Manuscript accepted on :11-03-2024
Published online on: 19-03-2024
Plagiarism Check: Yes
Reviewed by: Dr. Ahmed Flayyih Hasan Aljanabe
Second Review by: Dr. Nagham Aljamali
Final Approval by: Dr. Mariia Shanaida
Diyar Majid Jalil and Taghreed Altaei*
and Taghreed Altaei*
Faculty of Pharmacy, Isra University, Amman/ Jordan
Corresponding Author E-mail: tagreedaltaei@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2883
Abstract
Cardiotoxicity is a cause of death by drug overdose. Silymarin, a cytoprotective agent used in this research to protect against induced-cardiotoxic effects in Albino Wister rats; exhibited signs of heart damage, such as elevated levels of S100B, troponin I, and CK-MB. Seventy albino Wister rats of both genders were divided randomly with each group having 10 rats. Silymarin-treated, cardiotoxic-induced, and control groups were treated for ten days. The bioavailability of silymarin was assessed, and evaluation of the efficacy of silymarin on the biomarker S100B and cardiac biomarkers (Troponin I, and CK-MB), also the histopathological assessments of the heart, liver, and kidney, in addition to the coefficient correlation of the studied biomarkers were analyzed. Research’s outcome indicated that the sets subjected to silymarin presented substantial differences in rat weight and food consumption, compared to a decrease in cardiotoxic clusters. S100B plasma level was increased in cardiotoxic groups, reduced in those subjected to silymarin, and eliminated by pretreatment with silymarin. Troponin I & CK-MB expressively elevated significantly in cardiotoxic prompted rats, which declined with silymarin treatment and were prohibited in pretreatment by silymarin. The shielding characteristic of silymarin detected in end organs, like the liver, kidneys, and hearts when exposed to the cardiotoxic agent clozapine was extremely significant. The consequences of the histopathological examination of this study illustrated silymarin’s cardioprotective effects. A significant positive coefficient correlation of S100B with troponin I & CK-MB was recorded. In conclusion, silymarin reduces and prevents to a larger extent the cardiotoxicity brought about by clozapine and averts heart injury. The cardioprotective efficacy of silymarin is explained by its new mechanism of action as decreasing S100B, troponin I & CK-MB levels with a strong significant correlation to each other. The cardioprotective efficacy of silymarin gives promise for monitoring the cardiotoxicity adverse drug reaction induced by drugs.
Keywords
Cardiotoxicity; Cardiac function; S100B protein; Silymarin
Download this article as:| Copy the following to cite this article: Jalil D. M, Altaei T. Efficacy and Bioavailability of silymarin on Plasma S100B Level in Cardiotoxicity-induced Rats. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Jalil D. M, Altaei T. Efficacy and Bioavailability of silymarin on Plasma S100B Level in Cardiotoxicity-induced Rats. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/4ckYYrO |
Introduction
The concept of direct cardiac toxicity refers to the structural and functional changes that can occur in the cardiovascular system as a result of exposure to medicines. This phenomenon can be affected by drugs such as cancer drugs, antibiotics, and antipsychotics. Most drugs that are used for treating various diseases have cardiotoxicity side effects2. A cardiac adverse drug reaction (ADR) is a broad category of effects that can include myocardial infarction, heart failure, thrombosis, pericarditis, and arrhythmias. The underlying mechanisms are believed to include the disturbance of ionic processes, the induction of cellular damage by mitochondrial dysfunction, and hypercoagulability1.The main mechanisms that cause this are hypoxia, oxidative anxiety, and the production of free radicals3.
Clozapine is an atypical antipsychotic indicated medicinally for treatment-resistant schizophrenia. The pharmacological activities of clozapine are binding to dopamine D2 and D4 receptors, and anti-α-adrenergic, anti-muscarinic, anti-histaminic, and anti-serotoninergic effects4. It is known to increase the mortality rate due to heart inflammation. The presence of these conditions can obscure the supervision of patients, which can affect the clinical results5, 6. Clozapine is associated with the risk of myocarditis and cardiomyopathy7. Detecting cardiotoxicity in people administering clozapine who are at considerable risk can be done by measuring the levels of clozapine-N-oxide formation, and N-oxidation relative to N-desmethylation ratios during treatment by clozapine8.
Milk thistle is native to the Compositae family and is regarded as a type of liver disease treatment9. It is carefully studied for its ability to aid in treating liver illness10. According to Theophrastus, the plant has been used as a universal herb since the fourth century BC. The dried seeds of the plant contain various nutrients, such as flavonoids11.
Silymarin (SM) is a compound that contains four flavonolignans: silydianin, isosilychristin, silybin, and taxifolin12. It proves its cardiac protective effects during the coronary artery bypass graft (CABG) operation, due to its anti-inflammatory and antioxidant effects13. When exposed to acrolein-induced cardiotoxicity, the compound’s protective mechanisms were confirmed in a mouse model14.
The integrity of the Ca2+ channel is maintained by the presence of various proteins, such as those belonging to the Calmodulin superfamily15. The S100B protein is mainly concentrated in the astrocytes. It binds to calcium and is a vital component of the nervous system. The level of S100B protein in biological fluids can be regarded as a reliable indicator of distress in the nervous system. Recent studies have shown that the S100B protein is a damage-associated molecular pattern molecule.
The high concentration of the S100B protein can trigger tissue reactions that are related to damage16. The presence of S100B protein has been shown to play a role in various cellular processes. These include the regulation of cell differentiation, protein phosphorylation, and Ca2+ homeostasis. The large concentration of this protein in cells allows it to interact with other targeted proteins17, 18.
An assessment of the following was conducted for the first time to evaluate the impacts of silymarin efficacy as cardioprotective on S100B and cardiac function biomarkers levels in the induced-cardiotoxic model by clozapine in rats.
The efficacy of different doses of silymarin as protection against induced cardiotoxicity by evaluation of the biomarker S100B and cardiac function biomarkers (troponin I, CK-MB).
The Bioavailability of silymarin.
Examination of minute histopathology of body organs tissues such as heart, liver, and kidney.
The correlation of the biomarkers S100B, troponin I, and CK-MB levels amongst themselves to cardiotoxicity.
Materials and Methods
Materials
The chemical/ reagents used were rats, S100B, Elisa kit (AZ chemicals from Germany), CK-MB (Casabiotech from The USA), Troponin (Life Diagnostics from The USA), Dimethyl sulfoxide (Laboratory Rasayan from India), Ethanol absolute, Formaldehyde 37 – 40 %, Methanol, Phosphate buffer, acetonitrile, and HCL (AZ chemicals from Germany), Sodium chloride 0.9% w/v (KRxS from Germany), silymarin powder, Silybin, naringenin (internal standard, IS), Clozapine (Sigma–Aldrich, USA). Reference standards; Chroma Dex (Santa Ana, CA).
Methods
Rat and housing
The experiment used Seventy Albino rats, both male and female with weights of between 150-400 grams and an average age of 12-16 weeks. They were taken care of in ideal laboratory settings in the university’s faculty of pharmacy, Isra University. In quadruple, the rats were accommodated in cages where they could last at least one week before the start of the study. They were luminated at 12 hours intervals and the temperatures were maintained plus or minus 25 degrees and a humidity of between 10 and 50 percent. Rodent chow and tapped water were also available. The research was approved by an institutional Committee for Ethics in Animal Use from the University Faculty of Pharmacy (Protocol n. 001.11.2018) and was done according to the general ethical guidelines by N.I.H Publications No. 85-23, revised 1985).
Experimental design /study groups
A total of 70 albino Wister rats of both genders were divided randomly with each group having 10 rats. 3 silymarin doses were administered in a solution of 1 ml IP and DMSO (Dimethyl sulfoxide) as a solvent to groups 1 to 3. Groups 4-5 were cardiotoxic-induced and treated, while group 6 served as cardiotoxic-induced and 7 as negative control. The following is their randomization chat (randomized statistically by Microsoft Excel) in doses of milligrams per kilogram per day, for ten days.
(G1): 80 of silymarin.
(G2): 140 of silymarin.
(G3): 200 silymarin.
(G4): 25 Clozapine I.P. was injected in ten days and trailed by 140 silymarin (10 days).
(G5): 140 of silymarin (10 days), and then received 25 Clozapine I.P. (10 days).
(G6): 25 Clozapine I.P.
(G7): 0.5 ml of normal saline (control group).
Dosage and Trial Preparation
The solution of SM was prepared by reconstitution of SM powder with DMSO daily under sterile conditions before injection. The rats were anesthetized with ether (diethyl ether) solution (20 ml of ether on cotton in a jar) which required to render them unconscious, approximately 5 min. (3ml) the blood sample was withdrawn from the retro-orbital plexus by use of a capillary tube and transferred to an EDTA tube. Centrifugation of blood was instantly done for 10 minutes (1600RPM). Separation of plasma from the blood was done using the pipette to an Eppendorf covered by parafilm maintained at -20 degrees. The weights of the subjects were figured out by pre- and pro-research exercises. Every group except for controls had SM solution administered IP. The controls were injected with normal saline for the negative and clozapine for the positive control and left for ten days while receiving treatment dosages as per the schedule. On the tenth day, they were anesthetized using an ether solution. The process of withdrawing blood and centrifuging at 1600RPM for ten minutes was repeated and the separation and storage were at -20 degrees. A high concentration of ether was used to sacrifice the rats and their organs were removed by dissection and dried using filter paper. The organs and tissues were then stored in a 10% buffered formalin solution for histopathological sectioning studies. Rats in cardiotoxic clusters; blood samples were collected, and plasma was prepared. The rats were then sacrificed, and hearts were excised immediately, weighed, and prepared for histopathology analysis.
Bioavailability of silymarin
SM contents were ascertained by use of an HPLC-system (LC-2010A HT) with an Agilent Eclipse XDB-C18- column; (5 mm, 4.6_250 mm). The mobile phase; is methanol & pure water (46:54, v/v); at a flow rate of 0.8 ml per minute. Monitoring of effluent was conducted at 288 nm. Standardized silymarin, naringenin [the internal standard (IS) for quantification], Silybin concentrations in plasma samples were analyzed. Samples were added to IS (naringenin, 20 ng/mL in acetonitrile). It was then vigorously mixed for around ten minutes. Centrifugation was then performed at a rate of 16,000 x g for around five minutes. Finally, 5 μL of supernatant aliquots were directly injected into an LC- system for analysis.The pharmacokinetic parameters: such as maximum-plasma-concentration (Cmax), time of Cmax (Tmax), and area under plasma-concentration-time curve (AUC0-t) were calculated.
Evaluation of cardiac biomarkers
Assessment of the biomarkers; S100B, Troponin I, and CK-MB from the plasma was done as per the manufacturer’s instructions of Enzyme linked Immune Sorbent Assay.
Rat S100B assay
The quantitative sandwich immunoassay technique was utilized for the analysis. An antibody specific to S100B was pre-coated on a microplate. A pipette was used to place samples and standards into the wells, and an immobilized antibody was then bound to any S-100B protein. A biotin-conjugated antibody for S100B was then added to the wells after unbound substances were removed. Following a wash, a substrate solution was then added to the wells.
The intensity of the dye was measured after the development of the substance stopped. The concentration of S100B that could be detected was typically less than 0.78. The sensitivity of this test, which was determined as the Lower Limit of Detection (LLD) could be differentiated. The value of 20 replicates of zero-standard added using three standard deviations was then determined.
Troponin I assay
The procedure utilized two antibodies that can recognize Troponin’s epitope, which is characterized by a protease-resistant nature. One of these was immobilized on a microtiter well. The other was conjugated to horseradish peroxidase (HRP), and it was used for detection. Diluted and standard samples were then placed into the wells for one hour using an HRP conjugate. The Troponin molecules were then sandwiched between the detection and immobilization antibodies. Tetramethylbenzidine (TMB) was added to the wells for around 20 minutes. A blue-colored Troponin substance was then present, and its color was stopped by adding a stop solution. The absorbance at 450 nm was then measured. The Troponin concentrations were then calculated.
CK-MB assay
The activity of the CK-MB enzyme was estimated using a method that utilizes a quantitative sandwich immunoassay. This method involves coating an antibody specific to CK-MB on a microplate. A set of standards and samples was then placed into the wells. An immobilized antibody was then added to bind any CK-MB that was presented.
A biotin-conjugated anti-CK-MB antibody was added to the well after unbound substances were taken out. Following that, HRP was added to remove any avidin-enzyme reagent or substrate. As indicated earlier, the solution was added to wells, and the resulting color was proportional to the CK-MB’s initial bounded quantity. The absorbance was then measured at 450 nm, and the intensity of the dye, and the concentration of CK-MB were calculated.
Histopathology assessments
The organs collected were immersed in 10% formalin and a clearing agent, Xylene, was presented to penetrate the tissues. Lastly, paraffin was introduced to end the process of embedding to create a block of paraffin. Sectioning of blocks then occurred via a microtome into 4-5 µm thick sections before hematoxylin staining for further scrutiny. Each ventricle slice was fixed in a 10% formalin solution before embedding in paraffin. The specimen was assessed for normal histopathological structures connected with a myocyte cardiotoxic agent. The ventricle specimens were evaluated for typical histopathological features associated with clozapine-induced cardiotoxicity including inflammation, myocyte vacuolar degradation, necrosis of myofibers, and interstitial fibrosis).
Statistics
SPSS version24 was run to analyze the data. G*Power 3.1.9.4. software was used for Effect Size and Sample size calculations. Means and standard deviations were the main determinants in quantitatively explaining the validity. Statistical comparison between the means of two groups (i.e., means of two diverse groups or comparing means of one sample on two separate occasions) and autonomous samples was made by using the t-test. It determined the average values between the 2 groups. The change of means was ascertained using variance analysis. Pearson correlation coefficient helped in assessing the strength and the association between two numerical variables.
study outcomes
Seventy domestic Wister albino rats of varying ages 12-16 weeks, with weights of between 150-400 grams were utilized. Both genders were involved in a ratio of 9: 5.
The efficacy of different doses of silymarin as cardioprotection for cardiotoxicity-induced rats
Silymarin efficacy on the rats’ weight and food consumption
Figure 1Ashows thatin all the groups the weight of the rats as measured daily increased after the tenth day except for the cluster in groups 4 and 6, but this was determined to be insignificant statistically. The daily average food intake for the groups was slightly increased after treatment with silymarin, while groups 4, and 6 had less food intake, but were statistically insignificant, as shown in Figure 1B.
 |
Figure 1: The average rat’s weight [A], and the food consumption in all studied groups during the study period [B]. |
The Bioavailability of silymarin
It was figured out by measurements of plasma Cmax and Tmax from the groups treated with different doses of silymarin. Silybinin (isomeric-compound), was revealed as two-peaks noticed at about 19 & 21 min. The standard curves were linear (r=0.987). The mean silybinin conc. was 40.82±2.4, and silymarin was 68.67±1.7. The silymarin concentration was 58.6±2.9 in the first cluster (1) treated with 80 mg/kg, 74.9±2.2 in cluster (2); treated with 140 mg/kg silymarin, and 81.6±1.4 clusters (3); treated by 200 mg/kg silymarin. The highest concentration of plasma (Cmax) was 0.63±0.5μ/ml, and the time of Cmax (Tmax) was 1.50±0.4 hours. and AUC(0-t) was 2.12±0.53 for 140 mg/kg of silymarin, figure 2.
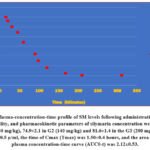 |
Figure 2: Plasma-concentration-time profile of SM levels following administration, relative bioavailability, and pharmacokinetic parameters of silymarin concentration were 58.6±2.9 in the G1 (80 mg/kg), 74.9±2.1 in G2 (140 mg/kg) and 81.6±1.4 in the G3 (200 mg/kg). |
The Efficacy of silymarin on cardiac biomarkers
S100B
Compared to the other groups, plasma S100B level in groups 4, and 6 was notably extremely significant numerically. Prior treatment with SM in the induced-cardiotoxic groups had different results; at a dosage of 140 mg per kg, writing down a significant reduction of S100B level compared to cardiotoxic cluster 6 and control, with 95% CI (p = 0.002), as illustrated in figure 3 (A).
Cardiac Troponin
Plasma troponin I was highly significantly increased with the cardiotoxic-induced group 6 (p=0.0001) and a highly significant increase in group 4 after induction of cardiotoxicity, then a significant decrease after treatment with silymarin, while in silymarin-treated groups was decreased with the dose 140 mg per kg; p< 0.001, no significant difference in group 5 compared with the positive-control, while negative-control; showed highly significant difference compared to cardiotoxic groups as showed in figure 3 (B).
Cardiac CK-MB
Assessment of plasma CK-MB illustrates significant differences in their levels among the cardiotoxic groups 4, 5, and 6 [p=0.007]. Plasma CK-MB was highly significantly increased in group 6 [p=0.0001] and a highly significant increase in group 4 after induction of cardiotoxicity, then a significant decrease after treatment with silymarin, while in silymarin treated groups was decreased with the 140 mg/kg dose (p < 0.001), comparison to cardiotoxic groups showed highly significant difference, while comparing to negative control showed no significant difference Figure 3 (C).
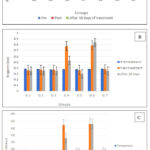 |
Figure 3: Plasma cardiac biomarkers; S100 (A), Troponin I (B), and CK-MB (C) levels pre- and post-treatment in all tested groups. |
Microscopically appearance and histopathology study
The microscopically histopathological section of all groups showed the tissue architecture. The heart-to-body weight was measured for all groups [H/B wt. ratio (g/Kg) x10-3] for the groups 1-7 as follows; 2.88 ±0.18, 2.86±0.1, 2.87±0.11, 3.3±0.2, 2.95±0.05, 3.97±0.11, 2.90±0.21, respectively.
Histopathology of the heart
Histopathological studies of heart sections of the studied groups; silymarin-treated groups (G1, 2, and 3) showed normal cardiac sections, figure 4 (A, B, and C).
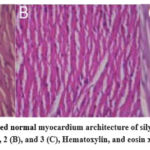 |
Figure 4: Sections showed normal myocardium architecture of silymarin-treated groups; 1 (A), 2 (B), and 3 (C), Hematoxylin, and eosin x400. |
Group 4 treated first with clozapine and then silymarin showed less inflammation than the clozapine-induced cardiotoxicity group (6). At the same time, the group (5), which was treated with silymarin first and then clozapine showed no cardiotoxicity, no cellular infiltration, or inflammation when compared to rats whose cardiotoxicity induction was done by clozapine (G 6); Inflammation abrasions were noted in both heart ventricles for the rats for which induction was done, there were signs of myocardial inflations of the cells, and myocarditis in the heart section. 5 (A-D).
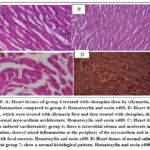 |
Figure 5: A: Heart tissues of group 4 treated with clozapine then by silymarin, showed less inflammation compared to group 6. Hematoxylin and eosin x400. |
Histopathology of the Rat’s Liver and Kidney
Histopathological sections of the rat’s liver for the tested groups showed no morphological changes, and normal architecture, the portal tract showed no fibrosis and no inflammation, and parenchyma showed no inflammation, no necrosis or apoptosis, and no bile stasis.
The microscopic features of the rat’s kidney for all groups showed normal architecture, the sections showed normal glomerulus, normal tubular, and no inflammation, or necrosis. The interstitium is normal, with no fibrosis, or inflammation, and a normal vessel.
Correlation study
The correlation study of plasma biomarker S100B levels to the studied parameters was estimated and analyzed according to their correlation coefficient with significance, and the correlation of Troponin I to CK-MB. It showed that there is a significantly very strong positive-correlation of plasma S100B & Troponin I in groups 4, 5, and 6, with correlation-coefficient {r = 0.990, p < 0.001}, as showed in figure 6 (A), while the correlation results showed that there was a very strong positive-correlation of plasma S100B conc. & CK-MB in groups 4, 5, and 6, the correlation- coefficient {r = 0.981, p < 0.001}, as shown in figure 6 (B). also, the correlation results showed that there is an extraordinarily strong positive-correlation of plasma Troponin I conc. & CK-MB in groups 4, 5, and 6, the correlation-coefficient {r = 0.984, p < 0.001}, as shown in figure 6 (C).
 |
Figure 6: A: The correlation study between plasma S100B concentrations and Troponin I level, a significantly very strong positive correlation of plasma S100B & Troponin I in groups 4, 5, and 6. |
Discussion
Evaluation of the contribution of cardiotoxicity induced by clozapine to cardioprotection by SM has been conducted for the first time in this study. Polypharmacy methods are to blame for the increased cardiac and cardiovascular problems. Drugs administered together with therapy worsen cardiac complications. Silymarin has a wide range of pharmacological effects including antioxidant activity19, anti-cancer effects against several human carcinoma cell lines20, 21, and stimulation of protein-synthesis, cell-regeneration from toxic liver damage, liver cirrhosis, and chronic inflammatory liver diseases22, 23, anti-inflammatory, immune-modulation effects, and neuroprotective24, and cardioprotection13, 25.
The main components of silymarin are silybin A and B accounting for 60–70% 26, the silymarin bioavailability in the silymarin-treated groups showed that Silybin, which is an isomeric compound, appeared as two peaks were detected at about 19, and 21 min., the standard curves were linear, silymarin concentration was high compared to silibinin with significance outcome. With a rise in dosage, the concentration of silymarin corresponded with the maximum concentration of plasma Cmax as well as the corresponding time Tmax and the area under the concentration of plasma curve AUC having compatibility with earlier studies.
Protein S100B is a calcium-tying protein in the semicircular lining of human heart cells27, 28, Continuous rise in the level of the protein characterizes its spread to the damaged tissues which clinically reflects an injury like hypoxia29, trauma30-32. Notably, protein serum level is top in numerous, neurologic, and circumstances33-35.
Focus on cardioprotective impacts of silymarin as a priority of the research being the first one having been undertaken with its relation to the plasma level of S100B protein. The plasma level was high in the rats induced with cardiotoxicity and the positive controls when a comparison was made with the other clusters of rats. The level of plasma was seen to be reduced when subjects were predisposed to silymarin in comparison to rats injected with cardiotoxic negative and positive controls at 95% CI, P equals 0.002).
Explanations are given in terms of research and clinical perspective for S100B, splicing operation of the artery side36, endarterectomy of the carotid37, and ischemia of myocardial38. The cardioprotection ability of silymarin was well demonstrated in this research where groups that were prior treated with it indicated reduced plasma levels. Serum S100B protein concentrations elevated in a key depression and following acute or chronic injection of antidepressants33, 39, 40, Inducing cardiotoxicity with clozapine has the level of serum increase while there was notably no observed elevation of the level of plasma in the clusters of rats studied.
Surgery pretreatment shields heart tissues from reperfusion damage, inflammation, and antioxidants when splicing coronary arteries in humans, Prior treatment of CK-MB and troponin I notably reduced silymarin41. In group 4 Troponin I was observed to elevate after inducing the rats with clozapine cardiotoxic but on silymarin treatment the level significantly decreased. while silymarin pretreated (group 5) showed a substantial variance explaining the cardioprotection nature of silymarin against the cardiotoxicity induction by clozapine, this study concurs with the one previously studied by Altaei (2012)13, 41.
The myocardium comprises numerous marker enzymes such as troponin and CK-MB, and once metabolically spoiled; it disposes of its contents into the extracellular fluid. The original increase in cardiac troponins after myocardial infarction happens concurrently with CK-MB, but it endures for longer than other enzymes42. Amplified actions of troponin, CK-MB could be noted in the plasma of clozapine-induced cardiotoxicity rats during the study. Prior injection of silymarin expressively dropped the release of biomarker enzymes. Assessment of plasma CK-MB displayed a substantial difference in levels of the cardiotoxic induced clozapine in clusters 4 and 5; A notably high rise at the positive cardiotoxic control cluster 6 while a high noteworthy raise in cluster 4 after inducing cardiotoxicity. After silymarin treatment, there was a substantial decline, while the previously treated cluster declined significantly in group 5. This concurs with earlier studies by Altaei, (2013)13, 41. CK-MB, and troponin I biomarkers levels increased, signifying an exhibition of myocardial necrosis—cellular injury with loss of purposeful integrity or cell membrane permeability43. High plasma CK-MB levels submit the existence of an injury inside the heart or necrosis when the comparison is done to the plasma troponin & CK-MB points in other clusters with the control clusters writing down a heart injury because of clozapine injected cardiotoxicity and demolishing cells. its deterioration in silymarin-treated clusters demonstrates silymarin cardioprotective. An increase in CK‑MB followed by an increase in the wet weight of the heart confirms the existence of cardiac hypertrophy and edema. The cardioprotective effects produced from the prior silymarin treatment are associated with the significance of reduced actions of troponin and CK-MB.
Researchers defined potentially deadly myocarditis, pericarditis, and eventual death as associates of clozapine injection44. This research concurs with the observation. scratches were found in both the left and right ventricles in comparison with the control subjects. The experiential examination of microscopical histopathology appeared normal in the end organ tissues building from silymarin-treated clusters. Heart-to-body weight was normal, while cardiotoxic-induced rats by clozapine showed heart injury and inflammation. The heart sections of the clozapine-induced cardiotoxicity (group 4) that were treated first by clozapine and then by silymarin showed less inflammation than the clozapine-induced cardiotoxicity group six (positive control); minimal myocardial damage which was characterized by mild interstitial edema and focal degeneration and necrosis of myofibers, while the pretreated silymarin group (5), which treated by silymarin first then treated by clozapine showed no cardiotoxicity.
Clozapine administration is known to produce free radicals via its quinine metabolites, which react with oxygen resulting in the enhanced production of reactive oxygen species (ROS). This ROS, the highly toxic by-products of aerobic metabolism are known to respond to cell membranes and macromolecules which enhance the creation of lipid peroxides, leading to tissue damage. Lipid peroxidation is an essential pathogenic issue in the necrosis of myocardium while the buildup of lipid hydroperoxides shows cardiac tissue injury 45-47. Silymarin injection could lower lipid peroxide levels 25. The prevention of damage to antioxidant balance in the regulation of inflammatory mediators is done by silymarin26. Inhibition of cellular penetration in prior silymarin-treated clusters and its purpose leading to in condensed production of responsive oxygen species, during ischemia, contributing to the cardioprotective nature of silymarin when subjected to myocardial ischemia and oxidative pressure by potential antioxidant action because of its capability to counteract the creation of free radicals by a high content of flavonoids.
Assessment of histopathological constraints in end organs like the kidneys wrote down an ordinary architecture, with no parameters of inflammation or destruction in all studied groups of rats. The results can explain silymarin hepato- and reno-defensive activities when subjected to clozapine. Silymarin has been hailed by researchers due to its capability to protect the liver from hepatoxic drugs and further occurrence of liver cancer48. This study agrees with that in its action as a hepatoprotective agent. The histopathology analysis of rat liver in cardiotoxicity-induced groups by clozapine showed normal architecture, and no inflammation or necrosis.
Koçarslan A. (2016)49 revealed an intraperitoneal silymarin injection decreases the chances of oxidative anxiety thereby protecting end organs like the liver from severe supraceliac abdominal reperfusion damage in the subjects.
Significance was noted as a strongly optimistic association in plasma S100B & Troponin I, CK-MB concentrations levels from clusters 4-6. Again, an examination of the correlation coefficient for plasma Troponin I and CK-MB points exhibited a solid confident link. This indicates that there is a correlation among the three biomarkers and may be effective in diagnosing heart damage or necrosis.
The distinct types of protein sensors that are involved in membrane repair are likely to coordinate their responses to diverse types of injuries. Defects in the repair genes are known to contribute to various forms of heart disease and muscle disease. The plasma membrane is a vital component of maintaining a healthy cellular environment and ensuring that cells survive. The influx of Ca2+ following membrane rupture is a signal that initiates the repair process. Multiple mechanisms have been identified that involve the recruitment of sensor proteins that are dependent on Calcium50.
The loss of S100A1 in the cell environment can result in the dysfunction of the Ca2+-controlled networks. This can lead to the failure of cardiomyocytes and endothelial cells. In addition, the lack of this receptor in ischemic myocardium can affect the cardiac fibroblasts’ function51, the present study agrees with that.
Acute inflammation during the initial stages of wound healing generates factors that are essential for tissue repair, but a prolonged inflammatory phase may lead to cell destruction and a changed constitution of the extracellular matrix with subsequent failure of epithelialization52. Pretreatment with silymarin normalized the clozapine-induced cardiotoxicity and the elevation of plasma levels of the diagnostic biomarker, suggesting that silymarin could maintain the membrane integrity, restricting the leakage of these biomarkers.
Silymarin could stabilize the myocardial membrane and associated enzymes which are connected to properties of anti-oxidant and anti-inflammatory. The authors agree with the evidence in the research that elucidates the instrument of silymarin antioxidant which is aligning its cardioprotective ability. Deterrence of cardiotoxicity by silymarin and shielding of myocardial necrosis can be described by antioxidant and anti-inflammatory significance impacts on S100B, troponin, and CK-MB levels.
Conclusion
The efficacy of silymarin presented cardioprotection for cardiotoxicity-induced rats by clozapine was proved for the first time in this research. A substantial alteration was observed in the plasma biomarkers S100B, troponin I and CK-MB exhibited a significant difference as compared to the baseline and controls. Also were proven to be supportive indicators in clozapine-induced cardiotoxicity accomplishment or myocardial wounds. The relationship of plasma S100B to cardiac biomarkers disclosed a significant very strong positive correlation. The cardioprotective efficacy of silymarin gives promise for preventing the cardiotoxicity adverse drug reaction and cardiac irreversible damage induced by drugs.
Acknowledgment
The authors would like to thank Isra University.
Conflict of Interest
The authors disclose that there is no conflict of interest.
Funding Sources
There is no funding Source.
References
- Alexandre Destere, Diane Merino, Thibaud Lavrut, Fanny Rocher, Delphine Viard, Milou-Daniel Drici, Alexandre O. Gérard, Drug-induced cardiac toxicity and adverse drug reactions, a narrative review. Therapies. 2023;ISSN 0040-5957, https://doi.org/ 10.1016/j.therap.2023.10.008. (https://www. sciencedirect. com/science/article/pii/S0040595723001695)
CrossRef - Shin DD, Brandimarte F, Luca L De, Sabbah HN, Fonarow GC, Filippatos G. Review of Current and Investigational Pharmacologic Agents for Acute Heart Failure Syndromes. Am J Cardiol. 2007; 4A-23A.
CrossRef - Lee CS. Mechanisms of Cardiotoxicity and the Development of Heart Failure Cardiotoxicity Heart Failure Pathophysiology Cardiomyopathy Toxicity. Crit Care Nurs Clin NA. 2015; Available from: http://dx.doi.org/10.1016/j.cnc.2015.07.002.
CrossRef - Novartis Pharmaceuticals Corporation. Clozaril (clozapine) [package insert]. U.S. Food and Drug Administration [updated December 2022]. Available from: https://www.accessdata.fda.gov/ drugsatfda_docs/ label/2022/019758s101lbl.pdf.
- Curto M, Girardi N, Lionetto L, Ciavarella GM, Ferracuti S, Baldessarini RJ. Systematic Review of Clozapine Cardiotoxicity. Curr Psychiatry Rep. 2016; Available from: http://dx.doi.org/10.1007/s11920-016-0704-3.
CrossRef - Li KJ, Gurrera RJ, Delisi LE. Potentially fatal outcomes associated with clozapine. Schizophr Res. 2018; 23–6. Available from: https://doi.org/10.1016/j.schres.2018.02.058.
CrossRef - De Las CC, Sanz EJ, Ruan C-J, de Leon J. Clozapine-associated myocarditis in the World Health Organization’s pharmacovigilance database: Focus on reports from various countries. Rev Psiquiatr Salud Ment. 2021;S1888–9891(21):00070–7. https:// doi.org/10.1016/j.rpsm.2021.07.004. 126. Alawami M, Wasywich C, Cicovic A, Kenedi C. A systematic review of clozapine induced cardiomyopathy. Int J Cardiol. 2014;176:315–20. https://doi.org/10.1016/j.ijcard.2014.07.103.
CrossRef - Brandi L. Bellissima, Kathryn E. Burns, Nuala A. Helsby, Ellen L. Kingston, Fintan Garavan, Malcom D. Tingle, Clozapine metabolism and cardiotoxicity: A prospective longitudinal study. International Journal of Cardiology.2024;131788:ISSN 0167-5273.https://doi.org/ 10.1016/j.ijcard.2024.131788. (https://www.sciencedirect.com/science/ article/pii/S0167527324001189).
CrossRef - Luper S. A review of plants used in the treatment of liver disease: part 1. Altern Med Rev. 1998;3(6):410–21.
- Schuppan D, Jia JD, Brinkhaus B, Hahn EG. Herbal products for liver diseases: a therapeutic challenge for the new millennium. Hepatology. 1999; 30(4):1099–104.
CrossRef - Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006: 491–504. Available from: http://icmr.nic.in/ijmr/ 2006/November/1103.pdf.
CrossRef - Rašković A, Stilinović N, Kolarović J, Vasović V, Vukmirović S, Mikov M. The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules. 2011; 16(10): 8601–13.
- Altaei T. Protective effect of silymarin during coronary artery bypass grafting surgery. clinical cardiology 2012; 34-38.
CrossRef - Elahe Taghiabadi, Mohsen Imenshahidi, Khalil Abnous, et al., “Protective Effect of Silymarin against Acrolein-Induced Cardiotoxicity in Mice,” Evidence-Based Complementary and Alternative Medicine, vol. 2012, Article ID 352091, 14 pages, 2012. https://doi.org/ 10.1155/2012/352091.
CrossRef - Berridge MJ. ―Inositol trisphosphate and calcium oscillations, ‖ Biochemical Society symposium, 2007. no. 74, pp. 1–7.
CrossRef - Michetti F, Clementi ME, Di Liddo R, Valeriani F, Ria F, Rende M, Di Sante G, Romano Spica V. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int J Mol Sci. 2023 May 31;24(11):9605. doi: 10.3390/ijms24119605. PMID: 37298554; PMCID: PMC10253509.
CrossRef - Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003; 60(6):540–51.
CrossRef - Botelho HM, Leal SS, Cardoso I, Yanamandra K, Morozova-roche LA, Fritz G, S100A6 amyloid fibril formation is calcium-modulated and enhances superoxide dismutase-1 (SOD1) aggregation. J Biol Chem. 2012; 1(1).
CrossRef - Köksal E, Gülçİn İ, Beyza S, Sarikaya Ö, Bursal E, Gu L. In vitro antioxidant activity of silymarin. J Enzyme Inhib Med Chem. 2009; 6366.
CrossRef - Yang S, Lin J, Chen W, Chiu J. Anti-Angiogenic Effect of Silymarin on Colon Cancer LoVo Cell Line. J Surg Res. 2003;138:133–8.
CrossRef - Singh RP, Agarwal R. Prostate Cancer Prevention by Silibinin. Curr Cancer Drug Targets 2004;1–11.
CrossRef - Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. PubMed Commons. 2001; 61(14): 2035–63.
CrossRef - Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Investig. 2002; 22(1):51–65.
CrossRef - Radko L, Cybulski W. Application of silymarin in human and animal medicine. J Preclin Clin Res, 2007;1(1): 22–6.
- Rao PR, Viswanath RK, Pradesh A. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Experimental cardiology. 2007;12(4):179–87.
- Milića N, Miloševića N, Suvajdžića L, Žarkovb M, Abenavolic L. New Therapeutic Potentials of Milk Thistle (Silybum marianum). Nat Prod Commun. 2013; 8(12):1801-10.
CrossRef - Tsoporis JN, Marks A, Kahn HJ, Butany JW, Liu PP, Hanlon DO, et al. Inhibition of Norepinephrine-induced Cardiac Hypertrophy in S100B Transgenic Mice. J Clin Invest, 1998;1609–16.
CrossRef - Gursoy M, Buyukuysal L. Mechanism of S100b Release from Rat Cortical Slices Determined Under Basal and Stimulated Conditions. Neurochem Res. 2010; 429–36.
CrossRef - Nagdyman N, Kömen W, Ko H, Müller C. Early Biochemical Indicators of Hypoxic-Ischemic Encephalopathy after Birth Asphyxia. Pediatr Res. 2001; 49(4):502–6.
CrossRef - Pelinka LE, Toegel E, Mauritz W, Redl H. Serum s 100 b : a marker of brain damage in traumatic brain injury with and without multiple trauma. Shock 2003; 19(3): 195–200.
CrossRef - Dimopoulou I, Korfias S, Dafni U, Anthi A, Psachoulia C, Jullien G. Protein S-100b serum levels in trauma-induced brain death. Neurology, 2003; 243–6.
CrossRef - Hachimi-idrissi S, Auwera M Van Der, Schiettecatte J, Ebinger G. S-100 protein as early predictor of regaining consciousness after out of hospital cardiac arrest. Resuscitation, 2002; 53: 251–7.
CrossRef - Rothermundt M, Arolt V, Wiesmann M, Missler U, Peters M, Rudolf S. S-100B is increased in melancholic but not in non-melancholic major depression. J Affect Disord, 2001; 66: 89–93.
CrossRef - Oliveira R, Gonc CA, Bello A. The ischemic rat heart releases S100B. Life Sci. 2005; 77: 882–9.
CrossRef - Tsoporis JN, Mohammadzadeh F, Parker TG. S100B : a multifunctional role in cardiovascular pathophysiology. Amino Acids, 2011; 843–7.
CrossRef - Boven WJP Van, Morariu A, Sacha ÞP, Gerritsen WB, Waanders FG, Korse TC, . Impact of Different Surgical Strategies on Perioperative Protein S100 A Release in Elderly Patients Undergoing Coronary Artery Bypass Grafting. Innovations (Phila), 2013; 8(3): 230–6.
CrossRef - Legge S Di, Piero V Di, Stani F Di, Gattuso RPR, Lenzi FBVGL. Carotid endarterectomy and gliofibrillar S100b protein release. Neurol Sci, 2003; 351–6.
CrossRef - Tsoporis JN, Mohammadzadeh F, Parker TG. Intracellular and Extracellular Effects of S100B in the Cardiovascular Response to Disease. Cardiovasc Psychiatry Neurol. 2010.
CrossRef - Manev R, Uz T, Manev H. Fluoxetine increases the content of neurotrophic protein S100 b in the rat hippocampus. Eur J Phar-macol, 2001; 2000–1.
CrossRef - Akhisaroglu, M. Manev, R. Akhisaroglu, E. Uz, T. & Manev, H. Both aging and chronic fluoxetine increase S100B content in the mouse hippocampus. Neuro Report, 2003;14(1):14–6.
CrossRef - D. Tagreed Altaei, D. Imad A. Jamal and D. Diyar Dilshad (March 13th, 2013). The Cardioprotection of Silymarin in Coronary Artery Bypass Grafting Surgery, Artery Bypass, Wilbert S. Aronow, Intech Open, DOI: 10.5772/55125. Available from: https://www.intechopen. com/books/artery-bypass/the-cardioprotection-of-silymarin-in-coronary-artery-bypass-grafting-surgery.
CrossRef - Kurian GA, Philip S, Varghese T. Effect of aqueous extract of the Desmodium gangeticum DC root in the severity of myocardial infarction. J Ethanopharmacol. 2005; 97: 457–61.
CrossRef - Nazmul M, Mpharm A, Hossain M, Mpharm R, Subhan N, Al A. Astaxanthin Prevented Oxidative Stress in Heart and Kidneys of Isoproterenol-Administered Aged Rats Astaxanthin Prevented Oxidative Stress in Heart and Kidneys of Isoproterenol-Administered Aged Rats. J Diet Suppl. 2017; 0211(May). Available from: http://dx.doi.org/10.1080/19390211.2017.1321078.
CrossRef - Markovic J, Mitrovic D, Sekuli S. Clozapine-Induced Pericarditis. Afr J Psychiatry (Johannesbg), 2011; (July): 236–8.
CrossRef - Neely, CF. DiPierro, FV. Kong, M. Greelish, JP. & Gardner, TJ. A1 adenosine receptor antagonists block ischemia-reperfusion injury of the heart. Circulation. 1996; 94: 376-80.
- Alankooshi, A. A., Alankooshi, A. A., Hasan, A. F., Tousson, E., El-Atrsh, A. & Mohamed, T. M. Impact of Coriander Seeds Extract Against Thyroidectomy Induced Testicular Damage and DNA Replication in Male Rats. OnLine Journal of Biological Sciences. 2023; 23(2): 193-201. https://doi.org/10.3844/ojbsci.2023.193.201.
CrossRef - Hameed, H. M., Hasan, A. F., Razooki, Z. H., Tousson, E. & Fatoh, S. A. Orlistat Induce Renal Toxicity, DNA Damage, and Apoptosis in Normal and Obese Female Rats. OnLine Journal of Biological Sciences. 2023; 23(1): 25-32. https://doi.org/10.3844/ojbsci.2023.25.32.
CrossRef - Wang X, Zhang A, Sun H. Recent Advances in Natural Products from Plants for Treatment of Liver Diseases. Eur J Med Chem. 2013; Available from: http://dx.doi.org/10.1016/j.ejmech.2012.12.062.
CrossRef - Koçarslan A, Koçarslan S, Aydin MS, et al. Intraperitoneal Administration of Silymarin Protects End Organs from Multivisceral Ischemia/Reperfusion Injury in a Rat Model. Braz J Cardiovasc Surg. 2016; 31(6): 434–439. doi:10.5935/1678-9741.20160072.
CrossRef - Li Z, Shaw GS. Role of calcium-sensor proteins in cell membrane repair. Biosci Rep. 2023 Feb 27; 43(2): BSR20220765. doi: 10.1042/BSR20220765. PMID: 36728029; PMCID: PMC9970828.
CrossRef - Rohde D, Busch M, Volkert A, Ritterhoff J, Katus HA, Peppel K, Most P. Cardiomyocytes, endothelial cells and cardiac fibroblasts: S100A1’s triple action in cardiovascular pathophysiology. Future Cardiol. 2015 May; 11(3): 309-21. doi: 10.2217/fca.15.18. Erratum in: Future Cardiol. 2015 Jul;11(4):502. PMID: 26021637.
CrossRef - Sameen S, Altaei T. Efficacy of topical zinc sulphate on wound healing of experimentally induced skin ulcers by Nicorandil and induction effect on transforming growth factor-β. American Journal of Clinical and Experimental Medicine. 2014; 2(6): 137-150.
CrossRef
list of Abbreviations
ADR: Adverse drug reaction.
AUC: Area under the curve.
Ca2+: Calcium ions.
CK-MB: Creatine kinase-muscle/brain.
Cmax: Maximum plasma concentration.
DMSO: Dimethyl sulfoxide.
HRP: Horseradish Peroxidase.
Tmax: time of maximum plasma concentration.







