef - Kuete V, Efferth T. Cameroonian medicinal plants: pharmacology and derived natural products. Front. Pharmacol. 2010; 1:123.
CrossRef - Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta. Medica. 2010; 76:1479–1491.
CrossRef - Kwon JE. Antimicrobial and antioxidant activity of the Dioscorea alata L. Korean J Microbiol. Biotechnol. 2010; 38(3): 283-288
- Scalbert A. Antimicrobial properties of tannins. Phytochem: 1991; 30:3875-3883
CrossRef - Cowan MM. Plant products as antimicrobial agents. Clin. Microbial. Rev. 1999; 12(4)564-582
CrossRef - Ravikumar S, Gnanadesign M, Suganthi P. Ramalakhsmi A. Antibacterial potential of chosen mangrove plants against isolated urinary tract infectious bacterial pathogens. Int. J Medical. Sci. 2010; 2(3):94-99








