Oluwafunbi Christianah Adeleye* and Ida Masana Risenga
and Ida Masana Risenga
Department of Animal, Plants and Environmental Science, Faculty of Science, University of the Witwatersrand, Johannesburg, South Africa.
Corresponding Author E-mail: 2415662@students.wits.ac.za
DOI : https://dx.doi.org/10.13005/bpj/2859
Abstract
The escalating global prevalence of diabetes mellitus presents a significant health concern, prompting exploration into alternative treatments. Recent research highlights the efficacy of newly developed bioactive medications sourced from plants in managing diabetes, surpassing currently used oral hypoglycemic drugs. Medicinal plants' therapeutic characteristics are from secondary metabolites and are greatly influenced by environmental factors. This study investigated the antidiabetic properties of Portulacaria afra, using various extraction solvents under different temperature settings with water deficit conditions, using an in vitro model. Aqueous, methanol, ethyl acetate, and n-hexane extracts from leaf, stem, and root were evaluated for antidiabetic potential under different treatments. Overall, extracts substantially increased in antidiabetic capacity compared to control samples. Aqueous leaf extracts at mid-range cold temperatures (10/15ºC) demonstrated the strongest antidiabetic activity, with an IC50 value of 2.33±0.832mg/ml after a 96-hour treatment. Under extreme cold temperatures (0/5ºC) with water deficit, ethyl acetate stem extracts showed the highest inhibitory action (IC50 2.85±0.111mg/ml). Aqueous stem extracts under hot temperatures showed the strongest inhibitory activity (IC50 1.70±0.666mg/ml) after a 48-hour treatment. Notably, the study provides the first data on the antidiabetic potential of P. afra's leaf, stem, and root extracts, particularly under temperature and water deficit conditions. This could be useful as leads worthy for further drug development against diabetes and related symptoms. The observed α-amylase inhibitory activity in aqueous and ethyl acetate stem extracts is most likely due to the polar compounds, establishing a foundation for future investigations.
Keywords
Antidiabetic activity; Extreme temperatures; Portulacaria afra; Solvents; Water-deficit; Whole plant
Download this article as:| Copy the following to cite this article: Adeleye O. C, Risenga I. M, The In Vitro Assessment of Antidiabetic Activity of the Plant Extracts Obtained from Portulacaria afra Jack. Grown under Concurrent Extreme Temperatures and Water-deficit Conditions. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Adeleye O. C, Risenga I. M, The In Vitro Assessment of Antidiabetic Activity of the Plant Extracts Obtained from Portulacaria afra Jack. Grown under Concurrent Extreme Temperatures and Water-deficit Conditions. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/43fVrqy |
Introduction
The significant role of herbal plants in the field of medicine is due to their inherent therapeutic efficacy. Despite the advancements in modern medicine, numerous diseases, including diabetes mellitus and its associated complications caused by the production of advanced glycation end products, are being treated with medicines of plant origin1. In developing nations, where most of the population has low resources and no access to contemporary treatments, plants remain important in the management of diabetes1. Due to the negative side effects of using insulin and oral hypoglycemic medications, the desire for alternate methods of treating diabetes, such as plant-based medications, has increased in industrialized nations2.
According to WHO recommendations, the use of plant-derived hypoglycemic treatments within traditional medicinal practices holds significant importance3. The antihyperglycemic effects of these treatments are due to their capacity to enhance pancreatic tissue functionality through increased insulin secretion or decreased intestinal glucose absorption. Therefore, treatment with herbal medicines protects β-cells and minimizes glucose fluctuation.
Diabetes mellitus (DM) is a lethal health condition marked by impaired insulin synthesis, which is either hereditary or acquired, and by diminished organ response to secreted insulin. Increased blood glucose levels brought on by such a shortage can harm various bodily systems, including blood vessels and nerves4.
The World Health Organization (W.H.O.) predictions show that the number of diabetic patients globally would surge from 171 million in 2000 to exceeding 366 million by 20305. While According to data from Statistics South Africa6, non-communicable diseases accounted for 58% of the country’s increase in mortality rates between 1997 and 2018. Of these, 12% of adult patients had diabetes, making the condition the second leading cause of death in South Africa. These predicted increases in global diabetes prevalence represent a significant burden on the health system.
The primary objective of treating diabetes mellitus is to successfully lower the increased blood sugar levels. To reduce post-prandial hyperglycemia, the predominant therapeutic strategies for managing diabetes centre on impeding the breakdown of dietary starch via carbohydrate hydrolysing enzymes such as α-amylase and α-glucosidase, alongside stimulating or enhancing insulin activity within target tissues through the utilization of oral hypoglycemic agents7.
Furthermore, the reduction of post-prandial hyperglycemia is achieved by delaying glucose absorption by blocking α-amylase or α-glucosidase in the gastrointestinal system. This procedure slows down the breakdown of carbohydrates, reducing the pace of glucose uptake which in turn dampens the postprandially induced rise in plasma glucose8. Acarbose, miliglitol, and voglibose, are some of the inhibitors currently in clinical use, these inhibitors’ primary negative effects are gastrointestinal, including bloating, stomach pain, diarrheal, and flatulence9.
Secondary metabolites with high therapeutic values like phenolics, flavonoids, glycosides, coumarins, saponins, terpenoids, alkaloids, and others are typically found in plants and contribute to their pharmacological qualities10. Also, plants contain antioxidants which are known to have therapeutic potentials for various diseases. The main function of natural antioxidants is to activate endogenous antioxidants to combat oxidative damage11.
Different factors such as environmental conditions, seasonal variations, plant parts, treatment/harvest period and extraction solvents affect both the growth of plants and the biosynthesis of phytochemicals production, chemical composition, antioxidant, and antidiabetic activities of plants12. Additionally, recent studies have shown that plant responses to stresses caused by combined antagonistic abiotic factors, are phenomenal compared to when exposed to single ones.
In this study we have selected Portulacaria afra, a succulent medicinal plant anda member of the Portulacaceae or Didiereaceae family. It is commonly known as elephant bush (English), spekboom, and porkbush (Afrikaans). P. afra is native to South Africa, Kenya, and Mozambique, it thrives mostly in subtropical biomes. 13. P. afra has recorded high phytochemical contents and pharmacological evidence have shown its antioxidant and antimicrobial properties Tabassum et al.14 and Adeleye & Risenga 15. The in-vitro antioxidant activity observed in P. afra whole plant extracts gives a preliminary prediction for the antidiabetic activity. To the best of our knowledge, there is no scientific documentation on the antidiabetic potential of Portulacaria afra leaf, stem, and root extracts.
Hence, the aim of our study is to assess the α-amylase inhibitory potential of the leaf, stem, and root extracts of P. afra using four distinct extraction solvents characterized by different polarities, and to investigate the effect of concurrent extreme temperatures (hot & cold) and water deficit on the antidiabetic activity of the different parts. This study is essential for determining exposure duration and temperature with water deficit-dependent effects on the plant extracts for the optimal antidiabetic activity, which could potentially be useful in drug production as a remedy for diabetes.
 |
Figure 1: Portulacaria afra leaf and stem. |
 |
Figure 2: Portulacaria afra root (arrow). |
Materials and methods
The chemicals and solvents utilized in this investigation were of analytical variety. The following items were procured from Sigma-Aldrich, USA, under CAS Number 1162-65-8: methanol, n-hexane, ethyl acetate, dimethyl sulfoxide (DMSO), potato starch, 3,5-dinitro salicylic acid (DNSA), sodium potassium tartrate, and sodium hydroxide.
The Conviron system specifications for Model No. PGW 40, Serial No. 170028, manufactured in China (280033R01) are as follows: Voltage – 230/400V, Frequency – 50Hz, Phase – 3, Total Input Amperage – 29.2A, Compressor Rated Load Amperage (R.L.A) – 65.5A, High Design Pressure – 400 PSI, Low Design Pressure – 200 PSI, Control Amperage – 2.0A, Lighting Amperage – 9.4A, Heater Amperage – 4.3A, Fan Horsepower – 2.6 HP, Drier Amperage – 10.9A, along with additional specifications for Receptacle Amperage, Glycol Pump Amperage, Motor Amperage, and Auxiliary Amperage. The Minimum Circuit Ampacity (MCA) required is 31.2A, with a Maximum Overcurrent Protection (MOP) of 32A, and a Short Circuit With-Stand Capacity of 5000 Amps RMS.
Plant Material collection
Portulacaria afra specimens were propagated via cuttings in July 2021 at the University of the Witwatersrand, Johannesburg, South Africa.
Cutting and growing of plants
Healthy cuttings displaying characteristics such as vibrant green leaves, absence of drooping, and no signs of fading foliage were selectively collected from a disease-free parent plant. Cuttings measuring 40–45 cm in length and 4 cm in thickness were harvested using sterile secateurs and subsequently transplanted into the greenhouse environment for cultivation16.
A total of 225 pots, each with a capacity ranging from 2 to 2.5 litres, were used to contain stem cuttings for root development. These pots were filled with Culterrean Potting’s professional cutting mix soil, chosen for its superior drainage, moist environment maintenance, and absence of potentially harmful pathogens detrimental to the initial rooting phase of the cuttings.
The cuttings were delicately inserted and secured within the partially filled potting mix soil before being surrounded by additional potting mix soil17.
The plants were further kept in the greenhouse at the University of Witwatersrand, Johannesburg, South Africa, for three months to allow them to adapt, develop new root systems, and produce new leaves along the stems18. In the greenhouse setting, plants monitoring was conducted while receiving a consistent watering regimen of 500 ml administered every two days due to their low-frequency watering requirements16.
Table 1: Experimental Design
|
|
Control |
Treatment A |
Treatment B |
Treatment C |
Treatment D |
|
Water deficit |
500ml of water every 2nd day. |
No water |
No water |
No water |
No water |
|
Temperatures (˚C night/day) |
25/27 |
0/5 |
10/15 |
30/40 |
35/45 |
|
Episodic Harvest frequency (hrs) |
Once off |
48, 96, 144 |
48, 96, 144 |
48, 96, 144 |
48, 96, 144 |
Sample size per harvest: n= 15/harvest
The settings for the conviron simulations used for the treatments were derived from forecasts regarding changes in the present and future climate conditions by the South African Department of Environmental Affairs 19, IPCC 20, and Mbokodo et al. 21 records. The temperature settings used in this study were chosen based on the length of heat and cold waves from earlier predictions, [(Control (25/27ºC); mid-range high (30/40ºC); and mid-range low (10/15ºC); extreme high (35/45ºC); and extreme low (0/5ºC)].
According to Janda et al.22, each plant was placed in a controlled climate simulation chamber (Conviron chambers, PGW40) with water deficit and high and low temperatures set simultaneously. Ambient conditions for CO2 (400 ppm), humidity (60%) and light (160 nits/level 1), pH, and salinity were maintained. It was arranged for the lights to be on for 12 hours per day, from 6 am to 6 pm while the South African Weather Service’s records23 were used to determine the control temperature ranges.
The plants were exposed to treatments and not watered for up to 144 hours (6 days), and the control samples were placed under 25oC (ambient) and watered every second day with 500ml of water24. The controls were maintained at a maximum nighttime temperature of 25°C (7pm to 5am) and a maximum daytime temperature of 27°C (5am to 7pm). Plants (A, B, C, and D) while receiving treatment did not receive any water. Samples for treatments A and B were subjected to cold temperatures of 0 and 10°C at night and 5 and 15°C during the day. For the C and D treatments, samples were exposed to hot temperatures of 30 and 40°C maximum at night and 35 and 45°C maximum during the day.
Five plants were harvested for all plant parts episodically 3 times for up to 6 days (144hrs), where plants were harvested every 48hrs (48, 96, 144) (Table 1). Harvested samples were oven airdried under 40°C for 2 to 3 days (Loveys et al., 2002). This procedure was repeated three times for each of the treatments. Where, n=45 control (25°), n=45 for (0/5°C), n=45 (10/15°C), n=45 (30/40°C), n=45 (35/45°C) with a total sample size of 225.
Sample preparation and extraction
The leaf, stem, and root of P. afra were gathered and cleansed using deionized water. Subsequently, these specimens were subjected to a four-day drying process at 40°C in a hot air dryer. Following this, an electric grinder was employed to finely powder the dried plant material. The powdered samples were then sealed in foil and stored in an airtight container within a dark cabinet at room temperature (32ºC) until further analysis commenced25.
Plant extract preparation
The crude plant extract was obtained by employing a series of sequential solvent extractions. A mixture of 80% methanol, n-hexane, ethyl acetate, and 100% distilled water (60°C), were chosen based on their respective polarities, was utilized26. Each extraction involved placing 3g of powdered plant material into individual containers and adding 30 ml of the designated solvent27. The methanol, n-hexane, and ethyl acetate extractions were put on a shaker, while the aqueous extracts underwent sonication at a temperature of 60°C for 45 minutes. The shaking process of all solvents lasted for 48 hours, except for the aqueous extracts28. Aqueous extracts underwent centrifugation to reduce viscosity, after which all extracts were filtered using filter paper into vials29. The resulting supernatant was discarded, and the vials were sealed with foil, and then refrigerated at 4ºC before subsequent analyses.
Preparation of buffer and reagent
The buffer and reagents were prepared according to the methodology outlined by Kamtekar et al.30 and Geethalakshmi et al.3 with slight modification.
The phosphate buffer solution was prepared by initially adding 800 ml of distilled water to a suitable container. Subsequently, 20.214 g of Sodium Phosphate Dibasic Heptahydrate powder and 3.394 g of Sodium Phosphate Monobasic Monohydrate were successively introduced into the 800 ml distilled water. The pH of the resulting solution was adjusted to the desired level of 6.9 using either HCl or NaOH. Distilled water was then incrementally added until the total volume reached 1 L.
For the preparation of the DNSA reagent, 1 g of 3,5-dinitrosalicylic acid was dissolved in 50 ml of distilled water while shaking with a magnetic stirrer on a hot plate set at 90-95˚C. Following this, 30 g of Potassium sodium tartrate tetrahydrate was dissolved in 20 ml of distilled water and gradually combined with the previous solution, resulting in the appearance of a milky yellow colour. Subsequently, 20 ml of 2(N) sodium hydroxide solution was added, leading to the development of a transparent orange colour. The final volume was adjusted to 100 ml using distilled water. After complete dissolution, the solution underwent filtration through filter paper, followed by transfer into a dark glass bottle for storage at room temperature (32ºC).
Evaluation of in vitro Antidiabetic Activity
In vitro inhibitory α-amylase assay
The determination of α-amylase inhibitory activity was conducted following the methodology outlined by Kumar et al.31 with few alterations. The leaf, stem, and root extracts of P. afra underwent drying, whereas aqueous extracts were subjected to lyophilization at -85º C.
The solvent-extracted residues were dissolved in a solution of 10% dimethyl sulfoxide (DMSO). Subsequently, all resulting extracts were standardized to a concentration of 100 mg/ml. Triplicates of five serial dilutions were meticulously prepared for each extract, encompassing a concentration range of 20 to 100 mg/ml.
In this study, 500 μl of plant extract was subjected to incubation alongside 500 μl of α-amylase solution for a duration of 10 minutes at a room temperature of 32 °C. The α-amylase solution, comprising 2 units/ml, was prepared by dissolving 0.001 g of α-amylase in a solution containing 100 ml of 0.02 M sodium phosphate buffer (pH 6.9) and 6.7 mM sodium chloride. Following this initial incubation period, 500 μl of a 1% starch solution, derived from the mixture of 1 g of potato starch with 100 ml of distilled water, heated and stirred for 15 minutes, was introduced into the mixture. Subsequently, the combined solution underwent a further 10-minute incubation period at room temperature (32°C).
To cease the reaction, 1 ml of DNSA reagent was introduced to the mixture, followed by a 5-minute incubation in a hot water bath (85°C). The termination of the reaction was indicated by a visible change in colour of the reaction mixture to orange red after the stipulated 5-minute incubation, upon which the mixture was removed from the water bath and allowed to cool to room temperature. The volume was then adjusted to 5 ml with distilled water. Blank experiments were conducted by substituting the enzyme with buffer, while the plant extract served as the positive control. Subsequently, the absorbance of the resultant solution was measured at 540 nm using a spectrometer. The absorbance was measured with a spectrometer at 540 nm. The inhibition (%) which is the sample concentration (mg/ml) required to decrease the absorbance by 50 % of alpha amylase was expressed with the following equation:
(Ac+) – (Ac–) – (As – Ab) ÷ (Ac+) – (Ac–) × 100.
Where, Ac+ is the absorbance of 100% enzyme activity (only solvent with enzyme),
Ac– is the absorbance of 0% enzyme activity (only solvent without enzyme),
As is the absorbance of test sample with enzyme,
Ab that absorbance of test sample without enzyme.
Statistical Analysis
The concentration values were graphed against the % inhibition values employing Microsoft Excel to derive a trend line equation which facilitating the calculation of IC50. Statistical analysis was done with a one-way analysis of variance (ANOVA) to ascertain significant differences. All experiments were conducted in triplicate, and data were presented as mean ± standard error (SE) when n = 3. Statistical significance was determined at P < 0.05, with Tukey test HSD in RStudio used to pinpoint specific significant differences.
Results and Discussion
Plants are exposed to a variety of biotic and abiotic elements as they grow and develop, and in response, they activate their defence mechanism. These fluctuating and sometimes harmful external environmental factors interact with plants32. Being non-motile organisms, plants have complex alternate defence mechanisms that use a wide range of chemical compounds as coping mechanisms for stressful situations. Secondary metabolites have a significant impact on how plants adjust to their environment32. The capacity of plants to synthesize these metabolites is nearly infinite.
Environmental factors, seasonal variations, plant part, and harvest/treatment period, affect the chemical composition, antioxidant, and antidiabetic activities of medicinal plants33.
Maintaining close to normal levels of glycemic control in both the fasting and post-prandial phases is the key therapeutic objective for diabetic patients. Numerous natural resources have been studied in relation to inhibiting the generation of glucose from carbohydrates in the gut or absorbing glucose from the intestine7. The α-amylase (α-1, 4-glucan-4- glucanohydrolases) is one of the main secretory products of the pancreas and salivary glands and is present in bacteria, plants, and higher animals. It aids in the digestion of starch and glycogen. Inhibiting their activity in the human digestive system is thought to be useful in managing diabetes by reducing the absorption of glucose produced when these enzymes break down starch7.
In this study, four extraction solvents were used to prepare P. afra leaf, stem, and root extracts under concurrent extreme hot and cold temperatures, mid-range high (30/40ºC); and mid-range low (10/15ºC), extreme high (35/45ºC); and extreme low (0/5ºC) with water deficit conditions. The extracts were evaluated for in vitro antidiabetic activity using the in vitro inhibitory α-amylase assay, where IC50 value is the amount of extract required to inhibit α-amylase activity by 50%. Lower IC50 values indicate stronger antidiabetic ability of the extracts, which was used to detect the potential antidiabetic effect of P. afra extracts. P. afra parts showed significant differences, with extracts showing significant differences (P < 0.05). The antidiabetic activity of each part of P. afra extract at different treatment periods is shown in Figures 3-6. A notable increasing trend of antidiabetic capacity was seen across all parts when compared to the control samples under simultaneous cold temperatures [mid-range low (10/15ºC); and extreme low (0/5ºC)] with water-deficit conditions. As shown in Figure 3, the aqueous leaf extracts under mid-range cold temperatures (10/15ºC) showed the strongest antidiabetic activity with IC50 value of (2.33±0.832mg/ml) amongst the plant’s parts after a 48-hour treatment period while the IC50 value for the control sample was (208.818±0.064mg/ml). Figure 4 shows the antidiabetic activity under concurrent extreme cold temperatures (0/5ºC) and water deficit, where ethyl acetate stem extracts showed the highest inhibitory action with IC50 value of (2.85±0.111mg/ml) against α-amylase after a 96-hour treatment period, while the IC50 value for the control sample was (324.16±0.111mg/ml). The variation observed in the activity among the plant organs at the different treatment periods suggests a possible corresponding shift in the accumulation of some compounds responsible for the activity12. Since most of these phytochemicals are produced in response to external stimuli such as light intensity, moisture/water deficit stress, and temperature amongst others34,35.
Similarly, Figures 5 and 6 show observed variations in the α-amylase inhibitory activity of the plant extracts under concurrent hot temperatures [mid-range high (30/40ºC); and extreme high (35/45ºC)] with water deficit conditions. A shift in antidiabetic activity was observed in the extracts in comparison with the control samples. The antidiabetic activity of the aqueous stem extract under the mid-range hot temperatures (30/40ºC) showed the strongest inhibitory activity with IC50 (1.70±0.666mg/ml) after a 48-hour treatment period while a similar trend was observed also in the aqueous stem extracts under the extreme hot temperatures (35/45ºC) with the highest inhibitory activity with IC50 (1.99±0.626mg/ml) after a 96-hour treatment period, with IC50 (10.61±0.127mg/ml) for the control sample.
Considering all these, the overall strongest inhibitory activity against α-amylase enzyme activity under both cold and hot temperatures with water deficit condition was found in the aqueous stem extracts under the mid-range hot temperatures (30/40ºC); with IC50 value (1.70±0.666mg/ml) after a 48-hour treatment period. Similar result was observed in Usman et al. 33 where the oil from (hot-dry) season harvested plants showed stronger antidiabetic activity than the essential oil from the harvested plants during the (wet-cold) season. Research by Chelladurai and Chinnachamy10 also reported the antidiabetic activity of aqueous stem extract of Salacia oblonga to be effective against α-amylase.
The marked variation in antidiabetic activity in this study may also be attributed to the polar and non-polar compounds present in the plant parts based on the extraction solvent. From a wide array of experiments, it has been established that methanol and water targets sugars, amino acids, and glycosides during phytochemical screening. Conversely, alkaloids, aglycones and glycosides are extracted with ethyl acetate while n-hexane extracts waxes, fats, fixed oils and volatile36,37.
The high presence of various phytochemicals like tannins, terpenoids, phenolics and flavonoids previously detected in P. afra parts from earlier findings 14, 15, have potential inhibitory effects on α-amylase enzyme due to their ability to bind with proteins, which in turn contributes to the lowering of postprandial hyperglycemia38.
Additionally, an extensive range of compounds, including arylpyrones and styrylpyrones, stibenes, tannins, coumarins, flavonoids, lignins, and lignans, fall within the phenolics category, being synthesized in reaction to abiotic stress39.
This correlates to this study’s observations and might explain the observed inhibitory effects across the different extracts of P. afra.
(More information advised to be written for Results)
The in vitro antidiabetic potential of all the extracts was determined by in vitro inhibitory α-amylase assay, where IC50 value is the amount of extract required to inhibit α-amylase activity by 50%. Lower IC50 values indicate stronger antidiabetic ability of the extracts, which was used to detect the potential antidiabetic effect of P. afra extracts. Figures 3-6 experimentally reveal the various changes observed in the antidiabetic activities of P. afra plant parts’ extracts with different solvents and treatment periods, under hot and cold temperatures [mid-range high (30/40ºC); and mid-range low (10/15ºC), extreme high (35/45ºC); and extreme low (0/5ºC)] concurrently with water deficit conditions. P. afra plant parts showed significant differences, with extracts showing statistically significant differences (p < 0.05), and were indicated by different letters (a, b, c, d) within the same column (Tukey’s post-hoc test).
 |
Figure 3: Graphical representation of antidiabetic activity in P. afra plant parts under 10/15°C with water deficit conditions.
|
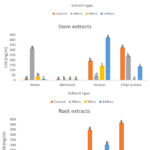 |
Figure 4: Graphical representation of antidiabetic activity in P. afra plant parts under 0/5°C with water deficit conditions.
|
 |
Figure 5: Graphical representation of antidiabetic activity in P. afra plant parts under 30/40°C with water deficit conditions.
|
 |
Figure 6: Graphical representation of antidiabetic activity in P. afra plant parts under 35/45°C with water deficit conditions.
|
Conclusions
The present study explores the potential antidiabetic activity of P. afra leaf, stem, and root with four extraction solvents and with focus on the inhibitory effects on α-amylase under extreme temperatures with water deficit condition. The results of this study also highlighted the effect of concurrent extreme temperatures with water deficit condition, treatment period, plant part and extraction solvent on the antidiabetic potential of P. afra.
The antidiabetic potential of P. afra leaf, stem and root extracts with the control samples were characterized by their inhibitory effect on α-amylase activity. P. afra leaf, stem and root have significant inhibitory effects against α-amylase enzyme. A marked variation in the antidiabetic activity were observed among the plant parts based on the extraction solvent and treatment period for all temperature ranges with water deficit condition. Of the extracts tested from P. afra, aqueous stem extracts under mid-range hot temperatures after a 48-hour treatment period showed the strongest inhibition on the enzyme (α-amylase) across all treatments, which may be attributed to the polar compounds present based on the solvent extraction and the temperature exposure duration. This study is the first to report the antidiabetic potential of the leaf, stem, and root of P. afra with the effect of concurrent extreme temperatures and water deficit.
Conclusively, the results of the study indicate that P. afra leaf, stem and root possess great antidiabetic potential from the in vitro assay performed. Therefore, the antidiabetic property can be credited to the rich phytochemicals and antioxidant activity possessed by the plant, according to earlier research. For future production of this species, this work has shown how environmental factors may influence the antidiabetic potential of specific plant sections. Subsequent research is necessary to isolate antidiabetic compounds and conduct in vivo studies.
Acknowledgements
Authors are grateful to God, and South Africa’s National Research Foundation (NRF) for supporting this work.
Conflict of interest
The authors declare that there are no conflicts of interest.
Funding source
This work was supported by South Africa’s National Research Foundation (NRF) under grant number: TTK201129577193
References
- Singab A. N, Youssef F. S, Ashour M. L. Medicinal plants with potential antidiabetic activity and their assessment. Med Aromat Plants. 2014; 3(151): 2167-0412.
- Poongunran J, Perera H. K. I, Fernando W. I. T, Jayasinghe L, Sivakanesan R. Alpha-glucosidase and alpha-amylase inhibitory activities of nine Sri Lankan antidiabetic plants. British Journal of Pharmaceutical Research. 2015; 7(5): 365-374.
CrossRef - Geethalakshmi R, Sarada D. V. L, Marimuthu P, Ramasamy K. α-Amylase inhibitory activity of Trianthema decandra L. International Journal of Biotechnology and Biochemistry. 2010; 6(3): 369-376.
- Das S. K, Samanta L, Thatoi H. In vitro antidiabetic and antioxidant potentials of leaf and stem bark extracts of a mangrove plant, Xylocarpus granatum. Journal of Herbs, Spices & Medicinal Plants. 2016; 22(2): 105-117.
CrossRef - American Diabetes Association. (2010). Diagnosis and classification of diabetes mellitus. Diabetes care, 33(Supplement_1), S62-S69.
- Statistics South Africa. Available from: https://www.statssa.gov.za/
- Thrikawala V. S, Deraniyagala S. A, Dilanka Fernando C, Udukala D. N. In Vitro α-Amylase and Protein Glycation Inhibitory Activity of the Aqueous Extract of Flueggea leucopyrus Willd. Journal of Chemistry. 2018.
CrossRef - Zinjarde S. S, Bhargava S. Y, Kumar A. R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC complementary and alternative medicine. 2011; 11(1): 1-10.
CrossRef - Michel C. G, Nesseem D. I, Ismail M. F. Anti-diabetic activity and stability study of the formulated leaf extract of Zizyphus spina-christi (L.) Willd with the influence of seasonal variation. Journal of ethnopharmacology. 2011; 133(1): 53-62.
CrossRef - Chelladurai G. R. M and Chinnachamy C. Alpha amylase and Alpha glucosidase inhibitory effects of aqueous stem extract of Salacia oblonga and its GC-MS analysis. Brazilian Journal of Pharmaceutical Sciences. 2018; 54.
CrossRef - Sartor T, Xavier V. B, Falcão M. A, Mondin C. A, Dos Santos M. A, Cassel E, Santarém, E. R. Seasonal changes in phenolic compounds and in the biological activities of Baccharis dentata (Vell.) GM Barroso. Industrial crops and products. 2013; 51: 355-359.
CrossRef - Rahali N, Mehdi S, Younsi F, Boussaid M, Messaoud C. Antioxidant, α-amylase, and acetylcholinesterase inhibitory activities of Hertia cheirifolia essential oils: Influence of plant organs and seasonal variation. International journal of food properties. 2017; 20(sup2): 1637-1651.
CrossRef - Oakes A. J. Portulacaria afra Jacq.: a potential browse plant. Economic Botany. 1973 Oct 1; 413-416.
CrossRef - Tabassum S, Ahmad S, Rehman Khan, K. U, Tabassum F, Khursheed A, Zaman Q. U, Bukhari N. A, Alfagham A, Hatamleh A. A, Chen Y. Phytochemical Profiling, Antioxidant, Anti-Inflammatory, Thrombolytic, Hemolytic Activity In Vitro and In Silico Potential of Portulacaria afra. Molecules. 2022 Apr 7; 27(8):2377.
CrossRef - Adeleye O. C, Risenga I. M. Screening of phytochemical profile and biological activities in the leaves, stems, and roots of South African Portulacaria afra using four extraction solvents. Biomedical and Pharmacology Journal. 2022; 15(3):1561-1572.
CrossRef - Thompson D. I, Mtshali N. P, Ascough G. D, Erwin J. E, Van Staden J. Flowering control in Watsonia: Effects of corm size, temperature, photoperiod, and irradiance. Scientia horticulturae. 2011; 129(3): 493-502.
CrossRef - Semwal V. K, Khanna-Chopra, R. Enhanced oxidative stress, damage and inadequate antioxidant defense contributes towards insufficient recovery in water deficit stress and heat stress combination compared to either stress alone in Chenopodium album (Bathua). Physiology and Molecular Biology of Plants. 2020; 26:1331-1339.
CrossRef - Raju L. K, Mana G. K, Cheruthazhakkatt S, Sarojini S. K, Balachandran I. Phytochemical contents and in vitro antioxidant activities of aqueous, hydroalcoholic and methanolic extracts of shankhapushpi (Clitoria ternatea. L) Plant cultivated under polyhouse and open field conditions. Journal of Herbs, Spices & Medicinal Plants. 2022; 28(3): 217-236.
CrossRef - South African Department of Environmental Affairs. South Africa’s draft National Climate Change Adaptation Strategy [document on the Internet]. c2019 [cited 2019 Jun 05]. Available from: https://www.google.com/ url?sa=t&source=web&rct=j&url =https://www.environment.gov.za/sites/default/files/legislations/session2_draftnational_adaptationstrategy.pdf&ved=2ahUKEwio3aXQitfiAhUvVRUIHdYkA9UQFjADegQIBhAB&usg=AOvVaw0tV53-1w9QW-kNo19rQeva
- IPCC 2018 “Global Warming of 1.5°C: Summary for Policymakers,” in Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Eds.
- Mbokodo I, Bopape M. J, Chikoore H, Engelbrecht F, Nethengwe N. Heatwaves in the future warmer climate of South Africa. Atmosphere.2020; 11(7):712.
CrossRef - Janda T, Szalai G, Tari I, Paldi E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta. 1999; 208: 175-180.
CrossRef - SAWS (2020) Weather questions. Retrieved July 23, 2020; from https://www.weathersa.co.za/home/weatherques [Google Scholar]
- Loveys B. R, Scheurwater I, Pons T. L, Fitter A. H, Atkin O. K. Growth temperature influences the underlying components of relative growth rate: an investigation using inherently fast‐and slow‐growing plant species. Plant, cell & environment. 2002; 25(8): 975-988.
CrossRef - Sabharwal H, Shukla G, Kondepudi, K. K, Maurya R, Kapila S, Dogra N. Phytochemical Analysis, and In Vitro Assessment of Extracts of Rhodobryum roseum for Antioxidant, Antibacterial and Anti-Inflammatory Activities. Journal of Herbs, Spices & Medicinal Plants. 2023; 1-19.
CrossRef - Koffi E, Sea T, Dodehe Y, Soro S. Effect of solvent type on extraction of polyphenols from twenty-three Ivorian plants. Journal of Animal and Plant Sciences (JAPS). 2010; 5(3): 550-558.
- Roghini R, Vijayalakshmi K. Phytochemical screening, quantitative analysis of flavonoids and minerals in ethanolic extract of Citrus paradisi. International Journal of Pharmaceutical Sciences and Research. 2018; 9(11): 4859-4864.
- Razmavar S, Abdulla M. A, Ismail S. B, Hassandarvish P. Antibacterial activity of leaf extracts of Baeckea frutescens against methicillin-resistant Staphylococcus aureus. BioMed research international. 2014.
CrossRef - Gnanasekaran N, Kalavathy S. Drought stress signal promote the synthesis of more reduced phenolic compounds (chloroform insoluble fraction) in Tridax procumbens. Free Radicals and Antioxidants. 2017; 7(1): 128-136.
CrossRef - Kamtekar S, Keer V, Patil V. Estimation of phenolic content, flavonoid content, antioxidant, and alpha amylase inhibitory activity of marketed polyherbal formulation. Journal of applied pharmaceutical Science. 2014; 4(9): 061-065.
- Kumar P, Mehta M, Satija S, Garg M. Enzymatic in vitro anti-diabetic activity of few traditional Indian medicinal plants. Journal of Biological Sciences. 2013; 13(6): 540-544.
CrossRef - Mahajan M, Kuiry R, Pal P. K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. Journal of Applied Research on Medicinal and Aromatic Plants. 2020; 18: 100255.
CrossRef - Usman L. A, Oguntoye O. S, Ismaeel R. O. Effect of seasonal variation on chemical composition, antidiabetic, and antioxidant potentials of leaf essential oil of Eucalyptus globulus L. Journal of Essential Oil-Bearing Plants. 2020; 23(6): 1314-1323.
CrossRef - Mirdehghan S. H, Rahemi M. Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Scientia Horticulturae. 2007; 111(2): 120-127.
CrossRef - Treutter D. Biosynthesis of phenolic compounds and its regulation in apple. Plant Growth Regulation. 2001; 34: 71-89.
CrossRef - Altemimi A, Lakhssassi N, Baharlouei A, Watson D. G, Lightfoot D. A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017; 6(4): 42.
CrossRef - Smetanska I. Sustainable production of polyphenols and antioxidants by plant in vitro cultures. Bioprocessing of Plant In Vitro Systems. 2018; 225-269.
CrossRef - Osadebe P. O, Omeje E. O, Uzor P. F, David E. K, Obiorah D. C. Seasonal variation for the antidiabetic activity of Loranthus micranthus methanol extract. Asian Pacific Journal of Tropical Medicine. 2010; 3(3):196-199.
CrossRef - Albergaria E. T, Oliveira A. F, Albuquerque U. P. The effect of water deficit stress on the composition of phenolic compounds in medicinal plants. South African Journal of Botany. 2020; 131:12-7.
CrossRef







